?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Data from two open-label trials (PRIOR and CURRENT) of women with postmenopausal osteoporosis or osteopenia were evaluated to assess whether monthly oral and quarterly intravenous (IV) ibandronate dosing improved self-reported gastrointestinal (GI) tolerability for patients who had previously experienced GI irritation with bisphosphonate (BP) use. In PRIOR, women who had discontinued daily or weekly BP treatment due to GI intolerance received monthly oral or quarterly IV ibandronate for 12 months. The CURRENT subanalysis included women receiving weekly BP treatment who switched to monthly oral ibandronate for six months. GI symptom severity and frequency were assessed using the Osteoporosis Patient Satisfaction Questionnaire™. In PRIOR, mean GI tolerability scores increased significantly at month 1 from screening for both treatment groups (oral: 79.3 versus 54.1; IV: 84.4 versus 51.0; p < 0.001 for both). Most patients reported improvement in GI symptom severity and frequency from baseline at all post-screening assessments (>90% at Month 10). In the CURRENT subanalysis >60% of patients reported improvements in heartburn or acid reflux and >70% indicated improvement in other stomach upset at month 6. Postmenopausal women with GI irritability with daily or weekly BPs experienced improvement in symptoms with extended dosing monthly or quarterly ibandronate compared with baseline.
Introduction
Declining postmenopausal estrogen levels lead to an increase in bone turnover and a decrease in bone mass. The resulting osteoporosis is a cause of substantial morbidity, reduction in quality of life, and increased mortality.Citation1,Citation2 Bisphosphonates (BPs), which are the treatment of choice, have proven efficacy in terms of bone turnover marker reduction, bone mineral density increase, and fracture risk reduction.Citation3–Citation6 However, their effectiveness in clinical practice is often compromised by poor adherence to dosing instructions and poor persistence with treatment.Citation7
Treatment discontinuation is associated with increased risk of fracturesCitation8–Citation10 and has been attributed to several causes, including patients’ experience of gastrointestinal (GI) side effects, such as esophageal irritation and ulceration, associated with oral BPs.Citation11–Citation13 The GI irritation observed with oral BPs is a result of direct contact between the drug and gastric mucosa.Citation14 BPs act as topical irritants on the gastric mucosa, leading to mucosal necrosis.Citation15 The effects can be minimized by following the dosing instructions, which are intended to minimize direct contact.Citation16 Less frequent administration may also help by allowing time for the gastric mucosa to recover between doses. In a database study, the risk of severe GI events was significantly lower for patients treated with ibandronate than with weekly BPs.Citation17 However, this study did not assess milder GI symptoms. The rate of GI adverse events was similar to placebo with all BPs in a number of randomized clinical trials (RCTs),Citation4,Citation5,Citation18–Citation21 in contrast to reports supporting a link between BP treatment and GI symptoms from routine clinical practice.Citation12,Citation22 This difference may reflect the generally healthier populations typically included in clinical trials compared with those treated in routine clinical practice, or factors such as better compliance with dosing instructions in clinical trials or under-reporting of adverse events in clinical trials.
Ibandronate, a nitrogen-containing BP indicated for prevention and treatment of postmenopausal osteoporosis, is available as monthly oral and quarterly intravenous (IV) formulations,Citation23,Citation24 thus allowing for the evaluation of GI symptoms with extended BP dosing regimens. The purpose of this investigation was to consider data from two clinical trials of ibandronate in order to assess whether extended BP dosing was associated with improved GI tolerability for patients who indicated previous GI irritation with daily or weekly BP use using self-reported questionnaires with questions specifically addressing GI symptoms.
Materials and methods
Study design
The frequency and severity of GI symptoms with ibandronate were assessed using questionnaires in two open-label, multicenter clinical trials, PRIORCitation25 and CURRENT.Citation26 PRIOR was a 12-month study that enrolled women who had discontinued daily or weekly BP treatment due to GI symptoms at least three months previously. The participants chose to receive either the 150 mg monthly oral or 3 mg quarterly IV ibandronate dose. CURRENT was a large, prospective, open-label, multicenter, six-month study designed to identify the level of patient satisfaction with once-monthly BP therapy in patients previously treated with weekly BPs, using the validated Osteoporosis Patient Satisfaction Questionnaire (OPSAT-Q)™.Citation27 In PRIOR, all patients were required to take supplemental calcium and vitamin D for the full duration of the study and the sponsor provided patients with a combination dietary supplement containing vitamin D 200 IU and elemental calcium 500 mg. In CURRENT, all patients were instructed to take supplemental calcium and vitamin D for the full duration of the study. In both studies, patients were instructed to take calcium and vitamin D in divided daily doses with a meal. Under no circumstances was the patient to take calcium, vitamin D, any other medication, or food/beverage (except water) together with study drug or during the predose or postdose fasting period.
Participants
All patients from PRIOR were included in this analysis. PRIOR recruited women who had discontinued previous daily or weekly BP treatment at least three months earlier due to GI intolerance.
The present analysis included data from a subset of patients from the CURRENT study with GI symptoms on weekly BPs at enrollment, who then received monthly oral ibandronate 150 mg for six months.Citation28 CURRENT included women currently receiving weekly BP treatment who switched to monthly ibandronate. Patients with contraindications to calcium or vitamin D; inability to stay in an upright position for 60 minutes; history of hyper-calcemia, renal disease, or liver disease; and a history of major upper GI disease (significant upper GI bleeding within the last year requiring hospitalization or transfusion; recurrent peptic ulcer disease documented by radiographic or endoscopic means; dyspepsia or gastroesophageal reflux uncontrolled by medication; abnormalities of the esophagus that delay esophageal emptying, such as stricture, achalasia, or dysmotility; and active gastric/duodenal ulcers) were excluded from CURRENT.
Patients who reported GI symptoms at baseline in CURRENT were identified for the present subanalysis based on their responses to the OPSAT-Q™. Patients with an OPSAT-Q™ score of 1 to 4 on 1 or more of the following questions: 11, 12, 14, 15 were included in the analysis ().
Assessments
GI symptoms were assessed with questions selected from the OPSAT-Q™ in both trials (). Scores from the selected OPSAT-Q™ questions were compared at screening (previous treatment) and months 1, 4, 7, and 10 in PRIOR, and at screening and month 6 in CURRENT. A five-point scale was used for each question. In PRIOR, a score of 1 for questions 11 to 13 indicated an answer of “extremely bothered,” while a score of 5 specified that the patient was “not bothered at all.” Similarly, for questions 14 to 16, which dealt with the frequency of GI symptoms, a score of 1 was awarded for an answer of “more than 3 days” and 5 for an answer of “0 days.”
Statistical analysis
The proportions of patients who reported improved, worsened, or unchanged GI symptoms on the OPSAT-Q™ questions at the end of the study compared with screening were analyzed for both studies. Additionally, for PRIOR, OPSAT-Q™ responses were transformed into an overall GI tolerance score on a scale of 0 to 100, with a higher GI tolerability score indicating less frequent and/or less severe symptoms:
Within-group comparisons of GI tolerance scores in PRIOR were conducted using t-tests.
Results
Patient demographics and baseline characteristics
Demographic and baseline characteristics are summarized in . In total, 147 participants in PRIOR (27.1%) chose oral and 396 (72.9%) chose IV ibandronate. The participants’ mean age was 65.7 years in the oral treatment group and 66.2 years in the IV treatment group. Most participants had a diagnosis of osteoporosis (84 oral [57.1%], 286 IV [72.2%]); the rest of the study population had a diagnosis of osteopenia. Detailed demographic and baseline data have been presented elsewhere.Citation25
Table 1 Baseline and demographic characteristics
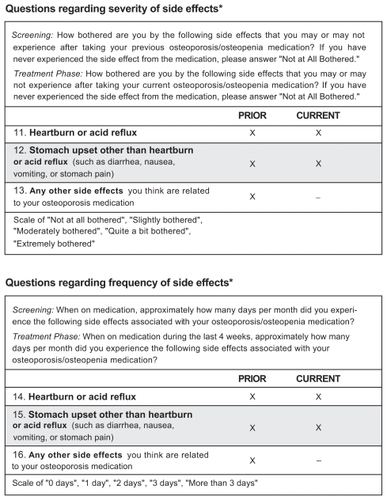
In the CURRENT study, participants with GI symptoms at baseline were identified by a score of 1 to 4 on at least 1 of the relevant OPSAT-Q™ questions. Overall 438, 339, 231, and 159 women had a score of at least 1 on questions 11, 12, 14, and/or 15, respectively. Detailed demographic and baseline data have been presented elsewhere.Citation28
GI symptoms
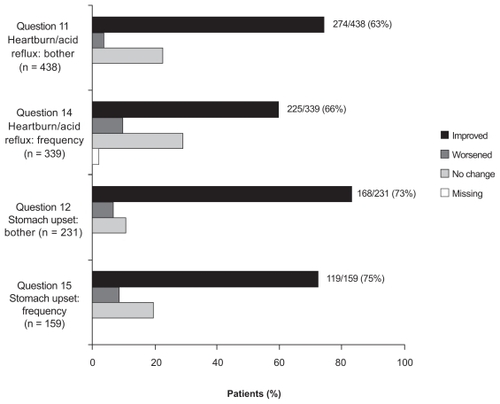
In PRIOR, over 75% of participants in both the oral and IV groups reported ≥10% increase in GI tolerability scores at all post-screening evaluations compared with screening (oral range: 77.9%–85.5%; IV range: 83.7%–85.8%). The majority of patients reported improvement in symptom severity scores (questions 11–13) from baseline at all post-screening assessments, with >70% of participants indicating improvement at month 1. The pattern of improvement in GI symptom frequency scores (questions 14–16) was similar to that in GI symptom severity scores. Over 90% of participants in each group reported improvements on each question at month 10 (). Mean GI tolerability scores were significantly higher at month 1 compared with screening for both the oral and IV treatment groups (oral: 79.3 versus 54.1; IV: 84.4 versus 51.0, respectively; p < 0.001 for both groups). The scores continued to increase for both groups at months 4, 7, and 10 and remained significantly higher compared with screening scores at all assessment points (p < 0.001 for both groups).
Figure 2 PRIOR: Change in self-reported gastrointestinal symptoms for patients with gastrointestinal symptoms at baseline (score of 1–4 on at least 1 of Osteoporosis Patient Satisfaction Questionnaire™ questions 11, 12, 13, 14, 15, 16) at Month 10.
A similar result was observed in the CURRENT study, where the majority of women with GI symptoms on their current weekly BP indicated improvements in degree of bother and frequency of GI symptoms six months after switching to monthly oral ibandronate (). Over 60% of patients reported improvements in heartburn or acid reflux (bother: 62.6%; frequency: 66.4%) and over 70% indicated an improvement in stomach upset other than heartburn or acid reflux (bother: 72.7%; frequency: 74.8%) at month 6.
Discussion
This analysis aimed to assess whether extended ibandronate dosing was associated with improved GI tolerability in patients who had previously experienced GI irritation with daily or weekly BP use. In both the PRIOR and CURRENT trials, women reported improvement in the GI symptoms they had encountered in previous treatment with daily or weekly BPs. The GI tolerability scores improved significantly for patients in the PRIOR study, and patients in both studies reported improvements in symptom severity and frequency scores.
Evidence from previous research on the occurrence of GI symptoms associated with oral BP treatment has been mixed. RCTs have generally reported a rate of GI adverse events similar to placebo with all BPs.Citation4,Citation5,Citation18–Citation21 However, after the introduction of daily alendronate, an increase in GI symptoms was reportedCitation29,Citation30 and results from later studies further supported the link between BP treatment and GI events.Citation12,Citation22 BPs have been shown to induce ulceration and necrosis in gastric mucosa.Citation15,Citation29 Although the mechanism of BP-induced GI irritation is not well understood, a study in human colon tumor cells suggests that BPs induce apoptosis and/or inhibition of proliferation of epithelial cells.Citation31 Another ex vivo study showed evidence of neutrophil accumulation and epithelial damage in the gastric mucosa of rats on contact with high concentrations of alendronate or pamidronate.Citation32
In order to minimize contact of BPs with gastric mucosa, the current administration recommendations for orally administered BPs were developed. The dosing instructions for weekly BPs state that the drug should be administered with a glass of water 30 minutes before the first food or beverage of the day and the patient should not lie down within 30 minutes after dosing.Citation16 However, despite the changes in the method of administration, recent data suggest that GI symptoms still account for a high proportion of discontinuations in clinical practice.Citation12,Citation13 IV administration, while requiring an injection, avoids contact of the BP with the gastric mucosa.
In a recent database analysis, fewer severe GI events occurred in patients receiving monthly oral ibandronate compared with weekly BPs, although the incidence of these events was low for all treatments.Citation17 In addition, 100% of patients receiving weekly BPs who had an event discontinued treatment. In contrast, only 44% of those receiving monthly ibandronate who experienced an event discontinued. A separate analysis has suggested that GI event rates may be lower with risedronate than with alendronate.Citation33
There are several possible reasons for the varying findings for BP-related GI events. RCTs employ stringent inclusion and exclusion criteria that exclude patients in poorer health, so the rate of GI symptoms may be lower in RCT populations than in the general population of patients receiving BPs. GI symptoms troublesome enough to prompt discontinuation may not be so severe that patients in an RCT report them. Furthermore, patients in normal clinical practice may follow administration guidelines less closely than those in an RCT, increasing the risk of GI symptoms. Most RCTs report GI events as adverse events.Citation4,Citation5,Citation18 The questionnaires used in PRIOR and CURRENT, with their specific GI-focused questions, may be a more sensitive tool for identifying GI symptoms than adverse event reporting.
PRIOR and CURRENT included distinct populations, both expected to be at risk of experiencing GI symptoms. Women in the PRIOR study had discontinued previous BP therapy due to GI symptoms, and women in the CURRENT subanalysis had experienced GI symptoms on their current BP before switching to oral ibandronate. Improvements reported in GI symptoms in this at-risk group may have clinical implications for other patients who have discontinued BPs due to GI symptoms or who are experiencing GI irritation with current BP treatment. Initiating monthly oral or quarterly IV ibandronate may be associated with improvement in self-reported GI symptoms. Since occurrence of GI events is associated with poor adherence to BP therapy, this may help these patients to persist with BP treatment, and therefore be more likely to realize the benefits of BPs in terms of fracture risk reduction.
The benefits of oral alendronate, risedronate, and ibandronate on fracture risk reduction for patients with postmenopausal osteoporosis were established in studies of daily formulations of each product. Subsequent studies compared the efficacy of longer dosing interval regimens of these products with the corresponding daily formulations in terms of BMD increase, which is associated with reduced fracture risk. Studies of daily alendronate and risedronate demonstrated that these regimens significantly reduce the risk of vertebral and nonvertebral or hip fractures compared with placebo.Citation5,Citation18,Citation20,Citation34,Citation35 Ibandronate 2.5 mg daily was shown to significantly reduce the rate of vertebral fractures compared with placebo,Citation4 and to significantly reduce the rate of nonvertebral fractures in a high-risk population.Citation36 Weekly alendronate, and both weekly and monthly risedronate provide similar cumulative doses to the corresponding daily regimens. For these products, the longer dosing interval regimens produced similar BMD increases from baseline to the corresponding daily formulations.Citation37–Citation39 Monthly ibandronate 150 mg provides a higher cumulative dose to ibandronate 2.5 mg daily, and was shown to provide a significantly larger BMD increase.Citation40 Recent pooled analyses of individual patient data from ibandronate studies have suggested that higher dose regimens, including monthly oral ibandronate 150 mg reduce the risk of nonvertebral fractures.Citation41,Citation42 In a recent database analysis, monthly ibandronate treatment was associated with a similar risk of nonvertebral fracture as weekly BPs.Citation43
A few limitations of this investigation should also be noted. The two studies included no comparators. It is not certain what outcomes would have resulted from rechallenge with a weekly BP (PRIOR) or continued treatment (CURRENT). CURRENT and PRIOR were open-label studies, so the possibility of bias being introduced by the inclusion of motivated patients cannot be excluded. The participants in PRIOR had a wide variation in the time between ending their previous treatment and entering the study. The baseline GI tolerance score reflected patients’ recollection of GI symptoms associated with previous treatment. The possibility that patients answered the questionnaire differently when reporting symptoms associated with ongoing ibandronate treatment in the study cannot be excluded. The CURRENT subanalysis was a post hoc analysis.
The results from the PRIOR and CURRENT studies suggest that women with GI tolerability issues on a daily or weekly BP regimen may experience improved symptoms with the less frequent dosing regimens of monthly oral or quarterly IV ibandronate. The improved GI tolerability associated with extended ibandronate dosing may help to improve adherence to BP therapy, thus, reducing fracture risk in women with postmenopausal osteoporosis.
Trial registry information
Details of CURRENT and PRIOR were posted prior to study enrollment and synopses of both studies have been posted on http://www.rochetrials.com/ and may also be accessed through the International Federation of Pharmaceutical Manufacturers and Associations trial portal (IFPMA; http://www.ifpma.org/clinicaltrials.html). The protocol numbers are ML18056 (CURRENT) and ML18058 (PRIOR).
Acknowledgments
The authors thank Andrew Cooper, BSc, of Envision Pharma, Southport, CT for his editorial assistance with this manuscript and Bann-mo Day, PhD, of Roche, Nutley, NJ, who performed the analyses.
Disclosures
This study was supported and funded by Roche and GlaxoSmithKline. Dr Derman has received research grants from Bristol-Myers Squibb, Procter and Gamble, and Wyeth; and is a consultant/speaker for Ortho-McNeil, Merck, Roche, Solvay, and Wyeth. Dr Kohles is an employee of Roche. Dr Babbitt has been on the speakers’ bureau at Merck, Procter and Gamble, GlaxoSmithKline, Lilly, and Kyphon; and has received research grants from Merck, Procter and Gamble, GlaxoSmithKline, and Lilly. This material was presented at the 56th Annual Meeting of the American College of Obstetricians and Gynecologists; May 3–7, 2008; New Orleans, LA, USA.
References
- EastellRTreatment of postmenopausal osteoporosisN Engl J Med1998338117367469494151
- GuyattGHCranneyAGriffithLSummary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fracturesEndocrinol Metab Clin North Am200231365967912227126
- BlackDMThompsonDEThe effect of alendronate therapy on osteoporotic fracture in the vertebral fracture arm of the Fracture Intervention TrialInt J Clin Pract Suppl1999101465012669740
- ChesnutCHSkagAChristiansenCEffects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res20041981241124915231010
- HarrisSTWattsNBGenantHKEffects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study GroupJAMA1999282141344135210527181
- McClungMRBisphosphonates in osteoporosis: recent clinical experienceExpert Opin Pharmacother20001222523811249544
- ChesnutCHTreating osteoporosis with bisphosphonates and addressing adherence: a review of oral ibandronateDrugs200666101351135916903769
- GoldDTMartinBCFrytakJRAmonkarMMCosmanFA claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosisCurr Med Res Opin200723358559417355739
- SirisESHarrisSTRosenCJAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: Relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc20068181013102216901023
- van den BoogaardCHBreekveldt-PostmaNSBorggreveSEGoettschWGHeringsRMPersistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: A database analysis studyCurr Med Res Opin20062291757176416968579
- EttingerBPressmanAScheinJChanJSilverPConnollyNAlendronate use among 812 women: prevelance of gastrointestinal complaints, noncompliance with patient instructions, and discontinuationJ Manag Care Pharm199845488492
- HamiltonBMcCoyKTaggartHTolerability and compliance with risedronate in clinical practiceOsteoporosis Int200314256262
- Penning-van BeestFJGoettschWGErkensJAHeringsRMDeterminants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosisClin Ther200628223624216678644
- LanzaFLGastrointestinal adverse effects of bisphosphonates: etiology, incidence and preventionTreat Endocrinol200211374315765619
- LichtenbergerLMRomeroJJGibsonGWBlankMAEffect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosaDig Dis Sci20004591792180111052322
- CryerBBauerDCOral bisphosphonates and upper gastrointestinal tract problems: what is the evidence?Mayo Clin Proc200277101031104312374247
- BlumentalsWAHarrisSTColeREHuangLSilvermanSLRisk of severe gastrointestinal events in women treated with monthly ibandronate or weekly alendronate and risedronateAnn Pharmacother200943457758519318598
- BlackDMThompsonDEBauerDCFracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research GroupJ Clin Endocrinol Metab200085114118412411095442
- EismanJARizzoliRRoman-IvorraJUpper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled studyCurr Med Res Opin200420569970515140336
- PolsHAFelsenbergDHanleyDAMultinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study GroupOsteoporos Int19999546146810550467
- ReginsterJMinneHWSorensenOHRandomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupOsteoporos Int2000111839110663363
- LanzaFLHuntRHThomsonABProvenzaJMBlankMAEndoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal womenGastroenterology2000119363163810982755
- Boniva (ibandronate sodium) injection US prescribing informationNutley, NJRoche Laboratories Inc2007
- Boniva (ibandronate sodium) US prescribing informationNutley, NJRoche Laboratories Inc2008
- LewieckiEMBabbittAMPiziakVKOzturkZEBoneHGAdherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluationClin Ther200830460562118498910
- BonnickSLSilvermanSLTannerSBPatient satisfaction in postmenopausal women treated with a weekly bisphosphonate transitioned to once-monthly ibandronateJ Womens Health2009187935943
- FloodEMBeusterienKMGreenHPsychometric evaluation of the Osteoporosis Patient Treatment Satisfaction Questionnaire (OPSAT-Q), a novel measure to assess satisfaction with bisphosphonate treatment in postmenopausal womenHealth Qual Life Outcomes200644216834773
- BinkleyNMartensMGSilvermanSLImproved GI tolerability with monthly ibandronate in women previously using weekly bisphosphonatesSouth Med J2009102548649219373149
- de GroenPCLubbeDFHirschLJEsophagitis associated with the use of alendronateN Engl J Med199633514101610218793925
- EttingerBPressmanAScheinJClinic visits and hospital admissions for care of acid-related upper gastrointestinal disorders in women using alendronate for osteoporosisAm J Manage Care199841013771382
- SuriSMonkkonenJTaskinenMNitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicityBone200129433634311595616
- WallaceJLDicayMMcKnightWBastakiSBlankMAN-bisphosphonates cause gastric epithelial injury independent of effects on the microcirculationAliment Pharmacol Ther199913121675168210594404
- MillerRGBologneseMWorleyKSolisASheerRIncidence of gastrointestinal events among bisphosphonate patients in an observational settingAm J Manag Care2004107S207215
- BlackDMCummingsSRKarpfDBRandomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research GroupLancet19963489041153515418950879
- McClungMRGeusensPMillerPDEffect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study GroupN Engl J Med2001344533334011172164
- ChesnutCHEttingerMPMillerPDIbandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONECurr Med Res Opin200521339140115811208
- BrownJPKendlerDLMcClungMRThe efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalcif Tissue Int200271210311112085156
- DelmasPDMcClungMRZanchettaJREfficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosisBone2008421364217920005
- SchnitzerTBoneHGCrepaldiGTherapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-weekly Study GroupAging (Milano)20021211210746426
- ReginsterJYAdamiSLakatosPEfficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2-year results from the MOBILE studyAnn Rheum Dis20066565465116339289
- CranneyAWellsGAYetisirEIbandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient dataOsteoporos Int200920229129718663402
- HarrisSTBlumentalsWAMillerPDIbandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studiesCurr Med Res Opin200824123724518047776
- HarrisSTReginsterJYHarleyCRisk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: The eValuation of IBandronate Efficacy (VIBE) database fracture studyBone200944575876519168160