Abstract
Candesartan cilexetil is a nonpeptide selective blocker of the angiotensin II receptor sub-type 1. It is a prodrug that is converted to its active metabolite during its variable absorption. It is highly protein bound with a small volume of distribution and a nine-hour half-life. Candesartan is one of two angiotensin receptor blockers approved for use in heart failure. MEDLINE was searched using OVID and PubMed to evaluate the evidence for using candesartan in patients with heart failure. Pharmacologic and pharmacokinetic evaluations, as well as clinical trials, were selected and are presented in this review. Clinical evidence supports the indication for use in systolic heart failure. Results for use in patients with diastolic heart failure were non-significant. Candesartan was well tolerated in the trials, with hyperkalemia, renal dysfunction, and hypotension being the most common adverse events. Use of angiotensin receptor blockers with angiotensin-converting enzyme inhibitors needs further study; however, candesartan appears to provide added benefit in this setting. Candesartan is a safe and effective option for patients with systolic heart failure. Data regarding other angiotensin receptor blockers is underway.
Introduction
Chronic heart failure (HF) affects approximately 5 million people in the US, and affects men and women almost equally (CitationAHA 2006). Improvements in the management of cardiovascular disease with medications and procedures prolong the lives of patients, leading to a larger elderly population with structural heart abnormalities. The burden of HF is increasing, as it is often a consequence of other forms of cardiovascular disease. It is estimated that by 2037 the prevalence of HF will double (CitationRich 1997). The financial burden is on the rise as well; direct and indirect costs of HF for 2006 are estimated to be US$29.6 billion in the US (CitationAHA 2006). In spite of the progress and cost, however, HF mortality remains high, with up to 20% of patients dying each year (CitationAHA 2006).
Management of HF has evolved substantially in the last two decades. Trials showing the benefits of angiotensin-converting enzyme inhibitors (ACE-I) in HF established ACE-I as a standard of care (CitationAdams et al 2006), reducing overall deaths by approximately 20% (CitationFlather et al 2000). In the early 1990s, the Metoprolol in Dilated Cardiomyopathy trial showed β-blockers may have beneficial rather than detrimental effects in patients with left ventricular (LV) systolic dysfunction who were already on established ACE-I therapy (CitationWaagstein et al 1993); this was confirmed by studies showing reduced mortality with carvedilol and metoprolol succinate (CitationPacker et al 1996; CitationMerit-HF 1999; CitationAdams et al 2006). Aldosterone antagonists (spironolactone and eplerenone) also prolong life in patients with advanced HF (CitationPitt et al 1999, Citation2003). Evaluation of angiotensin receptor blockers (ARBs) in HF has recently been conducted to address the continued high mortality rates in spite of the treatment advances (CitationCohn et al 2001; CHARM Program 2003; CitationPfeffer et al 2003). Additionally, approximately 20% of patients with LV dysfunction are not treated with ACE-I, usually because of intolerance (CitationBart et al 1999). Other common therapies used in HF are diuretics and digoxin for symptomatic management. Clearly, the magnitude of pharmacotherapy for chronic HF has expanded. Ascertaining appropriate evidence-based use of ARBs in HF, a possible addition to an already copious drug regimen, is critical to minimize risk and maximize therapeutic benefit. Within the class of ARBs, only two agents are approved for HF in the US: valsartan (Diovan®, Novartis Pharmaceuticals, NJ, USA) and candesartan (Atacand®, AstraZeneca, DE, USA).
Pathophysiology
Heart failure is not a single entity, but a cycle of damage and neurohormonal activation that begins with an adverse cardiovascular environment, causing or leading to cardiac injury. The initial damage can be caused by various conditions, including ischemia, uncontrolled hypertension, and autoimmune myocarditis (CitationWillerson 1995). The cycle culminates with systolic or diastolic dysfunction that decreases cardiac output and compromises organ perfusion. A compensatory response to improve organ perfusion begins with renin release and renin–angiotensin–aldosterone system (RAAS) activation. Renin is primarily released by juxtaglomerular cells of the kidneys; it catalyzes the conversion of angiotensinogen to angiotensin which is then converted to angiotensin II by the ACE (CitationDelgado and Willerson 1999).
Angiotensin II stimulates additional compensatory responses. By activating angiotensin type 1 (AT1) receptors, angiotensin II increases blood pressure by multiple mechanisms (CitationDostal 2000). First, it causes a rapid pressor response by direct vasoconstriction. Angiotensin II also activates the sympathetic nervous system, increases endothelin and vasopressin secretion, and increases peripheral noradrenergic transmission (CitationFarrell et al 2001). Constriction of the efferent renal arterioles increases glomerular filtration and peritubular oncotic pressure, promoting reabsorption of sodium and water in the proximal tubule. This compensatory response is protective initially, but chronically elevated angiotensin II can cause vascular and cardiac hypertrophy, vascular and cardiac fibrosis, and induction of cardiac arrhythmias (CitationKim and Iwao 2000).
Furthermore, angiotensin II stimulates the adrenal gland to release aldosterone. Aldosterone binds to mineralocorticoid receptors to promote sodium reabsorption, potassium excretion, and passive water retention. Fluid retention increases stress on the heart, further stimulating the hormonal activation and myocardial remodeling. Additionally, aldosterone synthetase is found in extraadrenal tissues such as the heart, blood vessels, and brain, resulting in aldosterone release via non-angiotensin II pathways (CitationWeber 2001).
In addition to sympathetic activation, cardiac injury also leads to remodeling of the myocardium. Acute injury, such as myocardial infarction, causes a decrease in pressure of the baroreceptors in the aortic arch, causing sympathetic nervous system stimulation. The sympathetic hormones (norepinephrine, vasopressin, and atrial naturetic peptide) increase heart rate, afterload, and cause myocardial remodeling (Jesseup and Brozena 2003). Norepinephrine also predisposes to ventricular arrhythmias and cardiac arrest. Myocardial remodeling worsens cardiac output, augmenting the cycle of RAAS activation and cardiac remodeling (CitationDelgado and Willerson 1999).
The interrelated hormonal activation and subsequent cardiac toxicity make identifying the appropriate targets to stop this cycle critical. Blocking both the receptors and the production of angiotensin II has been shown to stop the progression of HF and is an important component of HF therapeutics (CitationPitt et al 1997). Prior to development of these neurohormonal interventions, HF mortality was greater than 50% at 5 years (CitationAghababian 2002). By adequately blocking ACE, mortality can be reduced by approximately 30% (CitationGarg and Yusuf 1995) in spite of incomplete blockade of angiotensin II production. Blocking the angiotensin receptor sub-type 1 (AR1) receptor as an alternative or in addition to ACE inhibition is a reasonable target to attempt to slow the consequences of neurohormonal activation in HF.
Pharmacology
Blockade of angiotensin II production via ACE inhibition does not lead to complete blockade of the RAAS (CitationFrancis et al 2004). In fact, angiotensin II returns to pre-treatment levels in spite of chronic treatment with ACE-I (CitationBiollaz et al 1982; CitationUrata, et al 1990; CitationJorde et al 2000). This is due, in part, to angiotensin II generation through non-ACE dependent pathways which include enzymes such as cathepsin G, elastase, tissue plasminogen activator and chymase (CitationVolpe et al 2003).
Angiotensin II targets two major receptor subtypes, AT1 and AT2. The AT1 receptor is well characterized. Activation of the AT1 receptor leads to marked vasoconstriction, norepinephrine release, sensitization of blood vessels to norepinephrine, sympathetic activation, aldosterone secretion and vascular hypertrophy (CitationFrancis et al 2004). Although less well defined, it has been suggested that AT2 activation counteracts the effects of AT1 through vasodilatory and antigrowth effects (CitationVolpe et al 2003). Moreover, AT1 receptor blockade leads to increased circulating levels of angiotensin II available to exert these proposed beneficial actions unopposingly on AT2 receptors. However, accumulating evidence has challenged this counter-regulatory role of AT2. Animal models demonstrated that AT2 receptors may have cardiac hypertrophic and proliferative actions, while other reports suggest AT2 receptors demonstrate neutral effects on cardiac structure and function (CitationWiddop et al 2003; CitationLévy 2004; Citationd’Amore et al 2005). These observations suggest AT1 blockade could be beneficial in patients with HF; however, the effect of unopposed AT2 stimulation is still unclear.
Candesartan is a nonpeptide angiotensin II receptor antagonist that selectively inhibits the binding of angiotensin II to the AT1 receptor. In animal models, candesartan demonstrates insurmountable receptor antagonism with high affinity for and slow dissociation from the AT1 receptor (CitationEasthope and Jarvis 2002). AT1 receptor binding affinity for candesartan was approximately 80 and 100 times greater compared with losartan potassium and its active metabolite (E3174), respectively (CitationNishikawa et al 1997).
Pharmacokinetics
Candesartan cilexetil is administered orally as a prodrug and then rapidly and completely converted to its active form candesartan via ester hydrolysis during gastrointestinal absorption. Its pharmacokinetic properties have been evaluated in healthy volunteers. Absolute oral bioavailability of candesartan cilexetil is variable and has been cited as approximately 15% (CitationEasthope and Jarvis 2002) and as 42% (Citationvan Lier et al 1997). Absorption of candesartan is not significantly influenced by food (CitationRiddell 1997). It is highly protein bound (>99%) with a relatively low volume of distribution (0.13 L/kg) and reaches peak concentrations in approximately 3–5 hours (tmax) (CitationEasthope and Jarvis 2002). In a pharmacokinetic study of healthy volunteers, single and repeated administration of candesartan demonstrated similar dose-related results for area under the plasma concentration–time curve (AUC) and maximum serum concentration (Cmax), consistent with no accumulation of the drug at steady state (CitationHübner et al 1997). Almost all of candesartan is eliminated in the urine and feces (99%), with 70%–80% as unchanged drug and 20%–30% metabolized to an inactive metabolite (Citationvan Lier et al 1997; CitationEasthope and Jarvis 2002). Metabolism occurs via the cytochrome P450 isoenzyme 2C9, with a smaller contribution by de-ethylation and glucuronidation (Citationvan Lier et al 1997; CitationEasthope and Jarvis 2002). Candesartan’s half-life is nine hours; it is minimally removed by dialysis (CitationEasthope and Jarvis 2002).
Clinical trials review
Candesartan was approved for use in HF by the US Food and Drug Administration (FDA) in February 2005 (CitationFDA 2006b) and was approved in Europe in 2004. Evidence to support use in HF stems mainly from the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) programs, although other smaller trials have evaluated candesartan in HF as well (Table ).
Table 1 Summary of clinical trials evaluating candesartan in heart failure
Trials evaluating candesartan’s effects on surrogate endpoints in HF provided a foundation for large scale outcome studies. In the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) Pilot study (CitationMcKelvie et al 1999), 768 patients were randomly assigned to enalapril, candesartan, or enalapril plus candesartan over 43 weeks. Patients with New York Heart Association functional class (NYHA-FC) II–IV, 6-minute walk distance (6MWD) <500 meters, and ejection fraction (EF) <40% were randomized. Although there were no changes in NYHA-FC, quality of life (QOL), 6MWD or EF among the groups, there were differences among the groups in both end diastolic volume (EDV) and end systolic volume (ESV) over time compared with baseline values (p=0.007 and p=0.006, respectively) with candesartan and enalapril showing increases. The reduction in ESV suggests possible prevention of LV dilatation with the combination. Neurohormone levels were generally similar throughout the groups with a few exceptions. There were no significant differences in creatinine or patients with hyperkalemia, although potassium levels were higher in the combination group (p<0.05). Blood pressure declined more with the combination (p<0.01). No significant differences were found in mortality and hospitalizations among the groups. CitationMitrovic and colleagues (2003) also found comparable results when evaluating candesartan’s effects on similar surrogate endpoints. These findings suggested that candesartan may be safe and effective alone or in combination with an ACE-inhibitor in patients with HF.
CitationGranger and colleagues (2000) randomly assigned 270 patients with HF and a history of ACE-I intolerance to receive either candesartan 4 mg, 8 mg, or 16 mg versus placebo for 12 weeks, with a primary endpoint of tolerability (defined as percentage of patients completing the treatment period) (CitationGranger et al 2000). The most common reasons for ACE-I intolerance were cough (67%), hypotension (15%), and renal dysfunction (11%). No significant differences were found in discontinuation rates between the groups (candesartan 17.3% vs placebo 13.2%; no p-value reported). Symptomatic hypotension was more common with candesartan (p=0.03; candesartan 18.4% vs placebo 11.0%). No differences were found in major cardiovascular events (death, worsening HF, hospitalizations, myocardial infarction, stroke), NYHA-FC, QOL as assessed by the Minnesota Living with Heart Failure Questionnaire or SF-36 Health Survey, or the 6MWD. Blood pressure was reduced with candesartan (10/6 mmHg) with no changes in heart rate. Creatinine levels increased more than 1.1 mg/dL in 1.1% of candesartan patients; 2.2% of candesartan and placebo patients experienced hyperkalemia (potassium >5.5 mg/dL). These results also suggested that candesartan may be a safe and effective alternative for patients intolerant of ACE-inhibitors.
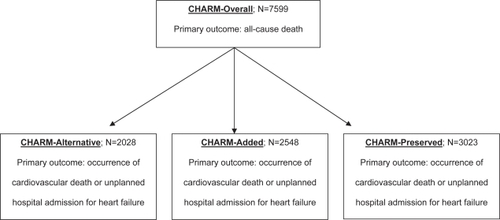
In the largest trial to date, candesartan was evaluated in Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity programs (CitationCHARM-Added 2003; CitationCHARM-Alternative 2003; CitationCHARM-Overall 2003; CitationCHARM-Preserved 2003). Overall, 7599 patients with chronic HF were assigned to one of three protocols: CHARM-Alternative, for patients with ACE-I intolerance; CHARM-Added, for patients established on ACE-I therapy; and CHARM-Preserved, for patients with isolated diastolic dysfunction. The primary outcome for the overall analysis was all-cause death, while each individual protocol’s primary outcome was occurrence of cardiovascular death or unplanned hospital admission for HF (see Figure ). The trial was powered towards the overall analysis: there was an 85% power to detect a 14% reduction in mortality at a significance level of 0.05. Patients were followed for a median duration of 37.7 months. At six months, the average dose in the candesartan and placebo group was 24 mg and 27 mg, respectively.
In the CHARM-Alternative protocol, 2028 patients intolerant of ACE-I were randomized to candesartan or placebo and were followed for 33.7 months. The most common reasons for ACE-I intolerance included cough (72%), hypotension (13%), and renal dysfunction (12%). There was a 23% relative risk reduction (RRR) in the primary endpoint (p=0.0004; placebo 40% vs candesartan 33%). There was no difference in the number of hospitalizations between the groups (p=0.06, placebo 1835 admissions vs candesartan 1718 admissions). More patients taking candesartan experienced a myocardial infarction compared to placebo (p=0.025; placebo 4.7% vs candesartan 7.4%), but there were no differences between the groups for stroke or coronary revascularization (p=0.42 and p=0.79, respectively). By study end, 24% of the candesartan group and 22% of the placebo group had permanently discontinued study medication (p=0.49). Angioedema occurred in three patients taking candesartan, all of whom were considered ACE-I intolerant because of angioedema on ACE-I. No cases were deemed life-threatening or lead to hospitalizations; in two cases, candesartan was resumed without recurrence.
In the CHARM-Added protocol, 2548 patients already receiving an ACE-I were randomized to candesartan or placebo and were followed for 41 months. There was a 15% RRR in the primary outcome (p=0.011; placebo 42% vs candesartan 38%). Similarly, there was a 16% RRR in cardiovascular death (p=0.029; placebo 27% vs candesartan 24%) and a 17% RRR in HF hospitalizations (p=0.014; placebo 28% vs candesartan 24%). There were fewer all-cause hospital admissions in the candesartan group (p=0.023; placebo 2798 admissions vs candesartan 2462 admissions). Fewer patients experienced a myocardial infarction with candesartan compared with placebo (p=0.012, placebo 5.4% vs candesartan 3.4%). There was no significant difference in all-cause death (p=0.086; placebo 32% vs candesartan 30%). Importantly, patients taking β-blockers, ACE-I, and candesartan did not have higher mortality than patients taking an ACE-I plus candesartan (p=0.20, placebo 39% and candesartan 35%). In a previous trial, a sub-analysis demonstrated that patients taking β-blocker, ACE-I and valsartan had worse outcomes (CitationCohn et al 2001). Although two large clinical trials have not confirmed this finding (CitationCHARM-Added 2003; CitationCHARM-Alternative 2003; CitationCHARM-Overall 2003; CitationCHARM-Preserved 2003; CitationPfeffer et al 2003), expert guidelines provide inconsistent recommendations regarding use of triple therapy (CitationHFSA 2006; CitationACC–AHA 2005).
In the CHARM-Preserved protocol, 3023 patients with isolated diastolic dysfunction were randomized candesartan or placebo and were followed for 36.6 months. There was no difference in the primary outcome between the treatment groups (p=0.118, unadjusted; p=0.051, adjusted; placebo 24% vs candesartan 22%). HF hospital admissions were lower for the candesartan group than placebo (p=0.014; placebo 566 admissions vs candesartan 402 admissions). However, there was no difference in all-cause hospitalizations. Patients taking candesartan were less likely to be diagnosed with diabetes (p=0.005; placebo 77 vs candesartan 47).
In the CHARM-Overall analysis, there was a 9% RRR in all-cause mortality that did not reach statistical significance initially (p=0.055; placebo 25% vs candesartan 23%), but became significant after adjustments for covariates (p=0.032; hazard ratio [HR] 0.9, 95% confidence interval [CI] 0.82–0.99). The lower mortality was largely attributed to fewer cardiovascular deaths (p=0.012; placebo 20% vs candesartan 18%). However, there were more deaths due to cancer in patients taking candesartan (p=0.038; placebo 1.6% vs candesartan 2.3%). Time to cardiovascular death or hospital admission for HF was reduced by 16% (p<0.0001; placebo 35% vs candesartan 30%). Although there were some differences in frequency of myocardial infarction within the individual protocols, there were no significant differences overall for incidence of myocardial infarction, stroke, or coronary revascularization. Fewer patients taking candesartan were hospitalized for any reason (p=0.015; placebo 7178 admissions vs candesartan 6690 admissions). In patients without a diagnosis of diabetes prior to the study, patients taking candesartan were less likely to develop diabetes during the trial (p=0.02; placebo 7% vs candesartan 6%). Treatment effect did not differ based on sex, age, EF, NYHA-FC, diabetes, or concomitant medications. More patients stopped taking candesartan than placebo due to hypotension, elevated serum creatinine, and hyperkalemia (p<0.0001 for all adverse effects; placebo 16.7% vs candesartan 21.0%).
A post-hoc evaluation of candesartan’s effect on cause-specific mortality in the CHARM-Overall program was performed (CitationSolomon et al 2004). The analysis demonstrated significant reductions in sudden death (HR 0.85, 0.73–0.99, p=0.036) and progressive HF (HR 0.78, 0.65–0.94, p=0.008), the two most common causes of death in patients with HF. Notably, however, there did not appear to be a difference in these causes of death in the patients specifically enrolled in the CHARM-Preserved protocol. Frequency of other common causes of death, such as stroke, myocardial infarction, and procedure-related mortality were similar to placebo.
The effect of candesartan on NYHA-FC was evaluated using the CHARM-Overall data (CitationO’Meara et al 2004). In the CHARM trial, NYHA-FC was assessed every two weeks during titration, then every four months thereafter. The investigators used two analyses to evaluate effect. The first was “last visit carried forward”, which carried forward the last available NYHA-FC documented when follow up data was not available due to study withdrawal or death. The second analysis was the “worst rank carried forward”, which assigned patients who died a NYHA-FC of V. For both approaches, candesartan showed more improvement and less deterioration in NYHA-FC (p=0.003 for last visit carried forward; p=0.003 for worst rank carried forward).
Granger and colleagues used the CHARM-Overall data to evaluate the effect of adherence to study medication on clinical outcomes (CitationGranger et al 2005). The proportion of study pills taken was assessed at each follow-up visit throughout the CHARM trial. The investigators defined good adherence as being adherent at least 80% of the time and poor adherence as being adherent less than 80% of the time; 89% of patients were at least 80% adherent, while 11% were less than 80% adherent. Good adherence was associated with lower all-cause mortality overall (HR 0.66, 95% CI 0.58–0.76, p<0.0001), as well as in each treatment group (candesartan HR 0.70, 0.57–0.86, p<0.001; placebo HR 0.62, 0.51–0.76, p<0.001).
Safety profile
Several trials have reported the safety of candesartan in HF. The RESOLVD trial found higher serum potassium when candesartan was used in combination with enalapril. Other expected side effects (hypotension, serum creatinine elevation) were similar to enalapril.
In the CHARM trial, patients treated with candesartan were more likely to experience serum creatinine elevations after six weeks (p<0.0001), a doubled serum creatinine (p=0.002), hyperkalemia (p=0.017), and lowered blood pressure (systolic blood pressure decreased by 5.2 mmHg, diastolic blood pressure decreased by 3.0 mmHg versus no change in placebo, p<0.001). By the end of the study, 23% of candesartan patients and 19% of placebo patients had permanently discontinued study medication (p=0.0001). Patients stopped the medication due to hypotension, increase in serum creatinine, or hyperkalemia (p<0.0001 for all causes). Angioedema occurred more in the candesartan patients (0.13% vs 0.08%), but no statistical data was reported.
Candesartan has been specifically evaluated in patients intolerant of ACE-I in two trials: SPICE and CHARM-Alternative (CitationBart et al 1999; CitationCHARM-Alternative 2003). The SPICE investigators found candesartan was associated with hypotension (3% vs 0%, no p-value) and serum creatinine elevation of 1.1 mg/dL in 1.1% of patients (vs no change in placebo-treated patients, no p-value). Renal insufficiency and hyperkalemia were similar between the treatment groups (no p-value). In CHARM-Alternative, patients who experienced ACE-I induced cough rarely experienced a recurrence on candesartan (0.3%). However, patients who experienced angioedema, hypotension, and renal dysfunction on an ACE-I had a 2.6%, 9.1%, and 23.1% chance, respectively, of experiencing that same side effect on candesartan. As such, ACE-I intolerant patients may tolerate candesartan, but patients who experienced hypotension or renal dysfunction on an ACE-I should be monitored closely if given candesartan as an alternative agent. Although the CHARM trial demonstrated a low recurrence of angioedema on candesartan, conclusions regarding the true rate and severity of recurrence cannot be made given the limited power of this part of the trial. As such, we recommend that patients who experience angioedema on an ACE-I should be given a combination of hydralazine and nitrate as an alternative rather than an ARB at this time.
The increased rate of cancer-related deaths in the CHARM trial has raised concerns. The investigators attribute this to chance. Unpublished candesartan data on file at AstraZeneca was pooled with the CHARM data to assess comprehensive experience, which showed no difference between investigator-reported neoplasms (p=0.6) or cancer-related deaths (p=0.4) (CitationCHARM-Overall 2003).
Special populations
Geriatric
CitationHübner and colleagues (1997) compared the pharmacokinetics of candesartan in healthy geriatric volunteers (average age 68) with young volunteers (average age 27). The maximum concentration (Cmax) was 50%–70% higher in elderly subjects after single dose administration of 4–16 mg of candesartan compared with young subjects; however, accumulation of candesartan did not occur after repeated once daily dosing. In addition, the time to reach serum concentration was similar between both groups. Area under the curve (AUC) increases were also observed, but no data was reported. The half-life (t½) was 9–12 h in the elderly volunteers compared with 9 h in younger subjects. No baseline or post-treatment information was provided regarding blood pressure or heart rate. In spite of the pharmacokinetic difference in elderly subjects, no dose adjustments are recommended for elderly patients. As with other antihypertensive agents in geriatric patients, it is reasonable to start candesartan at a low dose and titrate according to tolerability and blood pressure goals.
Renal dysfunction
The pharmacokinetics of candesartan cilexetil were studied in hypertensive patients with varying levels of renal function. The effects of single and multiple daily doses of candesartan 8mg were evaluated in patients with a creatinine clearance (CrCl) of >60 mL/min/1.73 m2 (group A), 30–60 mL/min/1.73 m2 (group B, moderate renal dysfunction), and 15–30 mL/min/1.73 m2 (group C, severe renal dysfunction) (CitationButer et al 1999). Patients with severe impairment displayed higher AUC and trough serum concentrations compared with normal renal function (p<0.05). The t½ increased with worsening renal status (group A 7.1 hours, group B 10.0 hours, and group C 15.7 hours), but no significant drug accumulation occurred. Patients with moderate to severe renal impairment experienced a significantly reduced mean arterial pressure versus those with normal renal function (p<0.05; group A −7 mmHg, group B −13 mmHg, group C −9 mmHg).
Candesartan was further evaluated in patients with varying degrees of renal function, including those on dialysis (normal CLcr >60 mL/min/1.73 m2; moderate dysfunction CLcr 31–60 mL/min/1.73 m2; severe dysfunction CLcr 15–30 mL/min/1.73 m2 with and without hemodialysis 2–4 days/week) (Citationde Zeeuw, et al 1997). A progressive increase in mean Cmax, t1/2, and AUC was observed with worsening renal impairment after multiple dosing of candesartan cilexetil 12 mg/day. The Cmax and AUC values were increased by 60% and 110%, respectively, in patients with severe impairment (CLcr 15–30 ml/min/1.73 m2, not requiring dialysis) compared with normal renal function; however, trough mean reductions in systolic and diastolic blood pressure were similar in both groups (no p-value). In hemodialysis patients who received 8 mg of candesartan, the mean extraction ratio and mean recovery of the drug were low: 0.016% and 0.18%, respectively. Higher AUC and Cmax values were observed compared with patients with normal renal function; however, no symptomatic hypotension was reported. In a dose escalation study, candesartan was assessed in hemodialysis patients (CitationOttosson et al 2003). Nine patients receiving chronic dialysis were titrated up to 16 mg of candesartan cilexetil with stable blood pressure; however four patients discontinued treatment due to hypotension. Trough plasma concentrations followed linear pharmacokinetics with little drug accumulation at the higher dosage of 16 mg/day. Although there are no specific recommendations for dosage adjustment in patients with renal insufficiency, candesartan should be used cautiously with close monitoring after initiation and during dose titration.
Hepatic dysfunction
Two studies showed no significant differences in pharmacokinetic parameters after administration of candesartan cilexetil 12 mg (either as single and multiple doses) in patients with mild to moderate hepatic impairment compared with healthy subjects (Citationde Zeeuw et al 1997; CitationHoogkamer et al 1998). However, clinically significant differences were found in another study evaluating a single dose of candesartan cilexetil 16 mg in patients with mild (Child Pugh A) to moderate (Child Pugh B) hepatic impairment versus healthy controls (CitationFDA 2006a). After single 16 mg dose, AUC values increased by 145% (p=0.027) and Cmax values increased by 73% (p=0.174) in patients with moderate liver impairment versus healthy controls. In patients with mild hepatic impairment, AUC and Cmax values increased to a lesser degree over healthy controls and were not statistically significant. Comparisons of adverse events or different clinical responses between patients with varying degrees of hepatic dysfunction were not provided in these studies. Given the absence of clinical markers to guide use, the pharmacokinetic data suggests that for mild hepatic impairment, no dose adjustment is necessary upon initiation, but patients with moderate impairment may need lower starting doses as a precaution. Investigations of candesartan cilexetil in patients with severe hepatic dysfunction are needed to ascertain safety in this population.
Conclusion
In summary, only valsartan and candesartan have prospective, randomized, controlled, clinical trial data showing improved outcomes in patients with HF. Both agents are approved to be used as an alternative to ACE-I. Candesartan is approved to be used in combination with an ACE-I, but valsartan is not. Both ARBs have been studied in combination with an ACE-I; however, in patients already established on and tolerating an ACE-I, only candesartan has been shown to further improve outcomes. Candesartan did not show statistical benefit in patients with preserved LV EF; however, the role of RAAS agents for isolated diastolic dysfunction needs further study. Other trials have looked promising in evaluating candesartan’s effects on surrogate endpoints in patients with preserved LV EF (CitationLittle et al 2004; CitationKasama et al 2005).
Patients who cannot tolerate an ACE-I due to cough, hypotension, or renal dysfunction should be given a trial on candesartan or valsartan. For those on established ACE-I therapy, a reasonable approach would be to add candesartan in patients who are on goal doses of ACE-I and β-blockers and who continue to experience disease progression (CitationACC–AHA 2005). Clinicians must be prudent about adding an ARB, as not all patients are candidates. Notably, the safety of triple therapy (ARB, ACE-I, plus β-blocker) has been questioned (CitationCohn et al 2001); as such, even though this finding was not confirmed, it is rational to reserve this therapy to patients in whom compliance with standard therapy has been reasonably confirmed, but who still experience disease progression. The safety of using ACE-I, ARBs, and aldosterone antagonists together is questionable and should be avoided (CitationACC–AHA 2005). Also, the data in special populations is limited in numbers and by lack of consistent peer review; if candesartan use is attempted in these groups, close monitoring of vital signs, laboratory values, and symptoms is essential. To summarize, candesartan significantly lowers cardiovascular mortality, overall mortality, and HF hospitalizations in patients with LV systolic dysfunction. As such, it is an appropriate alternative to ACE-I intolerant patients or to appropriately selected patients on established ACE-I therapy.
References
- AdamsKFLindenfeldJArnoldJMO2006Executive Summary: HFSA 2006 Comprehensive heart failure practice guidelineJ Cardiac Failure121038
- AghababianRV2002Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency departmentRev Cardiovasc Med3suppl 4S3912439425
- [ACC–AHA] American College of Cardiology–American Heart Association2005Guideline update for the diagnosis and management of chronic heart failure in the adultCirculation112182552
- [AHA] American Heart Association2006Heart disease and stroke statistics – 2006 updateDallasAHA
- BartBAErtlGHeldP1999Contemporary management of patients with left ventricular systolic dysfunction: Results from the Study of Patients Intolerant of Converting Enzyme Inhibitors (SPICE) RegistryEur Heart J2011829010448027
- BiollazJBrunnerHRGavrasI1982Antihypertensive therapy with MK 421: angiotensin II-renin relationships to evaluate efficacy of converting enzyme blockadeJ Cardiovasc Pharmacol4966726185790
- ButerHNavisGYWoittiezAJJ1999Pharmacokinetics and pharmacodynamics of candesartan cilexetil in patients with normal to severely impaired renal functionEur J Clin Pharmacol54953810192757
- [CHARM-Added] McMurrayJJVOstergrenJSwedbergK2003Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitorsLancet3627677113678869
- [CHARM-Alternative] GrangerCBMcMurrayJJVYusufS2003Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitorsLancet362772613678870
- [CHARM-Overall] PfefferMASwedbergKGrangerCB2003Effects of candesartan on mortality and morbidity in patients with chronic heart failureLancet3627596613678868
- [CHARM-Preserved] YusufSPfefferMASwedbergK2003Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fractionLancet3627778113678871
- CohnJNTognoniGfor the Valsartan Heart Failure Study Group2001A randomized trial of the angiotensin receptor blocker valsartan in chronic heart failureN Engl J Med34516677511759645
- d’AmoreABlackJMThomasWG2005The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophyHypertension4613475416286564
- DelgadoRMWillersonJT1999Pathophysiology of heart failure: a look at the futureTex Heart Inst J26283310217468
- de ZeeuwDRemuzziGKirchW1997Pharmacokinetics of candesartan cilexetil in patients with renal or hepatic impairmentJ Hum Hypertens11suppl 2S37429331004
- DostalDE2000The cardiac rennin-angiotensin system: Novel signaling mechanisms related to cardiac growth and functionRegul Pept9111110967197
- EasthopeSEJarvisB2002Candesartan cilexetil: an update of its use in essential hypertensionDrugs6212538712010090
- FarrellDMWeiCCTallajJ2001Angiotensin II modulates catecholamine release into interstitial fluid of canine myocardium in vivoAm J Physiol Heart Circ Physiol281H8132211454586
- FlatherMDYusufSKøberL2000Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patientsLancet35515758110821360
- FrancisGSWilson TangWHSonneblickEH2004Pathophysiology of heart failureFusterVAlexanderRWO’RourkeRAHurst’s: the heart11th edNew YorkMcGraw-Hill[online] Accessed 18 March 2006. URL: http//www.accessmedicine.com/
- GargRYusufF1995Overview of randomized trials of angiotensin-converting enzyme inhibitors on morbidity and mortality in heart failureJAMA2731450567654275
- GrangerCBErtlGKuchJ2000Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting-enzyme inhibitorsA Heart J13960717
- GrangerCBSwedbergKEdmanI2005Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomized, controlled clinical trialLancet36620051116338449
- [HFSA] Heart Failure Society of America20062006 Guideline executive summaryJ Card Fail22006121038
- HoogkamerJFWKleinbloesemCHOuwerkerkM1998Pharmacokinetics and safety of candesartan cilexetil in subjects with normal and impaired liver functionEur J Clin Pharmacol54341459696961
- HübnerRHögemannAMSunzelM1997Pharmacokinetics of candesartan after single and repeated doses of candesartan cilexetil in young and elderly healthy volunteersJ Hum Hypertens11suppl 2S1925
- JessupMBrozenaS2003Heart failureN Engl J Med34820071812748317
- JordeUPEnnezatPVLiskerJ2000Maximally recommended doses of angiotensin-converting enzyme (ACE) inhibitors do not completely prevent ACE-mediated formation of angiotensin II in chronic heart failureCirculation101844610694521
- KimsIwaoH2000Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseasesPharmacol Rev52113410699153
- LévyBI2004Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin systemCirculation10981314707017
- KasamaSToyamaTKumaduraH2005Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fractionJ Am Coll Cardiol45661715734608
- LittleWCWesley-FarringtonDJJoyleJ2004Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunctionJ Cardiovasc Pharmacol432889314716219
- MitrovicVWillenbrockRMiricM2003Acute and 3-month treatment effects of candesartan cilexetil on hemodynamics, neurohormones, and clinical symptoms in patients with congestive heart failureA Heart J145e14
- McKelvieRSYusufSPericadD1999Comparison of candesartan, enalapril, and their combination in congestive heart failure: the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) pilot studyCirculation10010566410477530
- [MERIT-HF] MERIT-HF Study Group1999Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF)Lancet3532001710376614
- MitrovicVWillenbrockRMiricM2003Acute and 3-month treatment effects of candesartan cilexetil on hemodynamics, neurohormones, and clinical symptoms in patients with congestive heart failureAm Heart J145e1412660683
- NishikawaKNakaTChataniF1997Candesartan cilexetil: a review of its preclinical pharmacologyJ Hum Hypertens11suppl 2S917
- O’MearaESolomonSMcMurrayJ2004Effect of candesartan on New York Heart Association functional class: Results of the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) ProgramEur Heart J251920615522471
- OttossonPAttmanPÂgrenA2003Candesartan cilexetil in haemodialysis patientsClin Drug Invest2354550
- PackerMBristowMRCohnJNUS Carvedilol Heart Failure Study Group1996The effect of carvedilol on morbidity and mortality in patients with chronic heart failureN Engl J Med3341349558614419
- PfefferMAMcMurrayJJVVelazquezEJValsartan in Acute Myocardial Infarction Trial Investigators2003Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both (VALIANT)N Engl J Med349189390614610160
- PittBRemmeWZannadF2003Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med34813092112668699
- PittBSegalRMartinezFA1997Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study: ELITE)Lancet349747529074572
- PittBZannadFRemmeWJ1999The effect of spironolactone on morbidity and mortality in patients with severe heart failureN Engl J Med3417091710471456
- RichM1997Epidemiology, pathophysiology, and etiology of congestive heart failure in older adultsJ Am Geriatr Soc45968749256850
- SolomonSDWangDFinnP2004Effect of candesartan on cause-specific mortality in heart failure patients: The Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) ProgramCirculation1102180315466644
- RiddellJG1997Bioavailability of candesartan is unaffected by food in healthy volunteers administered candesartan cilexetilJ Hum Hypertens11suppl 2S29309331002
- UrataHHealyBStewartRW1990Angiotensin II-forming pathways in normal and failing human heartsCirc Res66883902156635
- [FDA] US Food and Drug Administration2006aCandesartan cilexetil, Atacand®: clinical pharmacology and biopharmaceutics review [online]Accessed 18 March 2006. URL: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3877B1_04_FDA-Biopharm.htm
- [FDA] US Food and Drug Administration2006bCandesartan cilexetil, Atacand: Label revision, February 22, 2005Accessed 18 April 2006. URL: http://www.fda.gov/cder/foi/label/2006/020838s026lbl.pdf
- van LierJJvan HieningenPNSunzelM1997Absorption, metabolism, and excretion of 14C-candesartan and 14C-candesartan cilexetil in healthy volunteersJ Hum Hypertens11suppl 2S2789331001
- VolpeMMusumeciBPaolisPD2003Angiotensin II AT2 receptor subtype: an uprising frontier in cardiovascular diseaseJ Hypertens2114294312872031
- WaagsteinFBristowMRSwedbergKfor the Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group1993Beneficial Effects of Metoprolol in Idiopathic Dilated CardiomyopathyLancet342144167902479
- WeberKT2001Aldosterone in congestive heart failureN Engl J Med34516899711759649
- WiddopREJonesESHannanRE2003Angiotensin AT2 receptors: cardiovascular hope or hype?Br J Pharmacol1408092414530223
- WillersonJT1995Other cardiomyopathiesWillersonJTCohnJNCardiovascular medicineNew YorkChurchill Livingstone88894