Abstract
Overactive bladder is a dreadful syndrome that affects a considerable number of patients. Antimuscarinics are the mainstay of pharmacotherapy for this condition. Transdermal (TD) oxybutynin (OXY) bypasses the first-pass metabolism and reduces the formation of N-desethyloxybutynin, a compound believed to be associated with anticholinergic side effects. The 3.9 mg matrix TD system is applied twice weekly and transports OXY directly into the systemic circulation. The patch can be applied to abdomen, buttock, and hip, and provides continuous OXY delivery that minimizes peak and trough fluctuations in plasma levels. In clinical trials, TD and oral OXY produced a significant reduction in incontinence episodes, with no difference between oral and TD treatments. In addition, TDOXY was similar to tolterodine, and it produced a significant improvement in the number of urinary incontinence episodes, complete continence, and urodynamic and quality of life parameters compared with placebo. The incidence of anticholinergic adverse events with TDOXY was similar to placebo. Most common adverse events were mild–moderate skin reactions. Treatment satisfaction survey suggested patients’ preference to use the TD system in the future. Counseling on healthy skin care and appropriate product use can enhance patients’ knowledge about TDOXY for overactive bladder treatment.
Introduction
Overactive bladder (OAB) is defined as urgency, with or without urge incontinence, and usually with frequency and nocturia, in the absence of infection or other proven etiology (CitationAbrams et al 2002).
The National Overactive BLadder Evaluation (NOBLE) program examined the prevalence and burden of OAB in the US population (CitationStewart et al 2003). The overall prevalence of OAB was similar between men and women (16.0% and 16.9%, respectively), and prevalence of urge incontinence increased with age. Overactive bladder was associated with lower quality-of-life scores, higher depression scores, and poorer quality of sleep.
In Europe, a population-based survey revealed a 16.6% overall prevalence of OAB symptoms (CitationMilsom et al 2001). Similar to the NOBLE study, the prevalence of OAB symptoms increased with advancing age. Considering that only a few affected individuals currently receive treatment, there is a substantial need for improvement in the diagnosis and treatment of this condition.
Nonsurgical approach is the mainstay of OAB therapy, with fundamental pharmacologic treatment based on muscarinic receptor antagonism. A recent meta-analysis found that antimuscarinics confer quantifiable treatment benefit, in addition to a significant improvement in quality of life (CitationChapple et al 2005). Differences in the tolerability profiles of individual agents were thought to be of clinical significance and have a significant influence on adherence and overall efficacy.
Oxybutynin (OXY) is a competitive acetylcholine antagonist that relaxes the bladder smooth muscle. In patients with involuntary detrusor contractions, OXY increases bladder capacity and volume to first detrusor contraction, thus decreasing urinary urgency and frequency of urination (CitationWatson Pharma 2003). It is considered to be the earliest and most well-accepted agent for the treatment of OAB. However, anticholinergic side effects of orally administered OXY, primarily dry mouth, may limit its efficacy.
Pharmacokinetics
Oxybutynin is extensively metabolized, primarily by CYP3A4, found mostly in the gut wall and liver. N-desethyloxybutynin (DEO), one of the metabolites, is believed to be associated with anticholinergic side effects (CitationZobrist et al 2001). With oral administration, first-pass metabolism of OXY reduces its bioavailability to 6% and results in high levels of DEO (ratio to parent compound 11.9:1). In contrast, transdermally-delivered OXY is associated with a bioavailability of approximately 80% and low DEO levels.
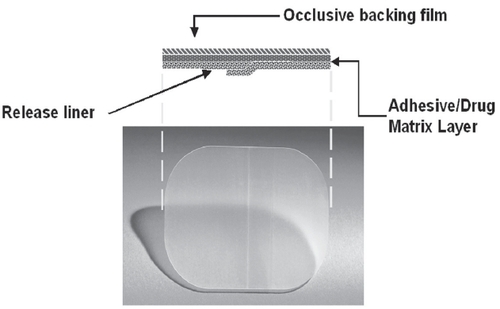
The transdermal (TD) OXY system (CitationWatson Pharma 2003) provides continuous and consistent delivery with twice-a-week application (). This matrix-type patch system transports OXY directly into the systemic circulation across intact skin.
Transdermal administration, unlike the oral route, bypasses the first-pass metabolism and reduces the formation of DEO (ratio to parent compound 1.3:1) (CitationWatson Pharma 2003). This preserves unmetabolized OXY in the circulation and results in greater systemic availability of the parent compound. Patch delivery of 3.9 mg/day produces OXY plasma levels similar to 10 mg/day of orally administered extended-release OXY (CitationAppell et al 2003). In addition, mean saliva output was greater during TD than extended-release oral treatment (15.7±9.3g vs 12.2±6.8g, respectively; p=0.02), based on a standardized saliva-production assay performed after achieving a pharmacologic steady state (CitationAppell et al 2003).
Pharmacokinetic studies in healthy volunteers demonstrated bioequivalent OXY absorption with a 39 cm2 (3.9 mg/day) patch application to abdomen, buttock, and hip (CitationZobrist et al 2003). In addition, a relationship between various sizes of TD systems was examined. This is to be expected since TDOXY is a matrix patch, with the therapeutic compound being found within the adhesive layer, and the adhesive surface area correlates directly with the compound dosage. Mean steady state plasma concentrations of OXY and DEO increased proportionally according to the patch size, while DEO to OXY ratios showed similar profiles.
Compared with oral administration, TD application offers several potential ways to improve the therapeutic value of OXY (CitationAppell et al 2003; CitationZobrist et al 2003):
avoidance of presystemic metabolism
reducing exposure to the DEO metabolite
greater saliva output
continuous delivery that minimizes peak/trough fluctuations in plasma levels that occur with intermittent oral administration.
TD formulation
Oxybutynin transdermal system is a matrix-type TD system containing OXY and triacetin (a skin permeation enhancer) dissolved in an acrylic block–copolymer adhesive with a surface area of 39 cm2 containing 36 mg racemic OXY, designed to continuously deliver OXY over a 3–4 day wear period (CitationZobrist et al 2001).
Clinical experience
Efficacy
TD vs immediate release OXY (CitationDavila et al 2001)
A total of 76 adults with history of urge or mixed urinary incontinence were randomized to receive TD or oral OXY for 6 weeks as part of a phase II clinical trial. All patients were responders to previous oral immediate-release anticholinergic therapy, with symptomatic improvement during a minimum of 6 weeks of oral OXY. Patients had to have at least 3 incontinent episodes daily, and a greater than 30% increase in urge incontinence episodes after a 2 week washout from current treatments.
Bladder symptoms and urodynamic findings were used to confirm diagnosis of motor detrusor instability. Patients were assessed in the clinic after 2, 4, and 6 weeks of treatment. Study participants completed a 3-day urinary diary to assess average daily incontinence episodes, a visual analog scale for efficacy, and an anticholinergic symptoms questionnaire to determine presence and severity of dry mouth and other symptoms.
Treatment was initiated at 1 of 3 dosing levels that matched prior OXY dose requirement. Maximal study doses were 5.2 mg/day TD and 22.5 mg/day oral OXY. Double-blind therapy included daily ingestion of oral OXY, and twice-weekly application of the TD system (ie, active TD and placebo oral, or placebo TD and active oral therapy). The starting dose was continued for the initial 2 weeks, followed by the investigator-adjusted dose titration based on the anticholinergic side effect profile. The goal was to reach the highest tolerable dose, primarily based on dry mouth severity.
The average number of incontinence episodes was reduced from washout to end of study by approximately 5 episodes/day in both groups (p<0.0001), with no significant difference between oral and TD therapy groups.
Visual analog scale analysis revealed no difference in mean continence score between TD and oral therapy upon study completion (p=0.9). The change in score for TD and oral groups from washout to study end was significant (5.8±4.2 vs 6.0±3.3cm respectively, p<0.0001). Urodynamics was repeated after dose titration.
Improvements in average bladder volume at first detrusor contraction were similar with TD and oral treatments (66±126 vs 45±163ml respectively, p=0.57). Mean maximum cystometric capacity increased 51±138ml in the oral (p=0.0538) and 53±88ml in the TD (p=0.0011) groups. Dry mouth severity was the primary outcome of interest in this study.
TDOXY vs placebo (CitationDmochowski et al 2002)
A 12-week, double-blind trial randomized 520 adults with OAB to receive 3 doses of TDOXY (1.3, 2.6, or 3.9 mg) or placebo twice weekly. A 2-week washout period was allowed for those receiving pharmacologic treatment. Patients completed a 7-day urinary diary at baseline, weeks 3, 6, 9, and 12. The Incontinence Impact Questionnaire (IIQ) and Urogenital Distress Inventory (UDI) quality of life instruments were assessed at baseline, and repeated at weeks 6 and 12.
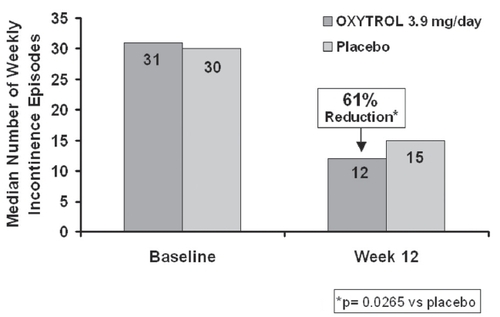
The double-blind period was completed by 447 patients (86%). Those treated with the 3.9 mg/day TD system had a significant reduction in the number of urinary incontinence episodes/week compared with placebo (). In the subset of 113 patients previously treated with anticholinergics, reductions vs placebo were also statistically significant in the 3.9 mg group. Treatment at the lower dosages did not achieve a statistically significant difference in weekly incontinence episodes from placebo.
Average daily urinary frequency reduction with this dose was −2.3±2.5 vs −1.7±3.0 with placebo, p=0.0457. The median increases in average urinary voided volume were also better than with placebo (24 vs 6 ml, p=0.0063). The 3.9 mg group had a significant improvement in the IIQ quality of life score over placebo, p=0.0327.
The double-blind portion of the trial was followed by a 12-week, open label, dose titration period. It was completed by 358 (87.1%) of 411 patients who entered the open-label extension. Patients in all arms experienced reductions from baseline in the number of incontinence episodes of approximately 3 episodes/day. The IIQ and UDI also indicated considerable improvements in quality of life, specifically with a decrease of 23 points in the Travel domain (p=0.0018) of the IIQ and a decrease of 25 points in the Irritative symptoms domain of the UDI, from baseline to last observation.
TDOXY vs tolterodine and placebo (CitationDmochowski et al 2003)
In a double-blind, double-dummy trial of prior responders to anticholinergic treatment, 361 adults with OAB were randomized to receive twice-weekly TDOXY 3.9 mg/day, daily long-acting tolterodine 4 mg, or matching placebos for 12 weeks. The median duration of prior treatment was >1 year.
Compared with placebo, there was a significant reduction in the median number of incontinence episodes with TDOXY (−3, p=0.0137) and tolterodine (−3, p=0.0011), with no difference between active treatments (p=0.5878). This corresponded to a 75% improvement in incontinence episodes with either tolterodine or TDOXY. Many patients achieved complete continence (39% with TDOXY, 38% with tolterodine; both p=0.014 vs placebo).
There was a significant improvement in total IIQ scores with both active treatments. The Global Assessment of Disease State scores also improved during TDOXY (p=0.0106) and tolterodine (p=0.0001) treatments, with no difference between them (p=0.1861).
Phase 3 clinical trials: combined results (CitationDmochowski et al 2005)
The data from two phase 3 trials (CitationDmochowski et al 2002, Citation2003) were pooled for further analysis. It included 242 patients who received TDOXY 3.9 mg/day, and 245 participants who received placebo. Over 90% of patients were female and white. Approximately 60% of participants had been on previous anticholinergic treatment.
Overall, baseline median number of daily incontinence episodes was 4.0. There was a 75% improvement with TDOXY treatment, as opposed to a 50% reduction with placebo (p=0.00004). Likewise, a decrease in daily urinary frequency was more evident with TDOXY compared with placebo (18% vs 8.7%, respectively; p=0.0023). There was a 15% increase in urinary voided volume with TDOXY, whereas only a 3.6% increase was noted with placebo (p<0.00001).
Following 12 weeks of treatment, there was a significant improvement in the mean IIQ quality of life score (−60.7 for TDOXY and −43.0 for placebo, p=0.0182).
Tolerability
TD vs immediate release OXY (CitationDavila et al 2001)
The three most commonly reported anticholinergic adverse events were reported more commonly in the oral group compared with the TD group, and included dry mouth (82% vs 39%), constipation (50% vs 21%), and somnolence (37% vs 18%). With direct prompted questioning regarding dry mouth, using the anticholinergic symptoms survey, TD treatment resulted in 39% of patients recording dry mouth, as opposed to a 94% incidence with oral treatment, at the highest tolerable dose (p<0.001). Better tolerability allowed 68% of TD system users to reach the maximum study dose, compared with only 32% of those in the oral OXY arm ().
During treatment, 38% of TD system users reported an improvement in constipation, relative to their prior immediate-release OXY regimen, compared with 5% in the oral group. Of those receiving a TD application, 61% revealed no skin erythema.
TDOXY vs placebo (CitationDmochowski et al 2002)
The most commonly observed treatment-related adverse events in the double-blind portion were application site reactions. In the 3.9 mg dose group, they included erythema (5.6%) and pruritus (16.8%). In the open-label extension, pruritus was the most frequently reported treatment-related event. The majority of patients experienced no or mild erythema.
Some patients used topical steroids or antihistamines (double-blind 4.4%, open-label 1.9% patients). Application site reactions caused treatment-related withdrawals of some patients (4.8% in double-blind, 3.6% in open-label extension).
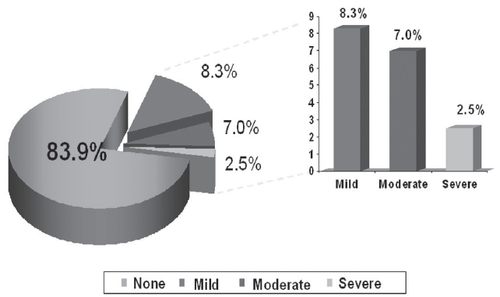
The most common anticholinergic adverse event for all doses was dry mouth (7.0% OXY vs 8.3% placebo, p=0.98). Central nervous system effects occurred infrequently.
TDOXY vs tolterodine and placebo (CitationDmochowski et al 2003)
Treatment-related systemic adverse events were more common with tolterodine, while application site reactions were more frequent with TDOXY. Erythema (8.3%) and pruritus (14.0%) were the most common treatment-related events in the TDOXY group. Dry mouth in this group was similar to placebo (4.1% vs 1.7%, p=0.2678). With tolterodine, most common treatment-related events were anticholinergic effects. Dry mouth in this group was more frequent than with placebo (7.3% vs 1.7%, p=0.0379).
Phase 3 clinical trials: combined results (CitationDmochowski et al 2005)
None of the patients receiving TDOXY discontinued treatment due to dry mouth; most discontinuations were because of application site reactions.
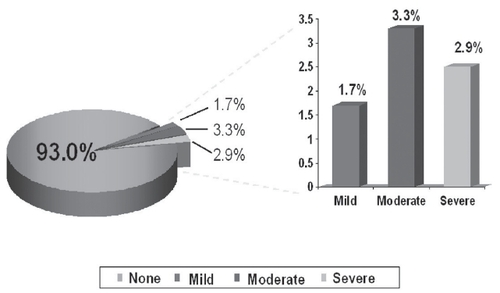
Erythema and pruritus was noted in 7.0% () and 16.1% () patients, respectively. Of these, mild-to-moderate erythema occurred in 71%, and mild-to-moderate pruritus occurred in 95% patients. Treatment discontinuations due to erythema were reported by 3.7%, and due to pruritus by 3.3% patients.
The incidence of application site reactions compares favorably with that of other studies of TD delivery systems. These localized, self-limited reactions may be improved in routine clinical practice through the use of multiple application sites, including the buttocks and hip areas, in addition to the abdomen, as used exclusively in the current trial. Long-term studies suggest that prolonged exposure to TD systems does not increase the incidence of application site reactions (CitationDmochowski et al 2003).
The overall incidence of anticholinergic adverse events (eg, dry mouth, constipation, dizziness, somnolence, abnormal vision, nausea) did not differ from placebo (12.8% vs 11.0%, respectively, p=0.5421).
Patient perceptions
Following the 12-week double-blind portion of the phase 3 trial (CitationDmochowski et al 2003), patients were eligible to enter an open-label study extension. These patients could volunteer to complete a patient satisfaction survey (CitationNewman 2003). A total of 185 questionnaires were analyzed to measure the satisfaction of using a skin patch for the treatment of urinary incontinence. Approximately two-thirds of the participants claimed that if they needed treatment for incontinence in the future, they would now prefer a TD system to other treatments.
The majority (72%) of patients reported that the application site-related itching and redness disappeared within a week. Many users (68%) described the patch as very easy to apply. Remembering to reapply the TD system twice a week was rated as the same as (45%) or easier than (33%) taking the daily pill.
Future directions
The Multicenter Assessment of Transdermal Therapy in Overactive Bladder with Oxybutynin (MATRIX) study is a 6-month, open-label, randomized, multicenter, prospective trial in a community-based population. This study is under way to assess safety, health-related quality of life, and other patient-reported outcomes in a large number of patients treated with TDOXY. Study entry data is already being reported and providing useful data regarding the current demographics of the populations of those suffering from OAB (CitationSand et al 2005).
Summary and expert opinion
Transdermal OXY presents a safe and effective treatment modality for patients suffering from OAB. This medication improves both objective and subjective aspects of OAB, including an improvement in patient’s quality of life.
Avoidance of first-pass liver metabolism allows for greater systemic availability of the parent compound, and diminishes formation of the metabolite, DEO, which is thought to be associated with anticholinergic side effects.
TDOXY offers efficacy comparable with currently available oral agents, while its anticholinergic adverse events profile is comparable with placebo. Most commonly seen treatment-related side effects are localized application-site reactions, which are reported as mild–moderate by most patients who develop erythema or pruritus. Appropriate patient counseling on healthy skin care, site rotation, prudent use of topical steroids when appropriate, and avoidance of local irritants can contribute to the successful incorporation of TDOXY into the OAB treatment plan.
Transdermal OXY usage in clinical practice has several limitations. Skin reactivity may pose an insurmountable barrier for some patients, despite the care recommendations noted above. Those patients requiring a higher dosage of OXY may not be able to achieve a clinically satisfactory response, as the dosage of OXY is related to the patch size. A dosage requirement of greater than 3.9 mg/d would require a larger patch, or multiple patches, possibly increasing the rate of skin reactivity and/or making this treatment modality financially unsuitable. Males may also be less suitable for patch therapy as the patch needs to be applied to a skin area devoid of hair. In addition, data is currently unavailable for pediatric use.
Since becoming Food and Drug Administration-approved and marketed in 2003, Oxytrol® use has been associated with various patient-reported beneficial features not captured in clinical trials. Most are related superficially to the use of OXY as the therapeutic agent, and many to the TD patch administration route. Oxybutynin lends itself to individualization in dosage, both on a chronic basis as well as for specific short-term usage. Since TD matrix dosing is dependent on patch surface area, many patients have increased their dosage by applying an extra patch or a portion of one (CitationRuscin et al 2004; CitationZinner et al 2004). The increased circulating OXY level can be expected to improve response rates, based on the previous phase II trial data. Other means of increasing circulating OXY include taking an oral dosage. This strategy has been utilized by taking half or one entire immediate release tablet, immediately prior to an activity when OAB symptoms would be particularly undesirable, such as airplane travel or going to a concert or opera. Rarely has extended-release dosing been used for this purpose.
There have been specialty-related prescriptive patterns seen for TDOXY. Gynecologists who prescribe matrix-type patches for hormonal replacement and contraception, have promptly adopted this agent. Urologists were much less familiar with patch therapy, having had less than desirable responses to the reservoir-type patches, and were significantly slower to adopt this patch.
Since the release of TDOXY, several new oral anticholinergic agents have become available. Overall efficacy and response rates for all oral agents are similar, with specific differences being noted based on particular study characteristics. The unique pharmacokinetic and clinical characteristics of TD delivery, including gel and intravaginal rings currently being clinically evaluated, may provide additional benefits for OAB sufferers () (CitationAlberti et al 2005)
Figures
Figure 3 Dry mouth tolerability in subjects treated with transdermal oxybutynin versus immediate-release oxybutynin.
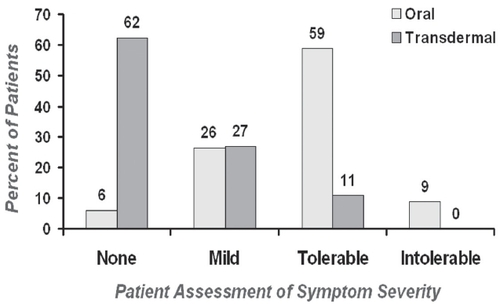
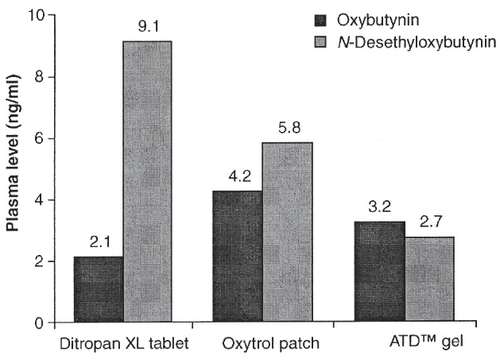
Figure 6 Oxybutynin and DEO levels based on route of administration. Copyright © 2005. Alberti I, Grenier A, Kraus H, et al. 2005. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin Drug Deliv, 2:935–50.
References
- AbramsPCardozoLFallM2002The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the international continence societyNeurourol Urodynam2116778
- AlbertiIGrenierAKrausH2005Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug deliveryExpert Opin Drug Deliv29355016296788
- AppellRAChancellorMBZobristRH2003Pharmacokinetics, metabolism, and saliva output during transdermal and extended-release oral oxybutynin administration in healthy subjectsMayo Clin Proc7869670212934778
- ChappleCKhullarVGabrielZ2005The effects of antimuscarinic treatments in overactive bladder: a systematic review and meta-analysisEur Urol4852615885877
- DavilaGWDaughertyCASandersSWTransdermal Oxybutynin Study Group2001A short-term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinenceJ Urol166140511435842
- DmochowskiRRDavilaGWZinnerNRTransdermal Oxybutynin Study Group2002Efficacy and safety of transdermal oxybutynin in patients with urge and mixed urinary incontinenceJ Urol168580612131314
- DmochowskiRRSandPKZinnerNRTransdermal Oxybutynin Study Group2003Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinenceUrology622374212893326
- DmochowskiRRNittiVStaskinD2005Transdermal oxybutynin in the treatment of adults with overactive bladder: combined results of two randomized clinical trialsWorld J Urol232637016151816
- MilsomIAbramsPCardozoL2001How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence studyBJU Int87760611412210
- NewmanDK2003Patient perceptions on new therapeutic options for the control of overactive bladderPoster presented at the 34th Annual Conference of SUNAMarch 26–30San Antonio, TX, USA
- RuscinJMStaskinDRSandPK2004Dose modulation of oxybutynin transdermal system for overactive bladderThe Consultant Pharmacist19938
- SandPKGoldbergRLucenteV2005Decreased sexual function in patients with overactive bladder: interim results from the matrix studyJ Pelvic Med Surg1183
- StewartWFVan RooyenJBCundiffGW2003Prevalence and burden of overactive bladder in the United StatesWorld J Urol203273612811491
- Watson Pharma2003Oxytrol Product InformationWatson Pharma, IncMorristown, NJ, USA
- ZinnerNRDavilaGAndersonRP2004Dose modulation using oxybutynin transdermal system for overactive bladder in older adultsJ Am Geriatr Soc52S767
- ZobristRHSchmidBFeickA2001Pharmacokinetics of the Rand S-enantiomers of oxybutynin and N-desethyloxybutynin following oral and transdermal administration of the racemate in healthy volunteersPharm Res1810293411496941
- ZobristRHQuanDThomasHM2003Pharmacokinetics and metabolism of transdermal oxybutynin: in vitro and in vivo performance of a novel delivery systemPharm Res20103912608543