Abstract
RETAANE® 15mg (anecortave acetate suspension) is under investigation to treat exudative age-related macular degeneration (AMD), the single largest cause of blindness in the Western world, affecting over 15 million people in the USA. RETAANE suspension is a unique synthetic cortisene and has antiangiogenic properties that were established in multiple experimental models of angiogenesis. The molecule acts at multiple sites of the angiogenic cascade. Clinical trials in patients with exudative AMD have demonstrated the excellent safety record of both the drug anecortave acetate and the posterior juxtascleral depot (PJD) administration procedure. A pivotal study comparing RETAANE suspension with placebo showed a significantly higher chance of maintaining vision in the treatment (73%) as compared with placebo (47%). Another study compared RETAANE suspension with Visudyne® photodynamic therapy, revealing no statistically significant differences between the two treatments over 24 months. AMD is a multi-faceted disease and therefore a molecule such as RETAANE suspension with a unique mechanism of action, demonstrated clinical efficacy, and retreatment every six months is an important potential treatment option which should be further investigated both as a monotherapy or in combination with other treatment strategies.
Introduction – current treatment options
Age-related macular degeneration (AMD) is the most serious threat to the vision of the elderly. It is the leading cause of severe and permanent central vision loss amongst those over 50 (CitationSwann and Lovie-Kitchin 1990). About 10% of the population over the age of 52 and up to 33% of those over 75 present with any signs of AMD. Over 15 million Americans are already afflicted with a form of the disease and the number is rising at the rate of 2 million new cases every year. According to the European office of AMD Alliance International, an estimated 2.8 million cases of wet AMD will be diagnosed in the 25 European countries within the next decade. With such an imminent threat to the vision of the elderly, significant research and development efforts are being devoted to finding effective treatment methods for wet AMD. Currently available treatments include laser photocoagulation, verteporfin photodynamic therapy (PDT) (Visudyne®, Novartis AG, Basel, Switzerland), pegaptanib sodium injection (Macugen®, Eyetech Pharmaceuticals/Pfizer), and anecortave acetate (RETAANE® suspension, Alcon Laboratories, Inc., Fort Worth, Texas, USA). In addition, modified PDT treatments such as the combination of PDT with intravitreal triamcinolone became very popular within the last year (CitationAugustin and Schmidt-Erfurth 2006).
Anecortave acetate is a promising new treatment option that is being clinically tested and recently approved in Australia for treating AMD patients with classic containing lesions. Until 2000, laser photocoagulation was the only approved treatment for choroidal neovascularization (CNV). Although effective in ablating CNV and attenuating vision loss over time, laser photocoagulation may be cytodestructive to retinal neurons and can result in an immediate and permanent loss of vision in the treated area, a trade-off unacceptable to many patients considering the procedure. Verteporfin PDT (Visudyne) was approved in 2000 following the demonstration that the procedure was effective in attenuating the rate of vision loss in AMD patients with classic containing lesions. The necessity of retreatments led to the investigation of combination strategies in multiple case series and different lesion types. Those combination approaches are now used by many clinicians on a routine basis (CitationAugustin and Schmidt-Erfurth 2006).
During the approval process, various pharmacological agents found to be effective in retarding vessel growth in animal models of ocular neovascularization were subsequently evaluated in AMD patients for inhibiting CNV growth and preservation of vision. In December, 2004, Macugen, an aptamer inhibiting vascular endothelial growth factor (VEGF) became the first pharmacological agent to be approved for treatment of CNV in all lesion subtypes in patients with exudative AMD.
At one year, Macugen was demonstrated to be more effective than sham injection at preserving vision. Patients treated with Macugen 0.3 mg had a 10% risk of experiencing severe vision loss (≥6 lines of vision) compared with 22% in the sham-injection group. Also at one year, 70% of patients in the treatment group had preserved vision compared with 55% in the sham-injected group (CitationGragoudas et al 2004). At the two year endpoint Macugen demonstrated a 45% treatment effect (difference between sham and active groups with less than 3 lines of lost vision). Subsequent approval of Macugen is expected in Europe in early 2006.
Other pharmacologic interventions for wet AMD including the VEGF inhibitor Ranibizumab (Lucentis®), and RETAANE, an angiostatic cortisene, are in late stages of clinical development/approval. Additionally, non-pharmacological approaches including transpupillary thermotherapy, radiation therapy, and surgical intervention are still being clinically evaluated. Table summarizes available and potential AMD treatment options. However, the results of those approaches are less promising as compared with drug therapies of this disease entity.
Table 1 CNV therapies currently approved or under clinical development
Pathophysiology of wet AMD
AMD is a complex, multi-faceted disease. While the definitive cause has not been elucidated, epidemiological studies have identified several risk factors. Age is by far the most prominent risk factor for AMD among males and females. Other risk factors associated with AMD include presence of soft drusen, macular pigmentary changes, CNV in the fellow eye, race, genetic predisposition, gender, iris color, obesity, and smoking (Table ).
Table 2 Summary of significant risk factors for age-related macular degeneration
Much is now known about the process for developing CNV in the wet (exudative) form of the disease. The multifaceted process of neovascularization can be broken down into several distinct stages (CitationClark 1997). A proangiogenic signal associated with late stage disease stimulates the production of trophic factors that initiates the growth of new blood vessels. The signal to release such growth factors is still speculative and may relate to hypoxia or inflammation associated with remodeling of the outer retina with immunologic phenomenons being involved as well (CitationKijlstra et al 2005). Synthesis of a variety of proangiogenic trophic factors has been proposed; with greatest evidence pointing to VEGF as an initiator of proliferation of CNV and subsequent vascular leakage. The trophic factors have been demonstrated to bind to receptors on vascular endothelial cells (VECs), leading to activation of genetic machinery leading to the production and release of enzymes such as matrix metalloproteinases (MMPs), which degrade the basement membrane and extracellular matrix which surrounds the VECs. Degradation of the extracellular matrix has been shown to be a prerequisite for proliferation and migration of VECs and subsequent tube formation, the result of which leads to the establishment of an immature blood vessel. With the development of a new basement membrane, the new capillary is stabilized and is capable of transporting blood through its lumen (Figure ).
Anecortave acetate – chemical properties, mechanism of action
New compounds known as cortisenes were discovered in 1985 and were found to have angiostatic properties in simple in vitro models of angiogenesis. These steroid-like compounds are devoid of clinically relevant glucocorticoid activity and therefore do not elevate IOP or cause cataracts (CitationCrum et al 1985). Further research led to the identification of anecortave acetate which is an antiangiogenic analog of cortisol (CitationBlei et al 1993).
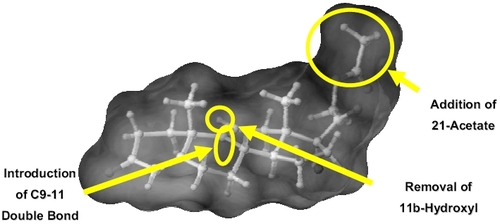
Anecortave acetate differs in structure from cortisol by the removal of 11-beta hydroxyl, addition of a double bond at the C9-11 position, and addition of an acetate group at the C21 position to enhance bioavailability. The resulting molecule is an angiostatic cortisene that imparts angiostatic activity, but does not exhibit typical glucocorticoid-receptor-mediated activity (Figure ). Anecortave acetate demonstrated a broad range of antiangiogenic properties in both in vitro and in vivo models of ocular neovascularization (Table ).
Table 3 Summary of the in vitro and in vivo models of neovascularization used to evaluate the antiangiogenic properties of anecortave acetate
Figure 2 The unique design of the blunt tinted posterior juxtascleral depot cannula ensures that anecortave acetate is delivered directly behind the macula while leaving the globe intact.
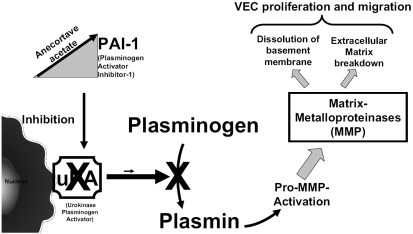
In vitro data indicated that the proliferation of cultured human VECs is inhibited by anecortave acetate. Anecortave acetate’s mechanism of action is in part through inhibiting the expression of urokinase plasminogen activator (uPA) and matrix metalloproteinases (MMPs), extracellular proteases necessary for the breakdown of the basement membrane and extracellular matrix necessary for subsequent migration of VECs into tissue stroma during blood vessel growth (CitationDeFaller and Clark 2000). Additionally, anecortave acetate has been demonstrated to stimulate the production of plasminogen activator inhibitor-1 (PAI-1), a specific inhibitor of uPA activity (CitationPenn et al 2001) (Figure ). In a rat model of retinopathy of prematurity (ROP) retinal levels of PAI-1 and mRNA increased 6- to 9-fold in 24 to 72 hours following intravitreal injection of anecortave acetate (CitationBenEzra et al 1997). Administration of anecortave acetate inhibits the activities of uPA and MMPs. As a result, proteolysis necessary for cellular migration cannot occur and angiogenesis is thus inhibited. Primary angiostatic effects of anecortave acetate on trophic factor release have been demonstrated as well. In one study using the rat ROP model, anecortave acetate reduced the expression of the proangiogenic insulin-like growth factor (IGF-1) and IGF-1 receptor by 36.9% and 50.5%, respectively (CitationLiu et al 2005). In a similar series of experiments using the same rat ROP model, injection of anecortave acetate also reduced VEGF protein at one and two days post-oxygen exposure. This model also demonstrated that anecortave acetate inhibited the transcription of VEGF mRNA, which is upregulated during angiogenesis (CitationYang et al 2005). Future studies are on-going to quantify specific VEGF mRNA splice-variants (Figure ).
Posterior juxtascleral depot (PJD)
Topical ocular administration and subconjunctival administrations of anecortave acetate did not achieve sufficient concentration of the drug to inhibit angiogenesis in the retina and choroid. Intravitreal injections of anecortave acetate provide therapeutic levels of the drug to the retina; however, routine intravitreal injections were undesirable due to the potential for serious complications such as endophthalmitis and retinal detachment and overall discomfort of the patient.
Sub-Tenon’s injections with a straight needle were found to provide therapeutic drug concentrations (≥0.1 uM) in both the retina and choroid, but these injections were highly variable in their placement. Poor drug positioning would result in an undesirably high incidence of treatment failures due to low drug bioavailability; therefore, a specialized cannula and depot delivery technique were developed for the administration of RETAANE.
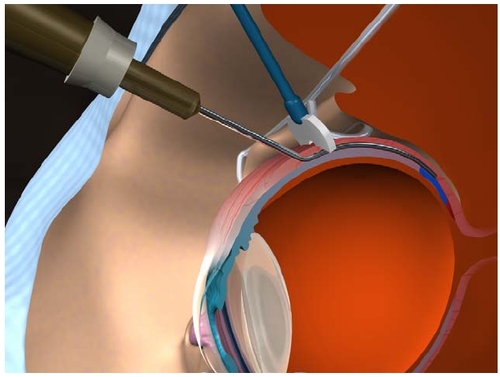
A 56-degree curved posterior juxtascleral cannula was developed to follow the curvature of the globe during insertion, underneath the conjunctiva and Tenon’s capsule, on bare sclera. It has a length that positions the tip directly behind the macula when it is fully inserted. The globe is not perforated during the procedure. The orifice of the cannula is located on the inferior surface of the tip to assure administration of anecortave acetate in direct apposition to the scleral surface (Figure ).
Figure 3 The counter pressure device prevents reflux of anecortave acetate when placed as posterior as possible in position with the posterior juxtascleral depot cannula.
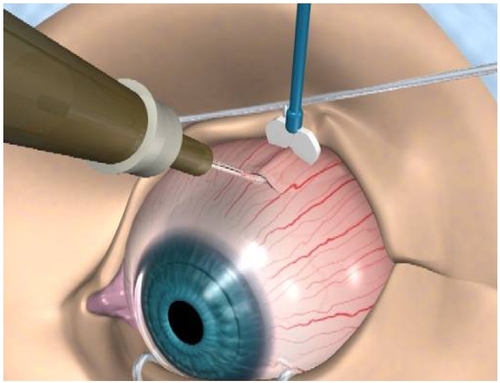
In contrast to the traditional sub-Tenon’s injections which missed the targeted area nearly 30% of the time, injections using posterior juxtascleral depot (PJD) delivered the entire dose over the macular region and directly onto the scleral surface. PJD administration results in no risk of endophthalmitis, retinal detachment, or IOP increase because the globe is not penetrated during administration. In clinical studies PJD resulted in no clinically relevant treatment-related adverse events and due to our current knowledge, re-administration of anecortave acetate is only required every 6 months.
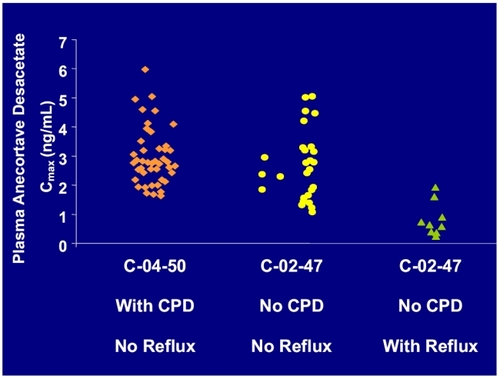
In the clinical studies it has been shown that anecortave acetate can reflux out the cannula incision during the PJD procedure. To address this issue, a counter pressure device (CPD) has been developed by the manufacturer of the drug to stop the reflux of the suspension, ensuring that the complete quantity of drug is delivered behind the macula. The CPD is positioned as posterior as possible from the incision site, perpendicular to the globe. The notch on the CPD straddles the cannula over the conjunctiva, which allows the procedure to be completed by one person and also increases stability. Studies on bioavailablility have been performed to further address this issue. Those studies have shown that patients that did not have reflux had greater systemic bioavailability as assessed by higher plasma concentrations of anecortave acetate (please see Figure ).
Finally, the PJD procedure does not require immediate follow-up visits and no safety issues with the procedure have been identified (Figure ).
The PJD procedure has been used in clinical trials to deliver more than 2500 juxtascleral depots in nearly 900 patients with no safety concerns reported (CitationRegillo et al 2005). Some patients have received as many as seven PJD administrations. To date, no globe perforations, damage to the optic nerve, damage to posterior ciliary arteries, or other significant concerns regarding the cannula have been raised by investigators during studies. The adverse events most often attributed to the PJD administration procedure in all treatment groups are shown in Table .
Table 4 Percentages of adverse events attributed to the posterior juxtascleral administration or sham procedure
No events associated with PJD were serious, almost all were mild and transient, with similar frequency across all groups, and did not interrupt treatment (CitationD’Amico et al 2003; CitationAugustin et al 2005).
Clinical trials
C-98-03: dose-response monotherapy study: RETAANE 15 mg (anecortave acetate suspension) or anecortave acetate (3 mg, 30 mg) versus placebo in AMD patients
One hundred twenty eight patients with exudative subfoveal AMD were enrolled in a double-masked, dose-response study, beginning in 1999, to evaluate the safety and efficacy of anecortave acetate. Patients were randomized to receive anecortave acetate (30 mg, 15 mg, or 3 mg) or placebo via PJD administration every six months for a total of 24 months. The primary efficacy variable was the mean change from baseline in best-corrected ETDRS logMAR visual acuity. The secondary efficacy outcomes were: (a) preservation of vision (loss of less than three lines), (b) clinically significant worsening of vision (loss of at least three lines), (c) severe vision loss (loss of at least six lines), and (d) changes in CNV.
The efficacy of anecortave acetate 15 mg for maintaining vision and suppressing lesion growth in patients with exudative AMD have been confirmed by long-term study results. Anecortave acetate 15 mg was superior at month 24 to other dosages tested and statistically superior to placebo in preserving visual acuity (p≤0.05). In the anecortave acetate group, 73% of patients maintained vision compared with 47% in the sham group. In the sham-treated group 23% of patients went on to severe vision loss compared with 6% in the anecortave acetate treated group (p≤0.05). Anecortave acetate also demonstrated the ability to inhibit the growth of classic CNV (p≤0.05) in eyes with all lesion types. Beginning at six months and continuing throughout the entire 24-month study, anecortave acetate 15mg was consistently more effective in stabilizing vision compared with placebo.
The following mild and transient conditions were noted post administration: ptosis, subconjunctival hemorrhage, ocular discomfort, ocular pruritus, hyperemia, and foreign body sensation (see also Table ).
The clinical efficacy observed in this study of a mixed population of AMD lesion types and sizes supports earlier observations that anecortave acetate is likely to inhibit CNV lesion growth regardless of lesion subtype.
C-00-07: anecortave acetate suspension and PDT combination trial
C-00-07 was a 6-month phase II study that evaluated the effect of anecortave acetate (15 mg or 30 mg) versus placebo following initial Visudyne PDT, for the treatment of patients with exudative AMD. This study enrolled 136 patients and was completed in 2002. Patients who received a single administration of anecortave acetate following initial treatment with PDT maintained better vision than patients who received PDT alone. Data from this study illustrated that anecortave acetate can be safely administered with PDT.
C-01-99: pivotal study versus Visudyne PDT: RETAANE suspension plus sham PDT versus Visudyne PDT plus sham PJD
This pivotal, non-inferiority study was designed to compare anecortave acetate 15 mg suspension to PDT with verteporfin. The 530 exudative AMD patients enrolled in this study were randomized 1:1 to either a PJD administration of anecortave acetate 15 mg every six months combined with a sham PDT treatment every three months per labeling guidelines, or to PDT every three months combined with a sham PJD administration every six months. This trial confirmed the excellent safety results reported in C-98-03 study. At 12 months 45% of anecortave acetate 15 mg-treated patients had preserved vision compared with 49% in the PDT group (p>0.05) which was not significantly different (CitationSlakter et al 2006). The study demonstrated that anecortave acetate and verteporfin PDT treatments are clinically equivalent throughout 24 months following treatment with respect to the percentage of patients with <3 line loss of logMAR visual acuity. The largest difference between the two treatment groups at 12 and 24 months was 3.5 letters which is not clinically significant. However, the drug did not meet the primary endpoint, which led to a further evaluation of the study. This led to interesting results regarding the reflux problem and treatment intervals which had a significant impact on the data. The primary endpoint would have been met with the statistical evaluation being performed with data from predominantly classic lesions only, which is the only lesion composition of the study population. This issue is extensively discussed by CitationSlakter and coworkers (2006). Furthermore, in a subsequent pharmacokinetic study, C-02-47, investigators looked at correlations between drug reflux and plasma levels of anecortave acetate and showed that there was a 4-fold lower plasma level of the drug in the eyes that experienced reflux.
When considering only those patients that did not have reflux in the C-01-99 study, the percentage of patients in whom vision was preserved increased from 45% to 50%. When data from patients who experienced reflux and retreatment after 6-months were excluded, 57% of anecortave acetate 15 mg-treated patients had preserved vision.
In this study it was noted that the degree of preservation of vision for both anecortave acetate 15 mg (45% with <3-line vision loss) and PDT was considerably less than previously observed in other trials including C-98-03 and the TAP study. Analysis of baseline data reveals that the lesions in this study were significantly smaller and younger at baseline than those in previous studies and included a high proportion of lesions with a retinal angiomatous proliferation (RAP) component. The high incidence of characteristics such as RAP, extensive subretinal hemorrhage/fluid and subfoveal hotspots along with the fact that these lesions were diagnosed early in the disease suggests that this study included unusually aggressive lesions (CitationSlakter et al 2005).
C-02-47: pharmacokinetics of RETAANE suspension in patients with AMD
This was a pharmacokinetics (PK) study in 20 patients with wet AMD who had two PJD administrations of RETAANE suspension over a 12 month period. The peak plasma levels were correlated with observed reflux. It was found that the patients for whom reflux was observed had plasma levels that were 4-fold lower than patients without reflux. These results highlighted the need for a CPD.
C-04-50: pharmacokinetics and reflux study with counter pressure device
Recognizing the need for a method to reduce reflux, the manufacturer of anecortave acetate (Alcon Laboratories, Inc.) initiated this study to determine the ability of a CPD to eliminate reflux. The study was done at the request of the FDA. This pharmacokinetic study was undertaken to determine whether reflux could be prevented and used serum drug levels as a quantitative, surrogate measure for the influence of reflux on drug absorption. Investigators evaluated and scored drug reflux following administration of RETAANE.
Reflux was effectively controlled in 100% of patients, with a reflux score of 0 for all eyes (as rated by the investigator), consistent with no loss of dose upon administration using the CPD. Mean plasma drug levels were increased 4-fold over drug levels observed with reflux in the C-02-47 trial. Controlling reflux with the CPD is expected to provide improved visual acuity outcomes based on improved outcomes in patients without reflux in protocol C-01-99 relative to those with reflux (Figure ).
Figure 4 Evidence supports use of counter pressure device (CPD) for managing reflux. Plasma concentrations of anecortave desacetate in patients with the CPD (C-04-50) were the same as the plasma concentrations in patients without the CPD and had no reflux (C-02-47). Patients (C-02-47) with reflux had 4 fold lower levels of anecortave desacetate than patients without reflux. In the C-04-50 study, the CPD prevented reflux in 100% of the patients.
C-02-60: the anecortave acetate risk reduction trial
Several studies have shown that patients with wet AMD in one eye are at high risk of developing CNV in the fellow eye with dry AMD. It was found that patients with both large drusen (larger than 63 μm) and retinal pigment epithelium hyperpigmentation in the fellow eye faced a 58% risk of developing CNV within 5 years. By comparison, patients without large drusen or hyperpigmentation in their fellow eye faced a much lower (10%) risk of developing CNV (CitationAbdelsalam et al 1999). Another study found that if the fellow eye had 5 or more large drusen, it was more than twice as likely to develop CNV compared with a fellow eye with fewer or no drusen. The chances of CNV development were also found to be higher in eyes with confluent drusen and focal areas of hyperpigmentation. Amongst the 670 patients observed in this study, 236 (35%) developed CNV in the better eye within 5 years (CitationBenEzra et al 1997).
These epidemiological studies have provided the baseline data necessary for undertaking clinical trials studying prevention of wet AMD. The studies using this clinical model are extremely costly due to the large numbers of patients that must be enrolled to have a reasonable probability of detecting a statistical difference between the treatment groups and the follow-up period is usually long.
The Anecortave Acetate Risk Reduction Trial (AART) was designed to demonstrate that anecortave acetate suspension (15 mg or 30 mg) is safe and effective in arresting the progression of non-exudative (dry) AMD in patients who are at-risk for progressing to exudative (wet) AMD. The primary outcome measure of this study is the percentage of patients with sight-threatening CNV in the study eye at month 48. The study is being conducted at over 100 centers across the US, Europe, Australia and South America. Enrollment of more than 2500 patients was completed at the end of 2005.
Role of RETAANE
As discussed above, RETAANE suspension is an effective and safe treatment for patients with wet AMD. Use as monotherapy is supported by the dose-response study (C-98-03) comparing efficacy of RETAANE 15 mg versus placebo and the demonstration of equivalence of RETAANE 15 mg and Visudyne PDT over 24 months (C-01-99). An alternative role for RETAANE 15 mg as an adjunct or in combination to anti-VEGF therapy may be considered because of its unique, multifactorial mechanism of action, 6 month duration of action and safety due to the PJD procedure. In addition, RETAANE suspension has been demonstrated to be safe for use with Visudyne PDT (see C-00-07 above). Not known at this time is whether RETAANE 15 mg given by PJD administration can potentiate the efficacy of intravitreal anti-VEGF therapy and reduce the frequency of intravitreal injections associated with the anti-VEGF approach. To further address this interesting issue, Alcon Research Ltd., is collaborating with the National Eye Institute to conduct the BRIDGE Study which is a randomized, masked, multi-center, phase II study of the effect of PJD delivery of RETAANE 15 mg on the response to intravitreal bevacizumab (Avastin™) in patients with subfoveal CNV secondary to AMD. The objective of this study will be to investigate whether RETAANE 15 mg (anecortave acetate suspension) reduces the frequency of intravitreal injections while maintaining vision and limiting disease progression in patients who are receiving intravitreal injections of bevacizumab (Avastin) for treatment of exudative AMD. Finally, due to the characteristics of RETAANE suspension (ie, mechanism of action, safety, and chronic duration following PJD administration), the drug may be suited for arresting the conversion of dry AMD to wet AMD in patients at risk for developing CNV associated with AMD (AART Study). If approved for arresting the progression of CNV, RETAANE 15 mg would be the only therapy, with the exception of AREDS vitamin supplement, to inhibit progression of any AMD subtype.
Regulatory status
RETAANE suspension was approved in Australia on December 12, 2005. On May 24, 2005 the US Food and Drug Administration (FDA) issued an approvable letter for the RETAANE 15mg (anecortave acetate suspension) new drug application (NDA).
In March 2006, Alcon has withdrawn the application for approval at the European Medicines Agency (EMEA).
Conclusion
A synthetic cortisone (anecortave acetate) has been shown to have antiangiogenic properties that have been established in multiple experimental models of angiogenesis. Clinical trials in patients with exudative AMD have demonstrated an excellent safety record both for anecortave acetate and the PJD administration, with no clinically relevant adverse events.
Co-administration of anecortave acetate 15 mg with PDT has been shown to be safe. Patients receiving one anecortave acetate administration and one PDT treatment showed a trend toward better visual outcomes than patients receiving multiple PDT treatments. In addition, treatment with anecortave acetate 15 mg reduced the need for future PDT treatments.
AMD is viewed as a multifactorial disease by most retina specialists, and as such it is likely that a combination of drugs will result in a better visual acuity outcome than a mono-therapy approach in this disease. In addition, a second goal of all new treatment strategies is the reduction of retreatments being necessary for a complete regression of the CNV. With its mechanism of action, excellent safety profile, and innovative delivery method once every six months, RETAANE suspension may function to assist not only in controlling the CNV either alone or as a component of combination therapy, but also as a treatment for reducing the risk of the development of wet AMD. The results from the AART study could thus be an important milestone for AMD treatment options.
References
- AbdelsalamADel PrioreLZarbinMA1999Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regressionSurv Ophthalmol4412910466585
- AugustinAJD’AmicoDJMielerWF2005Safety of posterior juxtascleral depot administration of the angiostatic cortisene anecortave acetate for treatment of subfoveal choroidal neovascularization in patients with age-related macular degenerationGraefes Arch Clin Exp Ophthalmol24391215290154
- AugustinAJSchmidt-ErfurthU2006Verteporfin therapy combined with intravitreal triamcinolone in al types of choroidal neovascularization due to age related macular degenerationOphthalmology11142216360209
- BenEzraDGriffinBWMaftzirG1997Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularizationInvest Ophthalmol Vis Sci581954629331259
- BleiFWilsonELMignattiP1993Mechanism of action of angiostatic steroids: Suppression of plasminogen activator activity via stimulation of plasminogen activator inhibitor synthesisJ Cell Physiol155568787684043
- ChristenWGGlynnRJMansonJE1996A prospective study of cigarette smoking and age-related macular degeneration in menJAMA2761147518827967
- ClarkAFPennJS2003eLetter to the Editor: PAI-1 and ocular angiogenesis [online] Accessed on 12 August 2005. URL: http://www.iovs.org/cgi/eletters/44/6/2791
- ClarkAFYorioT2003Ophthalmic drug discoveryNat Rev Drug Discov24485912776220
- ClarkAFBingamanDPKapinMA2000Ocular angiostatic agentsExp Opin Ther Patents10427448
- ClarkAFMellonJLiXY1999Inhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetateInvest Ophthalmol Vis Sci4021586210440274
- ClarkAF1997AL-3789: A novel ophthalmic angiostatic steroidExp Opin Invest Drugs6186777
- CrumRSzaboSFolkmanJ1985A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragmentScience230137582416056
- D’AmicoDJGoldbergMFHudsonH2003Anecortave acetate as monotherapy for the treatment of subfoveal lesions in patients with exudative age-related macular degeneration (AMD); Interim (month 6) analysis of clinical safety and efficacyRetina23142312652226
- DeFallerJMClarkAF2000A new pharmacological treatment for angiogenesisTaylorHRPterygiumThe Hague, NetherlandsKugler Pub15981
- GragoudasESAdamisAPCunninghamETJrVEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group2004Pegaptanib for neovascular age-related macular degenerationN Engl J Med35128051615625332
- HammWTJrMuellerHASlineyDH1976Retinal sensitivity to damage from short wavelength lightNature260153815821
- KijlstraALa HeijEHendrikseF2005Immunological factors in the pathogenesis and treatment of age-related macular degenerationOcul Immunol Inflamm1331115804763
- KleinRKleinBELintonKL1992Prevalence of age-related maculopathy: The Beaver Dam Eye StudyOphthalmology99933431630784
- LiuCGuXWangW-H2005Local delivery of anecortave acetate inhibits the expression of retinal IGF-1/IGF-1 receptor in the rat OIR model [abstract]Invest Ophthalmol Vis SciARVO 4135
- McNattLGWeimerLYanniJ1999Angiostatic activity of steroids in the chick embryo CAM and rabbit cornea models of neovascularizationJ Ocul Pharmacol Ther154132310530702
- NewsomeDASwartzMLeoneNC1998Oral zinc in macular degenerationArch Ophthalmol61928
- PennJSRajaratnamVCollierRJ2001The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurityInvest Ophthalmol Vis Sci422839011133880
- RegilloCDD’AmicoDMielerW2005Safety outcomes of clinical studies for anecortave acetate in patients with exudative age-related macular degeneration [abstract]Invest Ophthalmol Vis SciARVO 1373
- SeddonJMAjaniUASperdutoRD1994Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study GroupJAMA2721413207933422
- SeddonJMRosnerBSperdutoRD2001Dietary fat and risk for advanced age-related macular degenerationArch Ophthalmol1191191911483088
- SeddonJMWillettWCSpeizerFE1996A prospective study of cigarette smoking and age-related macular degeneration in womenJAMA276114168827966
- SlakterJSBochowTD′AmicoDJ2006Anecortave acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularization in age-related macular degenerationOphthalmology11331316368146
- SlakterJSJerdanJKaneFE2005Baseline characteristics of a Phase 3 clinical study of anecortave acetate 15 mg in patients with exudative age-related macular degeneration (AMD) [abstract]Invest Ophthalmol Vis SciARVO 1374
- SwannPGLovie-KitchinJE1990Age-related maculopathy; I. A review of its morphology and effects on visual functionOphthalmic Physiol Opt10149582196510
- TaylorHRMunozBWestS1990Visible light and risk of age-related macular degenerationTrans Am Ophthalmol Soc8817380
- VingerlingJRHofmanAGrobbeeDE1996Age-related macular degeneration and smoking. The Rotterdam StudyArch Ophthalmol114119368859077
- VingerlingJRKlaverCCHofmanA1995Epidemiology of age-related maculopathyEpidemiol Rev17347608654516
- YangRMcCollumGWBingamanDP2005The effect of anecortave acetate on VEGF message and protein levels in hypoxic Müller cells and in rat OIR [abstract]Invest Ophthalmol Vis SciARVO 4177