Abstract
Objective
To report our experiences in changing from intravitreal bevacizumab to ranibizumab in age-related macular degeneration (AMD).
Design
Retrospective case series.
Participants and methods
We retrospectively reviewed the records of 34 patients (36 eyes) who were treated with monthly injections of intravitreal bevacizumab for six months and then switched to monthly injections of ranibizumab for 12 months. Best-corrected visual acuity measurements (BCVA), contact lens biomicroscopy, optical coherence tomography (OCT), and fluorescein angiography were performed at the baseline examination and then monthly. Chi-square test was used for statistical analysis.
Results
Following bevacizumab treatment, retinal thickness decreased (P = 0.033) while BCVA improved (P = 0.040). Changing from bevacizumab to ranibizumab resulted in a transient decrease in BCVA (P = 0.045) and an increase in retinal thickness (P = 0.042). In addition, three eyes presented with a large subretinal hemorrhage. However, final retinal thickness was better than the initial thickness and the value following the bevacizumab course. No major ocular or systemic side effects were noted.
Conclusions
Ranibizumab was clinically effective in the long term but the change of treatment from bevacizumab to a half-size molecule with less half-life in the vitreous such as ranibizumab contributed to a transient “instability” in the eye which may have triggered the large subretinal hemorrhage. There is insufficient experience reported in the literature in switching from one agent to another. A prospective study with controls is necessary to determine whether it is safe to change from one medication to another.
Introduction
Age-related macular degeneration (AMD) is the most common cause of visual loss in patients aged over 65 years.Citation1 Neovascular AMD with the development of a choroidal neovascularization (CNV) in the macular area accounts for 80% of the severe loss of visual acuity due to AMD.Citation2,Citation3 Ranibizumab (Lucentis®), an isotype monoclonal antibody fragment, is a recombinant humanized immunoglobulin (Ig1) designed for intraocular use which binds to and inhibits the biologic activity of human vascular endothelial growth factor (VEGF) A. The latter contributes to the development and/or progression of choroidal neovascularization associated with neovascular (wet) AMD.Citation4,Citation5 Two-year results of the MARINA study and many other reports support the favorable results of ranibizumab.Citation6–Citation10
Bevacizumab (Avastin®) is a recombinant humanized full-length antibody that binds to all isoforms of VEGF, similar to ranibizumab. For the last two years, bevacizumab has been offered as an off-label intravitreal application for the treatment of wet AMD.Citation11–Citation14
We report our experience with patients who were treated initially with intravitreal bevacizumab and then switched to ranibizumab for a follow-up period of 18 months.
Participants and methods
We retrospectively reviewed the records of 34 patients (36 eyes) who were treated initially with intravitreal bevacizumab 1.25 mg/0.05 mL for six months (six-monthly injections) and then switched to ranibizumab 0.5 mg for 12 months (12 monthly injections) when the latter became commercially available in Greece. All patients were suffering from wet AMD and were older than 50 years. All types of neovascularization due to AMD were included in the study. Patients, who had photodynamic therapy with Visudyne® before starting the anti-VEGF treatment, were also included in the study. All patients had best-corrected visual acuity (BCVA) equal or better than 0.1. There were no eyes with evidence of other ocular disease than AMD during the 18-month follow-up period.
Nonstandarized Snellen BCVA, slit-lamp examination, contact lens biomicroscopy, optical coherence tomography (OCT), and fluorescein angiography (FA) were performed at baseline examination and on a monthly basis. An intravitreal injection was performed every month. In all cases, when changing from bevacizumab to ranibizumab, the f irst ranibizumab injection was performed one month after the last bevacizumab injection in order to avoid a time interval delay in which the eye was not “covered” by any anti-VEGF treatment.
All injections were performed under standard sterile conditions and topical antibiotics were administered for four days. All patients were examined 3–4 days after the injection.
The study was approved by the hospital ethics committee. All patients signed an informed consent form after detailed explanation of the procedure.
The chi-square test was used for statistical analysis. A P-value less than 0.05 was considered to be statistically significant.
Results
Thirty-four patients (36 eyes) were treated during this 18-month period. Mean age was 74.28 years. The youngest patient was aged 57 years and the oldest one was aged 89 years. Of the 34 patients, 16 were men (47.05%) and 18 were women (52.95%).
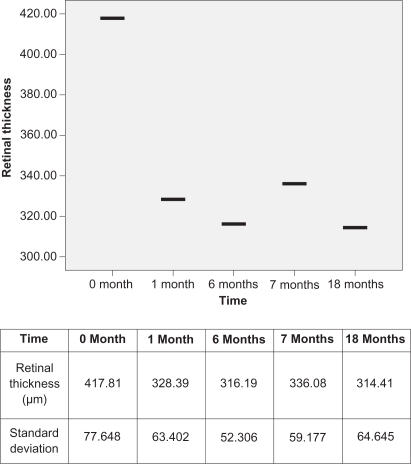
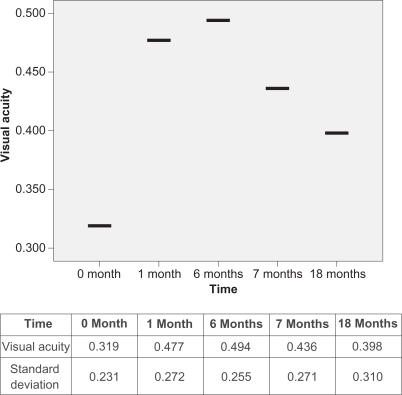
Initially, and before treatment, all 36 eyes had a mean retinal thickness of 417.81 μm and mean BCVA of 0.319. After the first injection of bevacizumab, the mean retinal thickness was decreased to 328.39 μm (P = 0.033), which was statistically significant. When the six-month bevacizumab course was completed, the mean retinal thickness had again decreased slightly to 316.19 μm. BCVA improved initially to 0.477, and showed a slight further improvement (0.494) at the end of six months. Comparing the values before treatment and after the six-month bevacizumab course, the difference was statistically significant both in retinal thickness (P = 0.005) and in BCVA (P = 0.040). Changing from bevacizumab to ranibizumab resulted in a transient decrease in visual acuity (0.494 to 0.436; P = 0.045) and an increase in retinal thickness (316.19 to 336.08; P = 0.042), which are both statistically significant parameters. This decrease in visual acuity lasted for a period of two months. The first ranibizumab injection was performed one month after the last bevacizumab injection in order to avoid a time interval in which the eye was not “covered” by any anti-VEGF treatment. Ranibizumab treatment was continued for one year for all eyes.
Final statistically significant results showed that retinal thickness was decreased in comparison to the starting values (417.81 μm to 314.41 μm; P = 0.004). In comparison to the value after finishing bevacizumab treatment and before starting ranibizumab treatment and while showing some improvement, this result was not statistically significant (316.19 μm to 314.41 μm; P = 0.09) ().
Although retinal thickness was significantly decreased at the end of the 18-month follow-up period, visual acuity did not improve as expected. In particular, final BCVA was 0.398. While being better than the initial measurement (0.319), the final BCVA was worse in comparison to that after finishing bevacizumab treatment and before starting ranibizumab treatment (from 0.494 to 0.398; P = 0.019) ().
Following each case in detail, during this 18-month period, we noted these results: Twenty-nine eyes (80.5%) showed immediate improvement both clinically (mean retinal thickness, 329.4) and in visual acuity (mean BCVA, 0.517) after the first bevacizumab injection while seven eyes (19.5%) remained stable (Mean retinal thickness, 324.0; mean BCVA, 0.314). On completion of the six-month bevacizumab course, in seven eyes which were stable initially, five showed improvements (mean retinal thickness, 255.0 μm; mean BCVA, 0.62) while two remained stable (mean retinal thickness, 254.0 μm; mean BCVA, 0.15). In changing from bevacizumab to ranibizumab, there was a transient decrease in mean BCVA and an increase in mean retinal thickness in OCT. In particular, one eye showed an increase in BCVA and a decrease in retinal thickness, 16 eyes showed deterioration in BCVA and increase in retinal thickness, and 19 eyes showed no change in BCVA with 17 of those having minor increase in retinal thickness while two noted a minor decrease. In addition, three patients (three eyes) from the deterioration group presented with a large subretinal hemorrhage within the first month of the first ranibizumab injection and although intravitreal injections of ranibizumab were continued, the hemorrhage expanded further, which resulted in a poor final BCVA. These patients were not on aspirin or other anticoagulant medication and did not have photodynamic therapy prior to the anti-VEGF treatment.
However, after one year of ranibizumab treatment, the final retinal thickness in OCT was improved from the initial measurement and was better than after the six-month course of bevacizumab. BCVA, although improved, did not follow these results. The latter can be explained because, in addition to the three eyes with large subretinal hemorrhage, 13 eyes with longstanding choroidal neovascular lesions were treated with multiple sessions of photodynamic therapy prior to the anti-VEGF treatment. This resulted in a final visual outcome that was worse than expected, although final retinal thickness did decrease.
Finally, no major ocular side effects such as endophthalmitis or retinal detachment were noted. Injection-site reaction and floaters were reported in almost all cases but were innocuous and resolved without treatment. Subconjuctival hemorrhage was noted in only a few cases. In addition, there were no cases of systemic adverse events such as myocardial or cerebral infarction or treatment emerged hypertension and no patient died during the 18-month study.
Discussion
Ranibizumab (Lucentis®), an isotype monoclonal antibody fragment, is a recombinant humanized Ig1 designed for intraocular use which binds to and inhibits the biologic activity of human VEGF A. Nearly 95% of patients with minimally classic or occultc choroidal neovascularization treated with intravitreal injections of ranibizumab lost fewer than 15 letters compared with 62.2% of patients receiving placebo injections.Citation6 Many other reports support these findings.Citation7–Citation10,Citation15
Bevacizumab is a recombinant humanized full-length antibody that binds to all isoforms of VEGF, similar to ranibizumab which has been offered as an off-label intravitreal application for the treatment of wet AMD.Citation11–Citation13,Citation16,Citation17
There is controversy over the use of ranibizumab and bevacizumab for treating AMD,Citation18 which is the leading cause of blindness in people aged older than 65 years.Citation1 Although both medications alone show very promising results and are now part of everyday practice, there has been no research documenting the results of change from one medication to another.
Recently, Stepien and colleagues reported that there were no apparent differences in visual acuity or injection rate in switching from bevacizumab to ranibizumab therapy.Citation19
Our study had a longer follow-up period and all patients had monthly injections. Using this protocol there was no time interval between changing anti-VEGF therapies and the eye was “covered” by anti-VEGF treatment during the entire follow-up period of 18 months. In comparison to Stepien and colleagues,Citation19 this study showed that switching from bevacizumab to ranibizumab resulted in a transient decrease of visual acuity and an increase of retinal thickness causing a transient “instability” in the eye.
The PIER study showed that reducing monthly injections after the first three months resulted in a decrease of visual acuity.Citation7 In our study, we injected bevacizumab monthly for six months followed by injections of ranibizumab monthly for one year. Thus, the decrease of visual acuity cannot be associated with any discontinuation of monthly anti-VEGF therapy.
Although a large subretinal hemorrhage may occur during anti-VEGF treatment without any obvious explanation,Citation20 the above mentioned transient “instability” may have triggered the large subretinal hemorrhage. The possibility of subretinal hemorrhage following change from intravitreal bevacizumab to ranibizumab has been reported.Citation21 In our three cases, patients were not on aspirin or other anticoagulant treatment and did not have photodynamic therapy prior to the anti-VEGF treatment. According to the literature, complications such as retinal epithelial tears are known.Citation22 In our cases, OCT covered the entire macular area, and did not show any signs of retinal tear, which could explain such a hemorrhage. It is known that the natural history and the visual outcome of a submacular hemorrhage in AMD are very poor.Citation23 However, Stifter and colleaguesCitation24 reported that intravitreal bevacizumab seems to be a promising therapeutic option in eyes with neovascular AMD and large submacular hemorrhages, with stabilization in BCVA and anatomic improvement. Our patients deteriorated further even although treatment was continued on a monthly basis.
In our study, eyes with longstanding choroidal neovascular lesions were treated with multiple sessions of photodynamic therapy prior the anti-VEGF treatment and had a less satisfactory visual outcome. It is known that photodynamic therapy improves the prognosis of exudative AMD. However, its effectiveness differs among different subtypes of choroidal neovascularization. However, according to the literature, histopathological studies described retinal pigment epithelium alterations and occlusion of choriocappilaris lumina caused by damage to endothelial cells.Citation25,Citation26 In our study, after 18 months of follow up, both bevacizumab and ranibizumab proved effective in reducing retinal thickness and controlling the disease. However, in those cases treated with photodynamic therapy prior to the anti-VEGF treatment, the final visual outcome was less satisfactory. The latter can be associated with damage of retinal pigment epithelium and the choriocapillaris by the photodynamic therapy, the long-standing disease, or both.
This study has obvious limitations such as the small number of patients, the nonstandardized visual acuity measurements and that it is retrospective. However, changing from one anti-VEGF agent to another is an aspect of anti-VEGF treatment which requires further investigation.
According to the literature, bevacizumab does not harm retinal function and is well tolerated by ganglion cells and photoreceptors.Citation27–Citation30 The molecular size of ranibizumab and bevacizumab and their half-life in vitreous are different.Citation28,Citation29
Ranibizumab was clinically effective in the long term in reducing retinal thickness, but the change of treatment from a larger molecule such as bevacizumab to a half-size molecule with less half-life in vitreous such as ranibizumab may have contributed to this transient increase in retinal thickness and decrease in visual acuity. The latter created a transient “instability” in the eye which may have triggered the large subretinal hemorrhage. One possible solution to the change of medication would be to increase the dose of ranibizumab on the month of shifting. A prospective study with large series of patients and controls may be necessary in order to determine whether it is safe to change from one medication to another, or it is preferable to continue treatment with the same anti-VEGF agent for this disease.
Disclosures
The authors report no proprietary or conflicts of interest in this work.
References
- AmbatiJAmbatiBKYooSHIanchulevSAdamisAPAge-related macular degeneration: etiology, pathogenesis, and therapeutic strategiesSurv Ophthalmol200348325729312745003
- BresslerNMBresslerSBFineSLAge-related macular degenerationSurv Ophthalmol19883263754132457955
- GuyerDRFineSLMaguireMGHawkinsBSOwensSLMurphyRPSubfoveal choroidal neovascular membranes in age-related macular degeneration. Visual prognosis in eyes with relatively good initial visual acuityArch Ophthalmol198610457027052423062
- GattoBCavalliMFrom proteins to nucleic acid-based drugs: the role of biotech in anti-VEGF therapyAnticancer Agents Med Chem20066428730116842232
- GaudreaultJFeiDRusitJSubocPShiuVPreclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administrationInvest Ophthalmol Vis Sci200546272673315671306
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- RegilloCDBrownDMAbrahamPRandomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1Am J Ophthalmol2008145223924818222192
- ChangTSBresslerNMFineJTDolanCMWardJKlesertTRImproved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trialArch Ophthalmol2007125111460146917998507
- BhatnagarPSpaideRFTakahashiBSRanibizumab for treatment of choroidal neovascularization secondary to age-related macular degenerationRetina200727784685017891007
- BrownMMBrownGCBrownHCPeetJA value-based medicine analysis of ranibizumab for the treatment of subfoveal neovascular macular degenerationOphthalmology2008115610391045.e517976724
- AveryRLPieramiciDJRabenaMDCastellarinAANasirMAGiustMJIntravitreal bevacizumab (Avastin) for neovascular age-related macular degenerationOphthalmology20061133363372.e516458968
- RichRMRosenfeldPJPuliafitoCAShort-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degenerationRetina200626549551116770255
- BashshurZFHaddadZASchakalAJaafarRFSaabMNoureddinBNIntravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective studyAm J Ophthalmol2008145224925618067876
- BuysYMBevacizumab: the need for controlled studies to move forwardCan J Ophthalmol200742678918059506
- SinghRPKaiserPKRole of ranibizumab in management of macular degenerationIndian J Ophthalmol200755642142517951897
- MelamudAStinnettSFekratSTreatment of neovascular age-related macular degeneration with intravitreal bevacizumab: efficacy of three consecutive monthly injectionsAm J Ophthalmol20081461919518455144
- DevenyiRGEditorial: update on the modern management of wet age-related macular degenerationCan J Ophthalmol200641213313816767199
- KleinRMKleinRBAvastin versus Lucentis: ethical issues in treatment of age-related macular degenerationRetina20072791163116518046218
- StepienKRosenfeldPPuliafitoCComparison of intravitreal bevacizumab followed by ranibizumab for the treatment of neovascular age-related macular degenerationRetina20092981067107319696701
- LevineJMarcusISorensonJSpaideRCooneyMFreundKBMacular hemorrhage in neovascular age-related macular degeneration after stabilization with antiangiogenic therapyRetina20092981074107919734761
- KaragiannisDMitropoulosPLadasILarge subretinal haemorrhage following change from intravitreal bevacizumab to ranibizumabOphthalmologica2009223427928219390227
- BakriSJKitzmannASRetinal pigment epithelial tear after intravitreal ranibizumabAm J Ophthalmol2007143350550717317396
- ScupolaACoscasGSoubraneGBalestrazziENatural history of macular subretinal hemorrhage in age-related macular degenerationOphthalmologica19992132971029885385
- StifterEMichelsSPragerFIntravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhageAm J Ophthalmol2007144688689217916314
- Schmidt-ErfurthULaquaHSchlotzer-SchrehardUViestenzANaumannGOHistopathological changes following photodynamic therapy in human eyesArch Ophthalmol2002120683584412049594
- SchnurrbuschUEWeltKHornLCWiedemannPWolfSHistological findings of surgically excised choroidal neovascular membranes after photodynamic therapyBr J Ophthalmol20018591086109111520762
- KaempfSJohnenSSalzAKWeinbergerAWalterPThumannGEffects of bevacizumab (Avastin) on retinal cells in organotypic cultureInvest Ophthalmol Vis Sci20084973164317118344448
- BakriSJSnyderMRReidJMPulidoJSEzzatMKSinghRJPharmacokinetics of intravitreal ranibizumab (Lucentis)Ophthalmology2007114122179218218054637
- GaudreaultJFeiDBeyerJCPharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbitsRetina20072791260126618046235
- LadasIDKaragiannisDARouvasAAKotsolisAILiotsouAVergadosISafety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injectionsRetina200929331331819287287