Abstract
Fluctuating Parkinson’s disease (PD) represents a clinical management challenge. The primary utility of levodopa in patients with PD is moderated by the “wearing off” phenomena seen with long-term use. COMT inhibitors slow down the rapid metabolism of levodopa, resulting in a more-sustained response to dopaminergic therapy. Tolcapone is a selective, reversible catechol-O-methyltransferase (COMT) inhibitor, shown to have both peripheral and central effects. In clinical trials, tolcapone has been shown to reduce “off” time, increase “on” time, improve patient and clinician assessments of disease severity, and improve patient quality of life. In a SWITCH study, tolcapone was associated with greater duration of “on” time than remaining on entacapone. Adverse effects of tolcapone are related to the class, with the exception of rare cases of hepatotoxicity. Tolcapone has been recently reintroduced on the European market and recent guidance from the US Food and Drug Administration has reduced the hepatic monitoring requirements for patients initiating tolcapone therapy. With proper monitoring, tolcapone is an effective, well-tolerated drug useful in the management of patients with fluctuating PD.
Introduction
Parkinson’s disease (PD) affects about 1% of adults over the age of 60; as industrial societies age, the prevalence of PD is expected to increase (CitationOlanow et al 2001). In the US alone, an estimated 60 000 new cases of idiopathic PD, characterized by resting tremor, rigidity, and bradykinesia, are diagnosed each year.
The dopamine precursor levodopa has been a mainstay of PD treatment for almost 40 years (CitationCotzias et al 1967). In fact, good response to levodopa helps distinguish idiopathic PD from other, similar movement disorders (CitationHughes et al 1992). However, levodopa therapy is eventually complicated by motor fluctuations, including “wearing off” and “on-off” phenomena (CitationFahn 1999). Drug-induced dyskinesias, including chorea and dystonia, also occur with long-term levodopa therapy in most patients, ultimately reducing quality of life and causing significant disability (CitationChapuis et al 2005).
Levodopa is routinely administered in combination with a decarboxylase inhibitor, many of which are available as co-formulations under a variety of brand names in the US and Europe. Decarboxylase inhibitors prevent conversion of levodopa to dopamine in the peripheral circulation, thereby allowing more levodopa to cross the blood–brain barrier into the central nervous system (CNS) (CitationOlanow et al 2001). Decarboxylase inhibitors also reduce nausea and vomiting that can occur due to stimulation of dopamine receptors in the area postrema that are not protected by the blood–brain barrier.
By blocking the decarboxylase route of metabolism, circulating levodopa is primarily metabolized by catechol-O-methyltransferase (COMT) to 3-O-methyldopa (3-OMD) (CitationKaakkola 2000). COMT inhibitors in combination with levodopa/decarboxylase inhibitor preparations are associated with an increase of CNS bioavailability of levodopa. Theoretically, COMT inhibitors that are active in the CNS would also reduce central metabolism of both levodopa and dopamine.
Tolcapone is a potent, selective reversible inhibitor of COMT (CitationZurcher et al 1990). Preclinical models showed tolcapone effectively inhibited COMT in the gut, brain, and liver (CitationBorgullya et al 1991; CitationDa Prada et al 1991; CitationZurcher et al 1991). In clinical trials, it has been shown to be effective adjunctive therapy for patients who are not obtaining optimal response to levodopa-based therapy. In 1998, 3 cases of acute hepatitis leading to mortality among patients receiving tolcapone led to the temporary suspension of availability of tolcapone in the EU (CitationEMEA 2004), as well as stringent monitoring requirements in the US (CitationFDA 1998). Since then, the compound has been returned to the EU approved drugs list, and the US Food and Drug Administration (FDA) requirements for liver monitoring have been eased (CitationFDA 2006). This article will review pertinent research related to appropriate selection of candidates for tolcapone use.
Clinical pharmacology of tolcapone
Pharmacokinetics
Tolcapone displays linear pharmacokinetics, independent of levodopa/carbidopa administration, and independent of sex, age, weight, or race (CitationTasmar PI 2006). Oral tolcapone is rapidly absorbed, with a tmax of about 2 hours and an elimination half-life (t1/2) of about 2 to 3 hours in both single doses and at the recommended dosing frequency of three times a day, both alone and in combination with levodopa and a decarboxylase inhibitor (Table ) (CitationDingemanse, Jorga, Schmitt, et al 1995; CitationDingemanse, Jorga, Zurcher, et al 1995; CitationDingemanse et al 1996; CitationJorga et al 1997). Tolcapone displays high plasma binding (>99.9%), mainly to serum albumin, leading to a limited volume of distribution at steady state (9L). The oral bioavailability of tolcapone is approximately 65%, which is decreased by about 10% to 20% if taken 1 hour before or 2 hours after a meal. Tolcapone can be taken without regard to meals (CitationTasmar PI 2006).
Table 1 Pharmacokinetic parameters (mean values) of oral tolcapone (TOL) in healthy volunteers.Table Footnotea TOL was administered alone or in combination with levodopa/benserazide 100 mg/25 mg (L-Dopa/Ben) or levodopa/carbidopa 100 mg/25 mg (L-Dopa/Car)
Tolcapone is extensively metabolized, primarily by glucuronidation into an inactive conjugate, with only 0.5% of the administered dose excreted unchanged in urine (CitationJorga et al 1999). In patients with moderate cirrhotic liver disease (Child-Pugh Class B), clearance and volume of distribution of unbound tolcapone was reduced by almost 50% (CitationJorga et al 1998). However, no clinically significant changes in pharmacokinetics were noted among patients with noncirrhotic liver disease. Tolcapone therapy should not be initiated in patients with active liver disease or who have elevated transaminase values (CitationTasmar PI 2006). Tolcapone pharmacokinetics are not altered by renal impairment, although caution is warranted among patients with severe renal dysfunction.
Pharmacodynamics
At clinically relevant concentrations, tolcapone inhibited COMT activity in in vitro assays of human liver, duodenum, kidney, and lung tissue (CitationDe Santi et al 1998). Tolcapone also inhibited COMT activity in erythrocytes among healthy volunteers receiving single or multiple doses of the drug (CitationDingemanse, Jorga, Schmitt, et al 1995; CitationDingemanse, Jorga, Zurcher, et al 1995; CitationDingemanse et al 1996; CitationJorga et al 1997). This dose-dependent inhibition was rapid, occurring within 2 hours of administration, and reversible, with ≥80% inhibition occurring with a single 200 mg dose.
Tolcapone may also block COMT activity in the CNS (CitationCeravolo et al 2002). A study of 12 patients with PD using positron emission tomography and radiolabelled fluorodopa showed evidence of central blockade of central COMT activity compared with placebo.
Mechanism of action – impact on levodopa pharmacokinetics
The mechanism of action of tolcapone is based on altering levodopa pharmacokinetics, as well as reducing the levodopa metabolite 3-OMD. Tolcapone prolongs the t1/2 of levodopa from approximately 2 to 3.5 hours (CitationDavis et al 1995a; CitationTasmar PI 2006). This results in an approximate doubling of levodopa relative bioavailability (area under curve). However, Cmax or tmax.of levodopa are unaffected. Studies in healthy volunteers and Parkinson’s disease patients have confirmed that the maximal effect occurs with 100 mg to 200 mg tolcapone. Plasma levels of 3-OMD are markedly and dose-dependently decreased by tolcapone when given with levodopa/carbidopa.
Clinical trials of tolcapone
Effects of COMT inhibition are seen shortly after administration, allowing for clinical efficacy of tolcapone to be investigated in single-dose trials where patients were observed in a controlled setting for several hours after acute administration. In a dose-ranging study, single doses of tolcapone were found to extend the time antiparkinsonian effects of levodopa/carbidopa by about 70% (CitationRoberts et al 1994). A separate study using levodopa/benserazide concurred with this finding, with tolcapone-treated patients displaying approximately 1 hour of extra “on” time versus placebo (CitationLimousin et al 1995). A dose-ranging study of levodopa/carbidopa plus the monoamine oxidase inhibitor selegiline found that the addition of a single dose of tolcapone, ranging from 50 mg to 800 mg, prolonged the antiparkinsonian effects of levodopa/carbidopa (CitationDavis et al 1995b).
In a study of tolcapone pharmacokinetics following 6 weeks of open-label therapy, improvements were seen in the Unified Parkinson’s Disease Rating Scale (UPDRS) at 7 time points following administration (CitationYamamoto et al 1997).
The approval of tolcapone in the US and Europe was based on evidence from 2 multicenter, randomized, double-blind, placebo-controlled clinical trials in patients with levodopa-induced motor fluctuations (CitationBaas et al 1997. CitationRajput et al 1997).
In the pivotal trial conducted in Europe, 177 patients were randomized 1:1:1 to receive tolcapone 100 mg or 200 mg three times daily (tid), or placebo (containing riboflavin to mimic urinary discoloration seen as a harmless side effect of tolcapone) (CitationBaas et al 1997). Levodopa could be adjusted according to patient response and adverse effects up until 2 weeks prior to the 3-month efficacy endpoint determination. Levodopa could not be increased above baseline prior to the 3-month time point. “On” time increased by 21.3% and 20.6% from baseline with 100 mg and 200 mg tolcapone (p<0.01 versus placebo). “Off” time decreased by 31.5% with the 100mg dose (p<0.5) and 26.2% with the 200 mg dose (p=NS). Tolcapone 200 mg tid was superior to placebo in UPDRS motor function score during “on” time. Tolcapone therapy was also associated with a statistically significant reduction in levodopa doses, with larger dose reductions seen with the 200 mg tid dose.
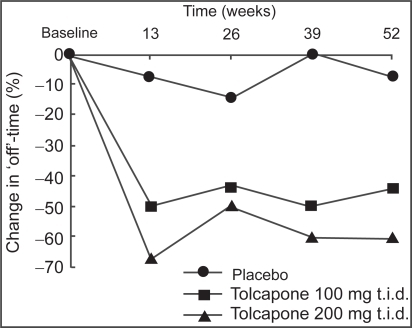
There were 202 patients enrolled in a similarly designed study conducted in North America (CitationRajput et al 1997). Total levodopa doses were significantly reduced in both tolcapone dose arms (p<0.01), as well as number of mean daily levodopa doses. In this trial, patients receiving tolcapone showed decreases in “off” time; however only tolcapone 200 mg tid reached statistical significance (p<0.01). At 3 months, a mean reduction in “off” time of 3.25 hours was noted with tolcapone 200 mg tid. Investigators’ global assessment (IGA) indicated that 68% of patients receiving tolcapone 100 mg tid, and 95% of those receiving tolcapone 200 mg tid displayed a reduced “wearing off” effect, compared with 37% of patients receiving placebo (p<0.01). Patients were allowed to continue on their assigned therapy for up to 12 months after randomization. For those that reached the 12-month time point, the IGA of overall efficacy showed improvement in 80% of patients receiving tolcapone 100 mg tid, and 88% of patients receiving tolcapone 200 mg tid, compared with 37% of patients receiving placebo. In addition, reductions in levodopa dosages noted at 3 months were maintained through the 12-month completion of the trial (Figure ).
Figure 1 Reductions in levodopa dosage seen at the end of 3 months were maintained throughout treatment in the patients who received tolcapone. Copyright © 1997. Permission requested from Rajput AH, Martin W, Saint-Hilaire MH, et al. 1997. Tolcapone improves motor function in parkinsonian patients with the “wearing-off” phenomenon: a double-blind, placebo-controlled, multicenter trial. Neurology, 49:1066–71.
The above trials were included in an analysis of treatment for levodopa-induced motor fluctuations recently completed by a committee of the American Academy of Neurology (AAN) (CitationPahwa et al 2006). The committee recognized tolcapone as having utility in reducing “off” time. Additional clinical trials of tolcapone that were not reviewed by the AAN committee have also been completed in patients with fluctuating PD, including a head-to-head study with the COMT inhibitor entacapone, as well as studies in patients with stable PD.
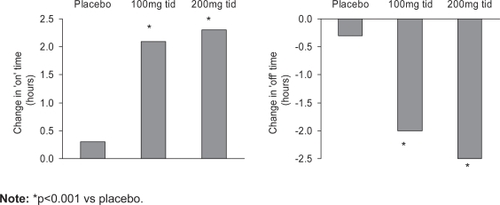
Tolcapone substantially increased “on” time and decreased “off” time in a study of 215 patients with fluctuating PD (CitationAdler et al 1998). Patients were randomized to tolcapone 100 mg, 200 mg, or matching placebo. The onset of response to tolcapone was rapid; improvements were seen at the first study visit 2 weeks after initiation of therapy. By week 6, on time had increased by more than 2 hours, and off time had decreased to a similar extent in both tolcapone groups (Figure ) (p<0.001 versus placebo). The improvement noted represented an approximately 38% improvement in off time. The total daily dose of levodopa required for patients receiving tolcapone 100mg tid was reduced by 23% (p<0.01 versus placebo). Patients receiving tolcapone 200 mg tid reduced their levodopa requirements by 29% (p<0.001 versus placebo). The total number of daily doses of levodopa was reduced significantly in both tolcapone groups.
Figure 2 Increased ‘on’ time, reduced ‘off’ time with tolcapone. Copyright © 1998. Permission requested from Adler CH, Singer C, O’Brien C, et al. 1998. Randomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Arch Neurol, 55:1089–95.
A 6-week placebo-controlled trial of tolcapone 50 mg, 200 mg, and 400 mg tid reported results from 151 patients (CitationKurth et al 1997). In this trial, investigators also conducted 10-hour evaluations of motor fluctuations at baseline and at the 6-week study visit. During this evaluation, patients were scored every 30 minutes for “off” time, “on” time, and “on” time with dyskinesia. The primary endpoints were changes in investigator-rated measurements of “on/off” time at the 6-week evaluation from baseline, as well as changes in the UPDRS motor subscale score. All three dosages of tolcapone reduced “off” time by about 40% from baseline (p<0.01 for all tolcapone arms vs placebo). Tolcapone also significantly increased “on” time for the 50 mg (p<0.05), 200 mg (p<0.01) and 400 mg (p<0.01) groups compared with placebo. In this study, there was no difference between the three tolcapone arms in “on” time. Patient diaries completed during the 6-week study supported the 10-hour evaluation finding of decreased “off” time and increased “on” time. The change in the area under the curve of mean UPDRS motor subscale scores favored all tolcapone doses (p<0.05). Patients receiving tolcapone also reported significant decreases in total levodopa dosing (p<0.01); reduction in the number of daily levodopa doses was statistically significant for patients receiving tolcapone 200 mg and 400 mg tid.
A European-Australian collaborative group reported a 6-week placebo-controlled trial of tolcapone 50 mg, 200 mg, and 400 mg tid versus placebo among 154 patients in a study that included a 2-week single-blind placebo run-in phase (CitationMyllyla et al 1997). The primary efficacy analysis was patient diary assessments of “on” and “off” time. In this study, improvements were seen in both primary efficacy variables. All tolcapone doses were statistically significantly more effective than placebo in increasing “on” time; however, only tolcapone 200 mg tid reached statistical significance versus placebo in reducing “off” time. The tolcapone 200 mg tid dose resulted in a 34% increase in “on” time and a 42% reduction on “off” time from baseline values. The greatest reduction in total levodopa daily dosage was also greatest in the tolcapone 200 mg tid group (p<0.01 versus placebo). The IGA for “wearing off” and symptom severity were both statistically significant for all tolcapone doses (p<0.05 versus placebo).
The UPDRS motor scores during off-period and the percentage of off time improved significantly using tolcapone in a small study of 40 patients (CitationShan et al 2001). However gait analysis did not confirm these findings in this trial. Secondary analyses of other UPDRS subscales of total scores in the above studies did not demonstrate statistically significant improvements over placebo (CitationKurth et al 1997; CitationMyllyla et al 1997; CitationRajput et al 1997; CitationAdler et al 1998; CitationShan et al 2001). Other secondary analysis of health-related quality of life found an improvement in one study (CitationBaas et al 1997), but not another (CitationAdler et al 1998).
Comparator trials
One other COMT inhibitor, entacapone, is approved for use in the US and Europe for patients with symptoms of levodopa “wearing off” (Comtan PI 2006). Recently, data were presented from the SWITCH study aimed at determining if switching from entacapone to tolcapone might improve patient outcomes (CitationSWITCH 2006). This was a double-blind, active-control study conducted at 32 centers in Europe and the US, enrolling 150 individuals who had 3 or more hours of “off” time despite optimized entacapone therapy. Patients were randomized to receive either entacapone 200 mg with each levodopa dose, or tolcapone 100 mg tid. Treatment duration was 3 weeks. In the per protocol analysis, a statistically significant greater proportion of patients switched to tolcapone had at least 3 hours a day of additional “on” time (p=0.018). Both groups saw an increase in “on” time; however, the tolcapone had a mean increase of 0.86 hours more than the entacapone group (p=0.042). More patients on tolcapone were judged to have a moderate or marked improvement on the IGA (27% for entacapone vs 40% for tolcapone; p=not significant). More patients responded to tolcapone, as defined by both at least 1 hour/day improvement in “on” time and an IGA rating of moderate or marked improvement (p=0.051). There was no difference between groups in the frequency or severity of adverse events, including dyskinesia, or in the incidence of liver enzyme abnormalities.
Other evidence in favor of the increased efficacy of tolcapone over entacapone was reported in a case study series of 40 patients who were switched from tolcapone to entacapone (CitationOnofrj et al 2001). Patients had been switched either due to intolerability or due to the temporary suspension of tolcapone availability in Europe. After 3 to 6 months of levodopa therapy adjustments, all patients received entacapone for 3 months. The authors reported that levodopa daily dosage was significantly reduced by both tolcapone and entacapone. “On” time was increased by 15% during tolcapone treatment (p<0.05), and by 8% during entacapone treatment. “Off” time was decreased by 16% during tolcapone and by 7% during entacapone treatment.
Tolcapone also appears to have greater long-term benefits compared with entacapone, according to an analysis conducted of data collected from 2 groups of patients enrolled in parallel long-term tolerability and efficacy studies of tolcapone and entacapone (CitationFactor et al 2001). The entry criteria, efficacy and tolerability analyses, and data collection points for the two trials were similar. In this analysis, tolcapone was more effective in lowering UPDRS motor and complication subscores, as well as duration of “off” time, and total levodopa doses. Improvements seen in UPDRS motor scores and change in levodopa dose remained below baseline level for 36 months in the tolcapone group; those same scores climbed above baseline in the entacapone group from the 6-month study visit onward.
Tolcapone 100 mg tid was compared with add-on therapy with the dopamine agonist pergolide (CitationKoller et al 2001). There was no placebo-controlled arm in this trial; however, both therapies showed similar efficacy in reducing “off” time, increasing “on” time, reducing total daily levodopa dosage, or in UPDRS scores, and IGAs. Tolcapone was more effective than pergolide in improving a quality of life measure, the PDQ-39 scale, at 12 weeks (p=0.0005).
An open-label comparative trial of tolcapone 200 mg tid versus the dopamine agonist bromocriptine was conducted in 146 PD patients (CitationTSG 1999). At the end of 8 weeks, both treatment groups showed similar results in “on” time, “off” time, and UPDRS subscale scores. Because bromocriptine must be titrated upward over a period of time, patients assigned to tolcapone showed greater improvements early in the course of therapy. The mean total daily levodopa dosage decreased by 16.5% in the tolcapone-treated patients, compared with 4% among patients receiving bromocriptine (p<0.01). In the tolcapone group, this reduction was achieved as early as week 1 and was almost complete by week 4.
Trials in stable PD
All of the trials reviewed above were conducted among patients with fluctuating PD associated with the levodopa “wearing off” syndrome. Tolcapone is approved for this indication in the US and Europe. Additional studies have been conducted to determine if tolcapone can provide benefits for patients with stable PD on levodopa therapy.
Tolcapone 100 mg and 200 mg tid were evaluated in patients on stable levodopa therapy in a 12-week crossover study (CitationSuchowersky et al 2001). All patients received tolcapone 100 mg tid for 4 weeks, then were randomly assigned to either continue on that dosage or to receive tolcapone 200 mg tid. At week 8, therapy was switched to the alternate dosage level in a double-blind fashion. During the 4-week open-label run-in, tolcapone 100 mg tid resulted in improvements in IGAs among 76% of patients. In addition, the mean total daily levodopa dose and mean UPDRS subscale scores decreased significantly (p<0.001 for all measures compared with baseline). During the double-blind treatment period, 61% of patients showed improvements in their IGA at either or both dosages. Other efficacy variables changed minimally; there was no difference between tolcapone dosages.
A double-blind, placebo-controlled trial investigated the effect of tolcapone 100 mg or 200 mg tid on activities of daily living and motor function in 298 patients with PD receiving levodopa but without motor fluctuations (CitationWaters et al 1998). At 6 months, both dosages of tolcapone produced significant reductions in the UPDRS subscales II and III and in the total score for subscales I to III. These improvements were maintained up to 12-months. At 6 months, both tolcapone groups had significant decreases in levodopa dosage compared with placebo. Both tolcapone groups reported decreases in mean total daily dose of levodopa, while the placebo group had a mean increase.
Tolcapone 200 mg and 400 mg tid were studied in patients whose “wearing off” symptoms had been stabilized by increasing their levodopa dosing (CitationDupont et al 1997). After a 1-week placebo run-in, patients were assigned to placebo or one of the tolcapone dosages. Levodopa dosage was reduced by 35% on day 1, then retitrated upward as required. After 6 weeks, the study was unblinded and the tolcapone groups crossed over to the other dose for exploratory purposes. Although not statistically different, both tolcapone groups showed greater reductions in levodopa dosage requirements compared with placebo. Tolcapone 200 mg tid showed statistically significant improvement in the UPDRS subscale II (p<0.05 versus placebo).
Safety and tolerability of tolcapone
Hepatotoxicity
During post-marketing surveillance, 3 cases of fatal hepatotoxicity were reported in patients who were not properly monitored. As a result, new FDA guidelines for liver testing were established. This also prompted the sponsor to conduct a review of the tolcapone safety database, representing approximately 100 000 patient-years of therapy (CitationWatts and Kricorian 2005). Although underreporting of adverse events may have occurred, a total of 219 cases of hepatobiliary events were recorded. Most of these cases were mild transient elevations in liver function tests <3 times the upper limit of normal (ULN). Most cases were either asymptomatic or resolved without treatment interruption. Only 21 cases (0.001%) reported elevations in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >3 times ULN; the median time to this adverse event was 81 days. After new monitoring guidelines were in place, no deaths related to hepatotoxicity occurred. In addition, asymptomatic elevations in liver function tests did not progress to acute hepatitis if treatment was withdrawn.
As a result of this analysis, the FDA has recently reduced the liver monitoring requirements for tolcapone use (CitationFDA 2006). The newly approved labeling states that serum glutamic pyruvic transaminase (SGPT)/ALT and serum glutamic oxaloacetic transaminase (SGOT)/AST levels should be determined at baseline, as well as periodically (ie, every two to four weeks) for the first six months of therapy. Periodic monitoring is recommended at intervals deemed clinically relevant after the first 6 months of therapy. In addition, patients can remain on tolcapone therapy if liver enzymes are below 2 times ULN and/or do not display symptoms of liver toxicity.
Other adverse events
New or worsening dyskinesia is the most common adverse event associated with tolcapone therapy. Worsening of dyskinesia most commonly occurs at the start of tolcapone therapy, and may be moderated by optimizing levodopa dosages (CitationBaas et al 1997; CitationRajput et al 1997; CitationAdler et al 1998). Dyskinesia and other dopaminergic adverse events are most likely due to the increase in levodopa concentrations that provide the rationale for tolcapone use.
Diarrhea is the most commonly reported nondopaminergic adverse event reported in clinical trials (CitationRajput et al 1997; Waters et al 1997; CitationAdler et al 1998). Table lists the common adverse events reported by the manufacturer in clinical trials.
Table 2 Adverse events associated with tolcapone (at least 5% in a tolcapone dosage group and at least one tolcapone dosage group > placebo) (CitationTasmar PI 2006)
In the comparative trial with entacapone, the frequency and severity of adverse events was similar between the two medications (CitationSWITCH 2006). In the trial comparing tolcapone with pergolide, tolcapone was associated with reduced risk of nondyskinesia adverse events (59% vs 77% for pergolide; p=0.001) (CitationKoller et al 2001). Dyskinesia occurred in 34% of tolcapone recipients and 25% of pergolide recipients. Incidence of nausea was also substantially higher with pergolide therapy (35%) compared with tolcapone (20%). In the trial comparing tolcapone with bromocriptine, nausea, orthostatic complaints, hallucinations, and peripheral edema were reported more often among bromocriptine patients, while muscle cramps, dystonia, and dry mouth were reported more often among tolcapone recipients (CitationTSG 1999).
Discussion
Tolcapone is an effective agent that can help improve the efficacy of levodopa-based therapy in patients with PD. It is especially useful, and indicated for, patients who are experiencing “wearing off” motor fluctuations from their levodopa therapy. Benefits of tolcapone have also been demonstrated among patients on stable levodopa therapy who have not yet begun to display motor fluctuations. There is also increasing interest in determining whether early administration of a COMT inhibitor (ie, at the first use of levodopa) might reduce the risk of eventual motor complications (CitationOlanow et al 2001). The primary adverse effect of tolcapone is dyskinesia, which is more of a problem among patients who present with dyskinesia prior to initiation of tolcapone therapy. Immediate reductions in levodopa dosages between 15% and 30% may be warranted to obviate this concern.
The goal of providing clinical benefits for PD patients over the longest period of time without undue adverse events remains elusive at best. The determination of when to begin levodopa-based therapy in patients with PD is complex. It is generally recommended that any pharmacotherapy not be initiated until patients begin to show functional deterioration (CitationOlanow et al 2001). Levodopa therapy may be delayed with early use of dopamine agonists in younger patients; however, eventually all patients receiving dopamine agonist therapy will require levodopa supplementation. Levodopa has the advantage of being almost universally efficacious among patients with idiopathic PD. However, the long-term outcome of levodopa-based therapy is clouded by the “wearing off’ phenomena. Future clinical research is warranted to determine whether early use of COMT inhibitors like tolcapone can delay the onset of levodopa-associated adverse effects.
References
- AdlerCHSingerCO’BrienC1998Randomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopaArch Neurol551089959708959
- BaasHBeiskeAGGhikaJ1997Catechol-O-methyltransferase inhibition with tolcapone reduces the “wearing off” phenomenon and levodopa requirements in fluctuating parkinsonian patientsJ Neurol Neurosurg Psychiatry6342189343116
- BorgulyaJDa PradaMDingemanseJ1991Ro 40–7592: catecholamine-O-methyltransferase (COMT) inhibitorDrugs Future1671921
- CeravoloRPicciniPBaileyDL200218F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson’s diseaseSynapse43201711793426
- ChapuisSOuchchaneLMetzO2005Impact of the motor complications of Parkinson’s disease on the quality of lifeMov Disord202243015384126
- CotziasGCVan WoertMHSchifferLM1967Aromatic amino acids and modification of parkinsonismN Engl J Med27637495334614
- Da PradaMZurcherGKettlerR1991New therapeutic strategies in Parkinson’s disease: inhibition of MAO-B by Ro 19–6327 and of COMT by Ro 40–7592Adv Behav Bio3972332
- DavisTLRoznoskiMBurnsRS1995aAcute effects of COMT inhibition on L-DOPA pharmacokinetics in patients treated with carbidopa and selegilineClin Neuropharmacol183337
- DavisTLRoznoskiMBurnsRS1995bEffects of tolcapone in Parkinson’s patients taking L-dihydroxyphenylalanine/carbidopa and selegilineMov Disord1034951
- De SantiCGiulianottiPCPeitrabissaA1998Catechol-O-methyl-transferase: variation in enzyme activity and inhibition by entacapone and tolcaponeEur J Clin Pharmacol54215199681662
- DingemanseJJorgaKMSchmittM1995Integrated pharmacokinetics and pharmacodynamics of the novel catechol-O-methyltransferase inhibitor tolcapone during first administration to humansClin Pharmacol Ther57508177768073
- DingemanseJJorgaKZurcherG1995Pharmacokinetic-pharmacodynamic interaction between the COMT inhibitortolcapone and single-dose levodopaBr J Clin Pharmacol40253628527287
- DingemanseJJorgaKZürcherG1996Multiple-dose clinical pharmacology of the catechol-O-methyl-transferase inhibitor tolcapone in elderly subjectsEur J Clin Pharmacol5047558739811
- DupontEBurgunderJMFindleyLJ1997Tolcapone added to levodopa in stable parkinsonian patients: a double-blind placebo-controlled study. Tolcapone in Parkinson’s Disease Study Group II (TIPS II)Mov Disord12692834
- [EMEA] European Medicines Evaluation Agency2004EMEA public statement on the lifting of the suspension of the marketing authorization for tolcapone (Tasmar) [online]. Accessed 22 May 2006. URL: http://www.emea.eu.int
- FactorSAMolhoESFeustelPJ2001Long-term comparative experience with tolcapone and entacapone in advanced Parkinson’s diseaseClin Neuropharmacol24295911586115
- FahnS1999Parkinson disease, the effect of levodopa, and the ELLDOPA trialArch Neurol565293510328247
- [FDA] US Food and Drug Administration1998Important drug warning [online]. Accessed 22 May 2006. URL: http://www.fda.gov
- [FDA] US Food and Drug Administration2006New monitoring requirements [online]. Accessed 2 November 2006. URL: http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Tasmar_PI.pdf
- HughesAJBen-ShlomoYDanielSE1992What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic studyNeurology42114261603339
- JorgaKMSedekGFottelerB1997Optimizing levodopa pharmacokinetics with multiple tolcapone doses in the elderlyClin Pharmacol Ther62300109333106
- JorgaKMKroodsmaJMFottelerB1998Effect of liver impairmenton the pharmacokinetics of tolcapone and its metabolitesClin Pharmacol Ther63646549663179
- JorgaKFottelerBHeizmannP1999Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyl-transferaseBr J Clin Pharmacol485132010583021
- KaakkolaS2000Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s diseaseDrugs5912335010882160
- KollerWLeesADoderM2001Randomized trial of tolcapone versus pergolide as add-on to levodopa therapy in Parkinson’s disease patients with motor fluctuationsMov Disord168586611746615
- KurthMCAdlerCHSt HilaireM1997Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease experiencing motor fluctuations: a multicenter, double-blind, randomized, placebo-controlled trialNeurology488179008498
- LimousinPPollakPPfefenJP1995Acute administration of levodopa-benserazide and tolcapone, a COMT inhibitor, (in) Parkinson’s diseaseClin Neuropharmacol18325865
- MyllylaVVJacksonMLarsonJP1997Efficacy and safety of tolcapone in levodopa-treated Parkinson’s disease patients with “wearing-off” phenomena; a multicenter, double-blind, randomized, placebo-controlled trialEur J Neurology4333341
- Novartis Pharmaceuticals USA2000Prescribing information for Comtan® (entacapone) tablets [online]. Accessed 22 May 2006. URL: http//www.comtan.com
- OlanowCWWattsRLKollerWC2001An algorithm (decision tree) for the management of Parkinson’s disease (2001): treatment guidelinesNeurology5611 Suppl. 5S1S88
- OnofrjMThomasAIaconoD2001Switch-over from tolcapone to entacapone in severe Parkinson’s disease patientsEur Neurol461116
- PahwaRFactorSALyonsKE2006Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology669839516606909
- RajputAHMartinWSaint-HilaireMH1997Tolcapone improves motor function in parkinsonian patients with the “wearing-off” phenomenon: a double-blind, placebo-controlled, multicenter trialNeurology491066719339691
- RobertsJWCora-LocatelliGBraviD1994Catechol-O-methyl-tranferase inhibitor tolcapone prolongs levodopa/carbidopa action in parkinsonian patientsNeurology4426858
- ShanDELeeSJChaoLY2001Gait analysis in advanced Parkinson’s disease - effect of levodopa and tolcaponeCan J Neurol Sci2870511252300
- SuchowerskyOBaileyPPourcherE2001Comparison of two dosages of tolcapone added to levodopa in nonfluctuating patients with PDClin Neuropharmacol421420
- [SWITCH] The Entacapone to Tolcapone Switch Study Investigators2006Entacapone to tolcapone switch: multicenter double-blind, randomized, active-controlled trial in advanced Parkinson’s diseaseMov Disord, Epub Nov 6.
- [Tasmar Pl] Valeant Pharmaceuticals International2006Tasmar® (tolcapone) tablets; prescribing information [online]. Accessed 15 May 2006. URL: http//www.tasmar.com
- [TSG] Tolcapone Study Group1999Efficacy and tolerability of tolcapone compared with bromocriptine in levodopa-treated Parkinsonian patientsMov Disord1438449918342
- WatersCHKurthMBaileyP1998Tolcapone in stable Parkinson’s disease: efficacy and safety of long-term treatment. Tolcapone Stable Study GroupNeurology505 Suppl 5S39S45
- WattsRKricorianG2005Evaluation of liver-related adverse events with tolcapone: a review of 7-years of worldwide safety dataPresented at the World Congress of Neurology5–11 November 2005Sydney, Australia
- YamamotoMYokochiMKunoS1997Effects of tolcapone, a catechol-O-methyltransferase inhibitor, on motor symptoms and pharmacokinetics of levodopa in patients with Parkinson’s diseaseJ Neural Transm104229369203084
- ZurcherGColziADa PradaM1990Ro 40–7592: inhibition of COMT in rat brain and extracerebral tissuesJ Neural Transm Suppl3237582089102
- ZurcherGDingemanseJDa PradaM1991Ro 40–7592, a potent inhibitor of extracerebral and brain catechol-O-methyltransferase: preclinical and clinical findingsAgnoliACampanellaGNew developments in therapy of Parkinson’s diseaseRome, ItalyJohn Libbey3743