Abstract
Gout is the most common inflammatory arthritis in an elderly population, and can be diagnosed with absolute certainty by polarization microscopy. However, diagnosis may be challenging because atypical presentations are more common in the elderly. Management of hyperuricemia in the elderly with gout requires special consideration because of co-medication, contra-indications, and risk of adverse reactions. Urate-lowering agents include allopurinol and uricosuric agents. These also must be used sensibly in the elderly, especially when renal function impairment is present. However, if used at the lowest dose that maintains the serum urate level below 5.0 to 6.0 mg/dL (0.30 to 0.36 mmol/L), the excess urate in the body will eventually be eliminated, acute flares will no longer occur, and tophi will resolve. Febuxostat, a new xanthine oxidase inhibitor, is welcomed, as few alternatives for allopurinol are available. Its pharmacokinetics and pharmacodynamics are not significantly altered in patients with moderate renal function or hepatic impairment. Its antihyperuricemic efficacy at 80 to 120 mg/day is better than “standard dosage” allopurinol (300 mg/day). Long-term safety data and efficacy data on tophus diminishment and reduction of gout flares have recently become available. Febuxostat may provide an important option in patients unable to use allopurinol, or refractory to allopurinol.
Introduction
Gout is the collective name for several disorders that are characterized by the formation and deposition of monosodium urate (MSUr) crystals. The condition is associated with recurrent episodes of acute joint pain due to the deposition of MSUr crystals in the synovial fluid. In addition to the effects observed in the joints, skin/subcutaneous tissue and kidneys may also be affected by tophaceous deposits, cellulitis, urate nephropathy, and/or kidney stones, respectively. In most cases, no identifiable underlying cause of gout is present, but evident factors are usually present that may contribute to increases in urate (uric acid) levels, such as reduced renal function, obesity, and the use of diuretics. Hyperuricemia may exist for several years to decades before the first symptoms of gout attacks appear; therefore it is a disease associated and correlated with aging.
Gout is one of the most common inflammatory arthritis affecting the elderly; however in general it appears to be poorly managed.Citation1–Citation3 Partly this is due to an absence of sufficient treatment strategies and guidelines. In 2006, the European League against Rheumatism (EULAR) published the first international recommendations for the diagnosis and treatment of gout.Citation4,Citation5 The development of these EULAR and British Society of Rheumatology (BSR) guidelines coincided with improved professional and patient education as well as the urge for improved professional performance. Until recently, allopurinol was the only antihyperuricemic drug worldwide available. Two uricosurics with barriers regarding availability (benzbromarone and probenecid) have been available for years, but their exact place in treatment strategy is not clear. In 2008 and 2009 respectively, European Medicines Agency (EMEA) and Food and Drug Administration (FDA) have approved febuxostat, a novel selective xanthine inhibitor, for the treatment of hyperuricemia in gout patients, respectively. Febuxostat greatly expands the treatment options for refractory or allopurinol-intolerant gout, given the huge prevalence of the disorder and good worldwide availability of only one antihyperuricemic drug.
The incidence and prevalence of gout in the elderly is increasing.Citation1,Citation6,Citation7 This appears related to improved lifespan leading to similar increases in age-related diseases (eg, cardiovascular diseases) and their associated adverse effects of treatment (eg, diuretics and low-dose salicylates) which can increase the risk of gout. “Elderly onset gout” differs from “classical” gout found in middle-aged men in several respects: no male predominance but an equal gender distribution, polyarticular presentation with upper-extremity-joint involvement, fewer acute gouty episodes, indolent clinical course, and an increased incidence of tophi.Citation1,Citation8 Several reviews have addressed the specific challenges of gout treatment in the elderly.Citation1,Citation8–Citation11 This review will focus on febuxostat for the management of gout in the elderly.
Gout pathophysiology
Uric acid is formed from nucleic acid either endogenously from cell breakdown or exogenously from metabolism of food. Cooling and acidification of the microenvironment, which can result in acute formation of urate crystals, reduce the solubility of MSUr. The gut excretes one-third of urate and two-thirds are excreted renally. Renal urate transport is typically explained by a 4-component model: glomerular filtration, a near-complete reabsorption of filtered urate, subsequent secretion, and postsecretory reabsorption in the remaining proximal tubule.Citation12 Recently, several new urate transporters have been identified as playing key roles in urate homeostasis, including URAT-1, and Glut9.Citation12,Citation13 The regulation of serum uric acid levels is under a strong genetic control. A recent meta-analysis of genome-wide association scans shows that common DNA variants at 9 different loci are associated with uric acid concentrations.Citation14
Excessive consumption of alcohol (particularly beer), sweetened soft drinks, fructose, meat, and seafood can also increase levels of serum urate (sUr).Citation12
Inhibition of urate transporters can be achieved by uricosurics, and production of uric acid can be inhibited using xanthine oxidase inhibitors, such as allopurinol. Febuxostat is a new selective inhibitor of xanthine oxidase. Uric acid deposits can also be lysed by the enzyme uricase, the coding gene for which became defective in humans in the Miocene because of an evolutionary mutation. The combined absence of uricase and almost total reabsorption of filtered urate explains that humans (and the greater apes) have 10-fold higher sUr levels than other mammals.
Drug-induced hyperuricemia
Chronic diuretic therapy is associated with reduced excretion of uric acid. Mechanisms are increased uric acid reabsorption in the proximal tubule secondary to volume depletion, and competition between the diuretic and uric acid for the organic acid secretory mechanism in the proximal tubule.Citation15 Low-dose diuretic therapy in hypertensive patients does not seem to alter serum urate levels significantly.Citation15,Citation16 Indeed, the requirement for anti-gout therapy in hypertensive patients is doubled for thiazide doses of ≥25 mg/day (in hydrochlorothiazide equivalents); no significant increase in risk is seen for lower doses.Citation15,Citation17 Similarly, low-dose therapy with a loop diuretic is not associated with hyperuricemia.Citation15,Citation18 However, low-dose diuretic therapy may be effective in hypertension but insufficient in patients with chronic heart failure who often additionally suffer from chronic renal failure.
Salicylates are known to interact with renal urate handling and low doses inhibit urate excretion. In one study it was found that even mini-dose aspirin (75 mg/day) was associated with a 15% decrease of urate excretion in elderly patients.Citation19 Thus, in elderly people with cardiovascular diseases not only diuretics, but also mini-dose aspirin is of importance.
Characteristics of gout presentation
Gout can be diagnosed with certainty only by identification of urate (MSUr) crystals, present in joints during acute attacks of gout, or in tophi. The clinical practitioner can confirm the presence and type of crystals by polarization microscopy.Citation4,Citation20,Citation21
The characteristic profile of gout is that of severe monoarthritis occurring within several hours. The first metatarsophalangeal joint is affected in 50% of gout attacks, and this is known as podagra. Gout may be localized in other joints, but shoulders, hips, and the vertebral column are rarely affected. The initial gout attack usually involves monoarthritis, but long-term gout over several years may become polyarticular and could lead to increasing joint damage. Similarly, a positive uric acid balance over a number of years can cause tophaceous deposits, possibly with periodic arthritis.
Urate production
Primary gout tends to involve low urate excretion, which is primarily originated in the proximal tubule. Only a minority of cases involves overproduction of urate. In some treatments of cancer (particularly lymphomas and leukemias), patients can develop tumour lysis syndrome including severe hyperuricemia with risk of urate nephropathy.
Urate nephropathy in gout
Aggressive chemotherapy among patients with chronic leukemia or malignant lymphoma could cause an excessive supply of uric acid resulting in acute urate nephropathy due to the deposition of urate crystals in collection ducts and ureters. Several kinds of urate crystals can be found, including uric acid crystals, amorphous urate, monosodium urate, and ammonium urate crystals.
In chronic hyperuricemia, the risk of developing renal crystals increases as serum urate concentrations rise. The risk is about 10% with serum urate 0.42 to 0.48 mmol/L, but can rise to 50% with serum urate concentrations >0.70 mmol/L. In the absence of stones or other risk factors (such as hypertension), the risk of urate nephropathy has generally been considered low.Citation22
Radiographic presentations of gout
X-ray examination at the initial onset of gout has revealed no abnormalities except for possible pre-existing arthrosis and soft tissue edema. Cartilage and bone might be affected by chronic and/or recurring arthritis, and subsequently exhibit narrowing of the joint cavity because of the disappearance of cartilage, and erosions or cysts because of contact with juxta-articular bone. These abnormalities and the appearance of the erosions may raise suspicions of gout, but erosions are a secondary manifestation and non-diagnostic characteristic of early gout. In chronic gout, however, some of these characteristic changes can help with diagnosis.
Treatment strategies for gout
Several approaches to the treatment of gout are available depending on the patient’s presentation of the disease. Optimal treatment often requires a combination of pharmacological intervention and lifestyle changes. Treatment should be tailored to the patient’s prognostic factors (high sUr, previous attacks, and radiographic signs), the clinical phase of the disease (acute, recurrent, tophaceous) and general risk factors, such as obesity, alcohol consumption, renal impairment, use of diuretics or other risk factors for secondary hyperuricemia. Primary prevention of gout often involves changes in lifestyle, such as a low-purine/weight-reducing diet or restricting alcohol intake; however, many patients are unlikely to undertake such changes until they are diagnosed with the disease, which often occurs when the symptoms are presented in the form of an attack of gout. Acute gout is usually treated by reducing the inflammation of the affected joint with non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, corticosteroids, and cooling.Citation5,Citation23–Citation26 Once the acute gout has subsided the objective is to prevent disease recurrence. This might involve lifestyle changes and low doses of NSAIDs or colchicine. In patients with high sUr levels who suffer from frequent attacks of gout, the use of urate-lowering drugs is warranted.
The following is an overview of the different drug classes and their potential use as part of the treatment strategies for gout. Information on these drugs is presented in and the current therapeutic strategy is summarized in .
Table 1 Antihyperuricemic drugs in gout
Table 2 Suggested experience-based strategy for initiation of antihyperuricemic therapy
Some drugs have a documented (modest) urate lowering side effect because of enhanced urate clearance by the kidney. These include losartan, fenofibrate, high dose aspirin and vitamin C.Citation27–Citation30 In patients with gout, these drugs might be preferred above other drugs in the same class.
Primary prevention of gout
Primary prevention of gout involves changes in lifestyle, such as changes to diet (low-purine/weight-reducing diet) and restricting alcohol consumption. No randomized studies have been conducted evaluating the effect of lifestyle changes on the incidence of attacks in patients with gout. Nevertheless, experts agree that lifestyle changes may have some effect. Physicians in daily practice also give lifestyle advice, when gout symptoms appear. However, fewer than 20% of patients with gout seeking medical advice are prepared to make long-term changes in lifestyle.Citation31 Recently, the negative role of meat, seafood and beer consumption, and the protective role of dairy products in the development of gout were demonstrated in a prospective study over a 12-year period among a population of around 47,000 healthy male subjects.Citation32
Treatment options for prevention of recurrence of gout
When gout has subsided, it is important to reduce the sUr concentration to prevent recurrence of gouty attacks. This involves restriction of alcohol, weight-loss in cases of obesity (not too rapidly as this can trigger gout), and ensuring adequate diuresis. Dietary measures are very important.Citation32 However, a strict low-purine diet can achieve only a limited reduction in sUr levels (≤0.10 mmol/L or 1.7 mg/dL), and many patients have difficulty with adhering to long-term dietary changes.Citation31 If possible, it is recommended that patients should discontinue diuretics if indicated for hypertension (find alternative antihypertensives), but should continue diuretics if indicated for overhydration/congesive heart failure. However, the effectiveness of these measures is limited and is not supported by controlled studies.
With tophaceous or recurrent gout (≥2 attacks per annum) the use of urate-lowering therapy may be warranted.Citation33 In contrast, asymptomatic hyperuricemia does not require specific treatment. The maintenance dose of urate-lowering drugs is preferably adjusted to the clinical effect: (1) prevention of gout attacks without using colchicine and/or NSAIDs, (2) disappearance of tophi based on the sUr-concentration with tophaceous deposits. Tophi may well disappear when lower sUr target values have been achieved to at least “normal” levels, but preferably low to normal (≤0.30 mmol/L).Citation34 In order to prevent further attacks of gout, stable sUr target values of at least ≤0.36 mmol/L are required, but in some patients ≤0.30 mmol/L may well be preferable; as has also been recommended by the British guidelines.Citation35–Citation38
There is some but not much evidence for these biochemical targets. But in practice they give clarity also to the patient. Another predefined aim of therapy particularly in elderly gout patients may be adhered to by clinicians treating elderly, ie, a normalization/any lowering of sUr to a level at which the gout patient does not need the chronic use of NSAIDs, glucocorticoids or colchicine; which was not specifically claimed for in aforementioned guidelines. Two main classes of drugs that reduce serum urate concentrations exist: xanthine oxidase inhibitors (XOi), such as allopurinol and febuxostat, and uricosuric drugs, such as benzbromarone and probenecid. XOi work by inhibiting uric acid production, thereby reducing serum urate concentrations. Uricosuric drugs inhibit the reabsorption of uric acid mediated by urate transporters. Rather few comparative randomized studies have been carried out comparing the effectiveness of these drugs.Citation39–Citation44
In the elderly, gout is often accompanied by renal function impairment and urate excretion is often low to very low. It must be kept in mind that when starting therapy with an XOi (uricostatics) it can take upto 8 weeks before a new steady state serum urate concentration is achieved. Secondly, when using allopurinol, the dosage should be tailored to renal function. However, since dosing tables for allopurinol are cautious and poorly investigated in clinically trials, these patients are often far from optimally treated.Citation45–Citation47 Possibly, febuxostat (in comparison to allopurinol) will provide a much more convenient and effective treatment option in this group of patients.
Special considerations when treating gout
Antihyperuricemic therapy might provoke arthritis or induce an attack of gout. For safety reasons, antihyperuricemic therapy should only be given after a gout attack has subsided, preferably with protection from colchicine, which should be initiated several days to 2 weeks earlier (0.5 mg twice daily).Citation5,Citation48 With normal renal function, administration of allopurinol could be started at a dose of 100 mg once daily, probenecid 250 mg twice daily and benzbromarone 100 mg once daily, and titrated every 2 weeks to standard dosage if tolerated. After 6 to 8 weeks, the efficacy can be evaluated and dose can be increased if necessary. Maintenance doses usually range from allopurinol 200 to 600 mg once daily, benzbromarone 100 to 200 mg once daily and probenecid 500 to 1000 mg twice daily. In case of impaired renal function, allopurinol dosage should be started 100 or 50 mg/day (depending on renal function), evaluated after 6 to 8 weeks and titrated carefully (with 100 or 50 mg/day).
When frequent attacks of gout without joint damage or tophi are present in patients with intolerance or allergy to allopurinol and uricosurics, prophylaxis with colchicine at low doses can be prescribed, eg, 0.5 mg once or twice daily in patients with good renal function. In exceptional cases, corticosteroids, or a combination of a uricosuricum and allopurinol, may be indicated for maintenance therapy. When a history of urolithiasis is present, adequate diuresis should be ensured and alkalization should be considered, especially if a uricosuric is prescribed.
Compliance is also a special consideration when supervising gout patients, and it is crucial to explain the dosing schedule and any potential side effect to the patient in order to prevent early withdrawal.
Xanthine oxidase inhibitors
Position of XOi
Allopurinol, a purine-analogue, is widely prescribed as the daily urate-lowering treatment (ULT) of first choice. Allopurinol has been proven to be effective in all cases of hyperuricemia. Because of the long half-life of the active metabolite oxipurinol (14 to 28 hours), allopurinol can be administered once daily. It can be given as a single dose of commonly 300 mg daily and may be titrated up to a maximum 800 mg daily if needed. In some patients only a daily dose of 100 mg may be adequate. Dosages above 300 mg/day are given 2 to 3 times daily.
By application of a dosage of 300 mg allopurinol the (British) target of sUr < 0.30 mmol/L can be reached in 20% to 26% of patients; but if tolerated, a dosage of 600 mg allopurinol is needed to reach aforementioned target in 78% of patients as demonstrated recently in a prospective randomized controlled trial (RCT).Citation43,Citation44 Approximately 20% of patients using allopurinol report adverse events, with 5% discontinuing its use.Citation49
Rare but life threatening is the allopurinol hypersensitivity syndrome.Citation50 Serious adverse reactions to allopurinol have been related to a decreased creatinine clearance rate and prolonged half-life of oxipurinol, and therefore it was proposed to adjust the allopurinol dose according to the rate of creatinine clearance.Citation51 The dosage of allopurinol in individual patients should be tailored based on serum urate and calculated creatinine clearance.
Currently, an alternative XOi may be prescribed, ie, febuxostat.Citation52,Citation53 Up to now the predominant alternative treatment option in cases of allopurinol intolerance has been a uricosuric therapy. In one recent RCT benzbromarone 100 to 200 mg daily showed similar success rates when compared with allopurinol 300 to 600 mg daily, and superior to the uricosuric probenecid.Citation43,Citation44 The long-term safety of benzbromarone however has not been a topic of research. As there was some doubt on its safety, it was withdrawn from the market in 2003.Citation54,Citation55
An emerging, potentially highly effective treatment is the use of uricase enzymes, such as rasburicase and pegloticase. In some individual cases and one clinical trial it is succesfully used to dissolve tophi.Citation56,Citation57
Febuxostat
Febuxostat pharmacology
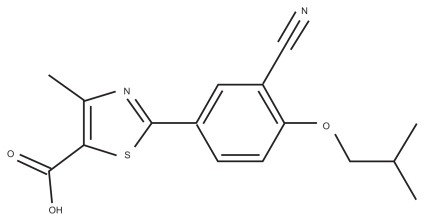
The active substance is a new chemical entity designated as 2-[3-cyano-4-(2-methlypropoxy) phenyl]-4-methlythiazole- 5-carboxylic acid (). It is a non-purine, selective xanthine oxidase/xanthine dehydrogenase inhibitor. It is packed in a dosage of 80 or 120 mg febuxostat.
Febuxostat 10 to 120 mg/day dose-dependently reduced mean sUr levels from baseline by 25% to 70% in healthy volunteers; 24-hour urinary uric acid excretion at day 8 was decreased by 46% to 66% relative to placebo.Citation58 Both effects seemed to plateau at dosages >120 mg/day.Citation58 Age (18 to 40 years versus ≥65 years) and sex had no clinically significant effect on the pharmacokinetic and pharmacodynamic properties of oral febuxostat 80 mg/day in healthy volunteers.Citation59
Absorption of febuxostat is rapid, with a time to Cmax of ≈1 hour. Febuxostat can be administered regardless of food or antacid intake.Citation60 Pharmacokinetic values are linear in the range of 10 to 120 mg. Febuxostat is highly (>98%) bound to human plasma proteins, mainly at the diazepam binding site. Elimination half-life of febuxostat is approximately 12 hours. Febuxostat is mainly eliminated by glucuronidation in the liver. Also, some quantifiable active metabolites 67M-1, 67M-2, and 67M-4 were found.Citation61–Citation63 No dosage adjustments are recommended in patients with mild to moderate renal impairment. Febuxostat inhibits cytochrome P450 isoenzyme 2D6, but interactions with CYP2D6 are not considered clinically significant. Just as in allopurinol, an important drug–drug interaction can occur with mercaptopurine and azathioprine due to inhibition of xanthine oxidase. Although no data are available yet, the combination of these drugs with febuxostat should be avoided.
Two phase I studies investigated the pharmacokinetics, pharmacodynamics, and safety of febuxostat in male and female subjects with normal or mild, moderate or severe renal function impairment.Citation64,Citation65 One study (n = 32) used febuxostat in the standard dosage of 80 mg/day.Citation64 Although plasma exposure to febuxostat and its metabolites was generally higher in subjects with increasing degrees of renal impairment, the percentages of decrease in serum uric acid were comparable regardless of the renal function group. A once-daily 80-mg dose of febuxostat appears to be safe and well tolerated in different renal function groups and does not appear to require any dose adjustment based on differences in renal function.Citation64
One open label phase I study (n = 27) investigated the pharmacokinetics and pharmacodynamics as well as safety of febuxostat in male and female subjects with normal liver function (n = 11) and subjects with mild (n = 8) and moderate (n = 8) hepatic impairment.Citation66 Caveat: patients with severe hepatic impairment, according to Child–Pugh classification, were excluded, and febuxostat 80 mg was given once daily for only 7 days. Overall, mild to moderate hepatic impairment did not significantly affect exposure to febuxostat or its active metabolites during this 1-week period.
Clinical efficacy
In a phase II dose-response study the efficacy of 40 mg, 80 mg, and 120 mg/day febuxostat was evaluated in 153 patients with hyperuricemia (baseline sUr ≥ 0.48 mmol/L) and gout.Citation67 Patients were aged 23 up to 80 years. Subjects received febuxostat (40 mg, 80 mg, 120 mg) or placebo once daily for 28 days and colchicine prophylaxis for 14 days prior to and 14 days after randomization. Significantly more patients receiving febuxostat than placebo achieved an sUr level of ≤ 0.36 mmol/L) at each visit (P < 0.001 for each comparison). The target sUr level (≤0.36 mmol/L) was achieved at study end in 0% of patients in the placebo group and 56%, 76% and 94% of patients in the 40 mg, 80 mg, and 120 mg febuxostat groups, respectively.
Subjects who completed this study were entered into a 5-year open-label extension study (FOCUS) and initially received febuxostat 80 mg daily.Citation68 Between weeks 4 and 24, dosing could be adjusted to febuxostat 40 or 120 mg. All subjects received gout flare prophylaxis during the first 4 weeks. Gout flares were recorded and treated throughout the study, and sUr, baseline tophi and safety were monitored. Among 116 subjects initially enrolled, dose adjustments were made for 44 (38%) subjects. As a result, 8 subjects received febuxostat 40 mg, 79 received 80 mg, and 29 received 120 mg daily maintenance dose. At 5 years, 93% (54/58) of the remaining subjects had sUr < 6.0 mg/dL (<0.36 mmol/L). Fifty-eight subjects (50%) discontinued prematurely; 38 did so in the first year. The primary reasons for discontinuation were: personal reasons 22 (19.0%), adverse event 13 (11.2%), gout flare 8 (6.9%), lost to follow-up 5 (4.3%), protocol violation 1 (<1%), other 9 (7.8%). Sustained reduction of sUr was associated with nearly complete elimination of gout flares. In 26 subjects with a tophus at baseline, resolution was achieved in 69% (18/26) by last visit on study drug at any point during the study. There were no deaths reported during the study. Long-term treatment with febuxostat resulted in durable maintenance of sUr < 6.0 mg/dL for most subjects. There was nearly complete abolition of gout flares in patients completing the study. Baseline tophi resolved in a majority of subjects.Citation68
In the phase III FACT trial, 762 patients with gout and with serum urate concentrations of at least 8.0 mg/dL (0.48 mmol/L) were randomly assigned to receive either febuxostat (80 mg or 120 mg) or allopurinol (300 mg) once daily for 52 weeks; 760 patients received the study drug.Citation69 Prophylaxis against gout flares with naproxen or colchicine was provided during weeks 1 through 8. The primary endpoint was a target sUr < 6.0 mg/dL (0.36 mmol/L) at the last 3-monthly measurements. The secondary endpoints included reduction in the incidence of gout flares and in tophus area. The primary endpoint was reached in 47% to 59% of patients receiving 80 mg of febuxostat, 44% to 74% of those receiving 120 mg of febuxostat, and 8% to 40% of those receiving 300 mg allopurinol (P < 0.001 for comparison of each febuxostat group with the allopurinol group). The rates of discontinuation due to adverse events were higher in both the 80-mg febuxostat group and the 120-mg febuxostat group than in the allopurinol group (). Categories and frequencies of treatment-related adverse events were not linked to discontinuation, but only published for all reported treatment-related adverse events.
Table 3 Efficacy and tolerability of febuxostat in randomized controlled trials
Febuxostat was concluded, at a daily dose of 80 mg or 120 mg, to be more effective than 300 mg allopurinol in lowering sUr.
Febuxostat reduced the median tophus area by 83% and 66% in patients in the 80-mg and 120-mg groups compared with 50% in patients receiving allopurinol. Similar reductions in gout flares occurred in all treatment groups: 64% and 70% in the 80-mg and 120-mg febuxostat groups, and 64% of patients receiving allopurinol. It was considered that an 8-week period of prophylaxis against gout flares due to urate mobilization probably was too short, as many gout attacks were noticed in the first weeks afterwards in all groups. This might be one of the reasons that no differences in reduction of gout flares were seen. Another point of discussion is the dose limit of allopurinol of 300 mg/day in this study, which is often considered as the “safe” maximum dosage.Citation70,Citation71 It is known that dosages up to allopurinol 600 mg/day are more effective, and the licensed maximum dosage is 800 mg/day (or 900 mg/day in some countries).Citation44
In the APEX trial, 1072 patients with gout, including persons with impaired renal function, and with sUr concentrations of at least 0.48 mmol/L were randomy assigned to receive either febuxostat (80 mg or 120 mg or 240 mg) versus allopurinol (300 mg or 100 mg) once daily for 28 weeks versus placebo.Citation72 Significantly higher percentages of subjects treated with febuxostat 80 mg (48%), 120 mg (65%), and 240 mg (69%) attained the primary endpoint of at least 3 monthly sUr levels <0.36 mmol/L compared with allopurinol (22%) and placebo (0%). A significantly higher percentage of patients with impaired renal function treated with febuxostat 80 mg (4 out of 9 = 44%), 120 mg (5 out of 11 = 45%), and 240 mg (3 out of 5 = 60%) achieved the primary endpoint compared with those treated with 100 mg allopurinol (0 out of 10 = 0%). Serious adverse events occurred similarly in all groups, although diarrhea and dizziness were more frequent in the 240 mg febuxostat group. Primary reasons for withdrawal were similar across groups except for gout flares, which occurred more frequent with febuxostat than with allopurinol. Schumacher et al concluded that at all doses studied febuxostat more effectively lowered and maintained serum urate levels <0.36 mmol/L than did allopurinol or placebo in subjects with hyperuricemia and gout, including in those with mild to moderate impaired renal function.Citation72
Subjects who completed the FACT or APEX trial were invited to enroll in an open-label extension study and assigned to fixed-dose daily ULT with febuxostat (80 mg or 120 mg) or allopurinol (EXCEL).Citation73 The majority of subjects were male, Caucasian, and in the age rabge of 45 to 65 years. ULT reassignment was permitted during months 1 to 6 to achieve sUr concentrations of 3.0 to 6.0 mg/dL (0.18 to 0.36 mmol/L). Flares requiring treatment, tophus size, safety, and sUr levels were monitored during up to 40 months of ULT maintenance. After 1 month of initial treatment, >80% of subjects receiving either febuxostat dose, but only 46% of subjects receiving allopurinol, achieved sUr < 6.0 mg/dL (<0.36 mmol/L). After ULT reassignment, >80% of all remaining subjects maintained the primary efficacy endpoint of sUr < 6.0 mg/dL at each visit. More subjects initially randomized to allopurinol required ULT reassignment to achieve sUr < 6.0 mg/dL compared with subjects receiving febuxostat. Maintenance of sUr < 6.0 mg/dL resulted in progressive reduction to nearly 0 in proportion of subjects requiring gout flare treatment. Baseline tophus resolution was achieved by 46%, 36%, and 29% of subjects maintained on febuxostat 80 mg, febuxostat 120 mg, and allopurinol, respectively. Overall adverse event rates (including cardiovascular adverse event rates), adjusted for 10-fold greater febuxostat than allopurinol exposure, did not differ significantly among treatment groups. Durable maintenance of goal range sUr level with either dose of febuxostat or in smaller numbers of subjects with allopurinol resulted in near elimination of gout flares and improved tophus status over time.
In October 2008 the most recent clinical trial of febuxostat was presented at the Annual Meeting of the American College of Rheumatology.Citation74 This 6-month phase III randomized, controlled, multicenter, double-blind trial comparing efficacy and safety of daily febuxostat and allopurinol in subjects with gout (CONFIRMS) trial randomized 2269 patients to receive febuxostat 40 mg/day, febuxostat 80 mg/day, or allopurinol 200 or 300 mg/day depending on renal function (patients with glomerular filtration rate of 30 to 59 mL/min received 200 mg/day of allopurinol). The primary endpoint was the proportion of patients achieving an sUr of less than 6 mg/dL (0.36 mmol/L) at the end of follow up. A secondary endpoint analyzed this same outcome in those patients with mild or moderate renal dysfunction defined as glomerular filtration rates of 60 to 89 and 30 to 59 mL/min, respectively. As in most of the previous febuxostat trials, a majority of the subjects were middle-aged, (mean age 52.8 years) white males. Baseline sUr was 9.6 mg/dL and gout duration on average was 11.6 years; 1483 individuals had mild or moderate renal dysfunction. Urate lowering efficacy was similar with allopurinol 300 mg/day (42% achieved primary endpoint) and febuxostat 40 mg/day (45% achieved primary endpoint) but febuxostat 80 mg/day was statistically superior to the other two groups (67% achieved primary endpoint). Among patients with renal dysfunction, febuxostat 80 mg/day achieved the primary endpoint in a higher proportion of patients (72%) than in those taking febuxostat 40 mg/day (50%) or allopurinol (42%). Rates of adverse events were comparable across groups and at all levels of renal function. Specifically, no difference was reported in the rate of cardiovascular events (6 events were reported: 3 in the allopurinol arm and 3 in the febuxostat 80 mg/day group). Five deaths occurred during the study, 1 in each febuxostat group and 3 in the allopurinol arm. CONFIRMS also provided additional evidence than that provided by the FACT and APEX trials supporting a stronger urate-lowering potency in the tested doses of febuxostat compared with allopurinol for those individuals with sUr greater than 10 mg/dL or with tophi.Citation74,Citation75
Efficacy and tolerability results of febuxostat in randomized controlled trials and long-term follow-up studies are summarized in and .
Table 4 Efficacy and tolerability of febuxostat in long-term clinical follow-up
Febuxostat tolerability and safety
In terms of safety, to date, results from clinical trials have shown that febuxostat is well tolerated with a safety profile comparable to that of placebo and allopurinol ( and ). The most commonly reported adverse drug reactions (investigator assessment) are liver function abnormalities (3.5%), diarrhea (2.7%), headache (1.8%), nausea (1.7%), and rash (1.5%).Citation76 In both the double-blind and extension phases, liver function test abnormalities were associated with colchicine administration.Citation62 Diarrhea, nausea, and vomiting are more frequent in patients concomitantly treated with colchicine.Citation76 Some serious rashes were reported with febuxostat in the APEX study.Citation71 Since allopurinol can cause life-threathening cutaneous reactions (Stevens-Johnson syndrome) in rare cases, special attention to this serious adverse event is necessary.Citation76
Some concerns about the safety of febuxostat have been expressed. Initial cardiovascular safety signals raised in the FACT trial and in the FDA clinical review of July 2006 have not been replicated in recently released phase III trial results. Postmarketing surveillance will be necessary to monitor rare but serious adverse events.
Febuxostat clinical utility in the elderly
Based upon currently available data and expert opinion, treatment with febuxostat should be considered indicated for patients with gout and associated hyperuricemia in several circumstances: allopurinol intolerance due to allergy or gastrointestinal complaints or biochemical side effects in blood counts or liver function tests. Currently, data are lacking about cross-allergy. Febuxostat may also be considered in mild to moderate renal impairment, ie, creatinine clearance 20 to 60 mL/min, as well as in mild to moderate non-alcoholic hepatic impairment, ie, with ALAT < twice upper limit of normal.
Before prescribing febuxostat, one should check documentation of allopurinol intolerance/ineffectivity/ contraindication. Tests should be performed regarding full blood count, liver enzymes (ASAT, ALAT), albumen, thyroid function (TSH), renal function (creatinine), plasma uric acid and in 24-hour urine sample: uric acid and creatinine clearance. Prophylaxis should be given using colchicine in appropriate dosage or alternatively an NSAID at least 3 days before starting febuxostat and prophylaxis should be continued until the target plasma uric acid has been reached (usually 3–6 months). When renal function is impaired (<50 mL/min) febuxostat should be started at a lower dose: 40 mg daily. During therapy, patients should be monitored according to national guidelines, eg, .
Table 5 Suggestions for monitoring febuxostat therapy
Conclusions
Reviewing the data, febuxostat can be regarded as a useful, novel, non-purine, selective and potent inhibitor of xanthine oxidase. Pharmacokinetics of febuxostat 10 to 120 mg daily are linear. No dose adjustment appears to be necessary in patients with mild-to-moderate renal impairment or mild-tomoderate hepatic impairment. Febuxostat 10 to 120 mg daily rapidly and sustainably reduces serum uric acid by 25% to 70%. Prophylaxis with colchicine or a NSAID is indicated preceding and during 6 months after initiating febuxostat. Febuxostat in clinical trials is well tolerated including those patients experiencing hypersensitity/intolerance to allopurinol.
Its antihyperuricemic efficacy at 80 to 120 mg/day is better than “standard dosage” allopurinol (300 mg/day). Febuxostat 80 mg daily appears to be equipotent to allopurinol 450 mg/day or benzbromarone 100 mg/day. Long-term safety and efficacy data on tophus diminishment and reduction of gout flares have recently become available. Efficacy and tolerability of febuxostat compared to a uricosuric drug is to be determined in future studies.
Elderly onset gout has distinct clinical features in comparison to gout in middle-aged men, and co-medication and co-morbidity should be carefully evaluated. When preventive treatment is considered in this population, the potential benefits should outweigh the risks.
Antihyperuricemic treatment success strongly depends on strategic use of the available drugs. This includes sufficient prophylaxis of gout flares during start of antihyperuricemic treatment, choice of drug tailored to patient characteristics, and dosage titration to target sUr levels. Febuxostat broadens the therapeutic options for antihyperuricemic in patients with gout. This is of clinical importance, especially for gout patients with currently unmet needs.
Disclosures
The authors have no conflict of interest to declare.
References
- SinghHTorralbaKDTherapeutic challenges in the management of gout in the elderlyGeriatrics200863713182018593209
- SaagKGChoiHEpidemiology, risk factors, and lifestyle modifications for goutArthritis Res Ther20068Suppl 1S216820041
- PascualESiveraFWhy is gout so poorly managed?Ann Rheum Dis200766101269127017881662
- ZhangWDohertyMPascualEfor EULAR Standing Committee for International Clinical Studies Including TherapeuticsEULAR evidence based recommendations for gout – Part I Diagnosis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT)Ann Rheum Dis200665101301131116707533
- ZhangWDohertyMBardinTfor EULAR Standing Committee for International Clinical Studies Including TherapeuticEULAR evidence based recommendations for gout – Part II Management: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT)Ann Rheum Dis200665101312132416707532
- ArromdeeEMichetCJCrowsonCSO’FallonWMGabrielSEEpidemiology of gout: is the incidence rising?J Rheumatol29112403240612415600
- WallaceKRiedelAJoseph-RidgeNWortmannRIncreasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care populationJ Rheumatol20043181582158715290739
- FamAGGout in the elderly. Clinical presentation and treatmentDrugs Aging19981332292439789727
- Ene-StroescuDGorbienMJGouty arthritis. A primer on late-onset goutGeriatrics2005607243116026179
- HoskisonKTWortmannRLManagement of gout in older adults: barriers to optimal controlDrugs Aging2007241213617233545
- De LeonardisFGovoniMColinaMBruschiMTrottaFElderly-onset gout: a reviewRheumatol Int20072811617653719
- ChoiHKMountDBReginatoAMPathogenesis of goutAnn Intern Med2005143749951616204163
- EnomotoAKimuraHChairoungduaAShigetaYJutabhaPChaSHMolecular identification of a renal urate anion exchanger that regulates blood urate levelsNature2002417688744745212024214
- KolzMJohnsonTSannaSMeta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrationsPLoS Genet200956e100050419503597
- SpiekerLERuschitzkaFTLüscherTFNollGThe management of hyperuricemia and gout in patients with heart failureEur J Heart Fail20024440341012167377
- BagatinJSardelicSPivacNComparison of chlorthalidone, propranolol and bopindolol in six-month treatment of arterial hypertensionInt J Clin Pharmacol Res19981873789675624
- GurwitzJHKalishSCBohnRLThiazide diuretics and the initiation of anti-gout therapyJ Clin Epidemiol1997509539599291881
- DunnCJFittonABrogdenRNTorasemide. An update of its pharmacological properties and therapeutic efficacyDrugs1995491211427705212
- CaspiDLubartEGraffEHabotBYaronMSegalRThe effect of mini-dose aspirin on renal function and uric acid handling in elderly patientsArthritis Rheum200043110310810643705
- DieppePAInvestigation and management of gout in the young and the elderlyAnn Rheum Dis19915042632662029210
- PascualETovarJRuizMTThe ordinary light microscope: an appropriate tool for provisional detection and identification of crystals in synovial fluidAnn Rheum Dis198948129839852559666
- KanellisJKangD-HFeigDIWortmannRLBeckerMARyanLMAsymptomatic hyperuricemiaCrystal-Induced Artrhopathies: Gout, Pseudogout and Apatite-Associated SyndromesNew YorkTaylor and Francis Group20068485
- SchlesingerNDetryMAHollandBKLocal ice therapy during bouts of acute gouty arthritisJ Rheumatol200429233133411838852
- JanssensHJJanssenMvan de LisdonkEHvan RielPLvan WeelCUse of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trialLancet200837196271854186018514729
- RubinBRBurtonRNavarraSSmugarSSTershakovecAMEfficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trialArthritis Rheum200450759860614872504
- SchumacherHRJrBoiceJADaikhDIRandomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritisBMJ200232473521488149212077033
- ElisafMTsimichodimosVBairaktariESiamopoulosKCEffect of micronized fenofibrate and losartan combination on uric acid metabolism in hypertensive patients with hyperuricemiaJ Cardiovasc Pharmacol1999341606310413068
- KaTInokuchiTTsutsumiZTakahashiSMoriwakiYYamamotoTEffects of a fenofibrate/losartan combination on the plasma concentration and urinary excretion of purine basesInt J Clin Pharmacol Ther2006441222616425967
- GaoXCurhanGFormanJPAscherioAChoiHKVitamin C intake and serum uric acid concentration in menJ Rheumatol20083591853185818464304
- ChoiHKGaoXCurhanGVitamin C intake and the risk of gout in men: a prospective studyArch Intern Med2009169550250719273781
- LevinsonWCohenMSBradyDTo change or not to change: “Sounds like you have a dilemma”Ann Intern Med2001135538639111529714
- ChoiHKAtkinsonKKarlsonEWWillettWCurhanGPurine-rich foods, dairy and protein intake, and the risk of gout in menN Engl J Med2004350111093110315014182
- FerrazMBO’BrienBA cost effectiveness analysis of urate lowering drugs in nontophaceous recurrent gouty arthritisJ Rheumatol19952259089148587081
- Pérez-RuizFCalabozoMPijoanJIHerrero-BeitesAMRuibalAEffect of urate-lowering therapy on the velocity of size reduction of tophi in chronic goutArthritis Rheum200244735636012209479
- SarawateCAPatelPASchumacherHRSerum urate levels and gout flares: analysis from managed care dataJ Clin Rheumatol2006122616516601538
- ShojiAYamanakaHKamataniNA retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapyArthritis Rheum200451332132515188314
- YamanakaHTogashiRHakodaMOptimal range of serum urate concentrations to minimize risk of gouty attacks during antihyperuricemic treatmentAdv Exp Med Biol199843113189598023
- JordanKMCameronJSSnaithMfor British Society for Rheumatology and British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group (SGAWG)British society for Rheumatology and British Health Professionals in Rheumatology guideling for the management of goutRheumatology (Oxford)20084681372137417522099
- SutariaSKatbamnaRUnderwoodMEffectiveness of interventions for the treatment of acute and prevention of recurrent gout – a systematic reviewRheumatology (Oxford)200645111422143116632483
- Pérez-RuizFAlonso-RuizACalabozoMHerrero-BeitesAGarcía-ErauskinGRuiz-LuceaEEfficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic goutAnn Rheum Dis19985795455499849314
- BeckerMASchumacherHRJrWortmannRLFebuxostat compared with allopurinol in patients with hyperuricemia and goutN Engl J Med2005353232450246116339094
- ReindersMKvan RoonENHoutmanPMBrouwersJRJansenTLBiochemical effectiveness of allopurinol and allopurinol-probenecid in previously benzbromarone-treated gout patientsClin Rheumatol20072651459146517308859
- ReindersMKVan RoonENJansenTLThAEfficacy and tolerability of urate lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinolAnn Rheum Dis2009681515618250112
- ReindersMKHaagsmaCJansenTLTAA randomized controlled trial with dose escalation on the efficacy and tolerability of allopurinol 300/600 mg/day versus benzbromarone 100–200 mg/day in gout patientsAnn Rheum Dis200968689289718633127
- DalbethNKumarSStampLGowPDose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with goutJ Rheumatol20063381646165016783857
- ChungYStockerSLGrahamGGDayROOptimizing therapy with allopurinol: factors limiting hypouricemic efficacyAm J Med Sci2008335321922618344696
- PanomvanaDSripraditSAngthararakSHigher therapeutic plasma oxypurinol concentrations might be required for gouty patients with chronic kidney diseaseJ Clin Rheumatol200814161118431090
- BorstadGCBryantLRAbelMPScroggieDAHarrisMDAllowayJAColchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritisJ Rheumatol200431122429243215570646
- WortmannRLRecent advances in the management of gout and hyperuricemiaCurrent Opin Rheumatol2005173319324
- ArellanoFSacristánJAAllopurinol hypersensitivity syndrome: a reviewAnn Pharmacother19932733373438453174
- HandeKRNooneRMStoneWJSevere allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiencyAm J Med198476147566691361
- CHMP 2008Committee for medicinal products for human use summary of positive opinion for adenuric2122008 URL: http://www.emea.europa.eu/pdfs/human/opinion/Adenuric_8075108en.pdf
- CDER 2009Center for Drug Evaluation and Research URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149534.htm
- JansenTLReindersMKVan RoonENBrouwersJRBJBenzbromarone withdrawn from the European market: another case of “absence of evidence is evidence of absence?”Clin Exp Rheumatol200422565115485024
- LeeMHGrahamGGWilliamsKMDayROA benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients?Drug Saf200831864366518636784
- MoolenburghJDReindersMKJansenTLRasburicase treatment in severe tophaceous gout: a novel therapeutic optionClin Rheumatol200625574975216247589
- VogtBUrate oxidase (rasburicase) for treatment of severe tophaceous goutNephrol Dial Transplant200520243143315673692
- BeckerMAKisickiJKhosravanRFebuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteersNucleosides Nucleotides Nucleic Acids2004238911111116
- KhosravanRKukulkaMJWuJTJoseph-RidgeNVernilletLThe effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidaseJ Clin Pharmacol20084891014102418635756
- KhosravanRGrabowskiBWuJTJoseph-RidgeNVernilletLEffect of food or antacid on pharmacokinetics and pharmacodynamics of febuxostat in healthy subjectsBr J Clin Pharmacol200865335536317953718
- SchumacherHRJrFebuxostat: a non-purine, selective inhibitor of xanthine oxidase for the management of hyperuricaemia in patients with goutExpert Opin Investig Drugs2005147893903
- HairPIMcCormackPLKeatingGMFebuxostatDrugs200868131865187418729537
- BruceSPFebuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and goutAnn Pharmacother200640122187219417132810
- MayerMDKhosravanRVernilletLWuJTJoseph-RidgeNMulfordDJPharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairmentAm J Ther2005121223415662289
- HoshideSTakahashiYIshikawaTPK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairmentNucleosides Nucleotides Nucleic Acids2004238911171118
- KhosravanRGrabowskiBAMayerMDWuJTJoseph-RidgeNVernilletLThe effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidaseJ Clin Pharmacol20064618810216397288
- BeckerMASchumacherHRJrWortmannRLFebuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with goutArthritis Rheum200552391692315751090
- SchumacherHRBeckerMALloydEMacDonaldPALademacherCFebuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety studyRheumatology (Oxford)200948218819419141576
- BeckerMASchumacherHRJrWortmannRLFebuxostat compared with allopurinol in patients with hyperuricemia and goutN Engl J Med2005353232450246116339094
- MorelandLWFebuxostat – treatment for hyperuricemia and gout?N Engl J Med2005353232505250716339099
- RundlesRWMetzENSilbermanHRAllopurinol in the treatment of goutAnn Intern Med19666422292585322938
- SchumacherHRJrBeckerMAWortmannRLEffects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, doubleblind, parallel-group trialArthritis Rheum200859111540154818975369
- BeckerMASchumacherHRMacDonaldPALloydELademacherCClinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with goutJ Rheumatol20093661273128219286847
- BeckerMSchumacherHRJrEspinozaLA phase 3 randomized, controlled, multicenter, double-blind trial comparing efficacy and safety of daily febuxostat and allopurinol in subjects with goutPresented at: the Meeting of the American College of RheumatologyOctober 24–29, 2008San Francisco, California URL: http://acr.confex.com/acr/2008/webprogram/Paper3414.html
- GaffoALSaagKGFebuxostat: the evidence for its use in the treatment of hyperuricemia and goutCore Evidence200942536
- European Medicines Agency (EMEA)Summary of product characteristics 19/05/2009 Adenuric-H-C-777-T-13. URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/adenuric/emea-combinedh777en.pdf