Abstract
The leading treatments for postmenopausal osteoporosis are the nitrogen-containing bisphosphonates, which are required long term for optimal benefit. Oral bisphosphonates have proven efficacy in postmenopausal osteoporosis in clinical trials, but in practice the therapeutic benefits are often compromised by patients’ low adherence. Nonadherence to bisphosphonate therapy negatively impacts outcomes such as fracture rate; fractures are in turn associated with decreased quality of life. The most common reason cited by patients for their nonadherence is that the strict dosing instructions for bisphosphonates are difficult to follow. One aspect of bisphosphonate administration that can be changed is dosing frequency and several studies have evaluated patient preferences for different dosing schedules. Studies have shown a preference for a weekly bisphosphonate regimen versus daily dosing and it has been demonstrated that this preference for reduced dosing frequency impacts on adherence. Ibandronate is the first nitrogen-containing oral bisphosphonate for osteoporosis that can be administered in a monthly regimen and two robust clinical studies demonstrated a strong patient preference for this monthly regimen versus a weekly regimen. It is important that physicians consider patient preference when prescribing treatment for osteoporosis to ensure that the disease is effectively managed for the long-term benefit of the patient.
Introduction
Osteoporosis affects one in three postmenopausal women but the nature of this generalized, initially asymptomatic, chronic disease means that many patients are unaware that they have this disease until they experience their first fracture. Osteoporosis is characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures (CitationWHO 2003a). Bone loss is the result of an imbalance in bone turnover, with bone resorption occurring at a faster rate than new bone formation. The resulting reduction in bone mass and accompanying damage to bone microarchitecture increases the risk of fracture. The most common form of osteoporosis, that experienced by postmenopausal women, results from reduced estrogen production following the menopause; by increasing bone resorption, estrogen deficiency disrupts the fine balance of the bone remodeling cycle. The spine (vertebral fractures), hips, and wrists (nonvertebral fractures) are the most common sites of osteoporosis-related bone fractures (ie, fractures that are out of proportion to the level of external trauma), although osteoporosis-related fractures can occur at almost any skeletal bone site.
Patients who suffer a vertebral fracture are subsequently at an increased risk of further fractures of all types, including hip fractures (CitationJohnell et al 2001). Large prospective fracture studies have demonstrated an increased mortality rate following vertebral fracture. In the Study of Osteoporotic Fractures (SOF), women with at least one prevalent vertebral fracture experienced a 23% greater age-adjusted mortality rate than age-matched controls in the general population (CitationJohnell et al 2004). In patients with postmenopausal osteoporosis in the Fracture Intervention Trial (FIT) (CitationMelton 2000) and the European Prospective Osteoporosis Study (EPOS) (CitationKanis et al 2004), it was demonstrated that the presence of a vertebral fracture increases the relative risk of mortality by approximately 60%.
In addition to mortality, vertebral fractures are associated with debilitating pain (lasting several weeks or months, with chronic pain lasting for many years), kyphosis (curvature of the spine, leading to height loss, abdominal protrusion, and a hump at the top of the spine), disability, and restricted movement (CitationRoss 1997; CitationCenter et al 1999; CitationLips et al 1999; CitationCummings and Melton 2002; CitationHodgson et al 2003; CitationJalava et al 2003; CitationLips 2003; CitationNaves et al 2003). There are also psychological effects; patients may experience anxiety about their loss of independence, as well as having a fear of falls and further fractures. Nonvertebral fractures, especially hip fractures, are devastating; around half of the patients who experience a hip fracture will never be able to walk again without assistance (CitationJohnell 1997) and as many as 30% of hip fracture patients require permanent institutional care (CitationLips et al 1999).
Population-based outcome modeling estimates that Caucasian women over 50 years of age have a one-in-three risk of at least one vertebral fracture and a one-in-five risk of at least one hip fracture in their remaining lifetime (CitationChrischilles et al 1991). The incidence of osteoporosis-related fractures increases with age, so as the population ages, the number of osteoporosis-related fractures is projected to increase dramatically (CitationCooper et al 1992; CitationMelton et al 1992). Overall, 40% of women with postmenopausal osteoporosis will suffer one or more fragility fractures during their remaining lifetime (CitationMelton et al 1992). In the year 2000, an estimated 75 million people had osteoporosis in Europe, the US, and Japan (CitationMadhok et al 2000). During 2002, the direct impact of this disease translated to an estimated treatment cost in the US of $17.5 billion (CitationMelton 2003), and in 2003, in the EU, the estimated total direct cost of osteoporosis-related fractures was 25 billion (CitationIOF 2003). Due to the increasing burden of osteoporosis, there is a real need for effective treatments.
Bisphosphonates for the treatment of osteoporosis
The aim of pharmacological intervention in postmenopausal osteoporosis is to reduce the frequency of fractures and, consequently, reduce the related burden on patients and healthcare services and improve patients’ quality of life. The leading treatments for postmenopausal osteoporosis are the nitrogen-containing bisphosphonates. These antiresorptive agents reduce postmenopausal bone loss by inhibiting osteoclast activity and reducing the rate of bone resorption. This shifts the balance in favor of bone formation, so that bone mass is increased (CitationRussell and Rogers 1999). In numerous robust clinical trials, the nitrogen-containing bisphosphonates alendronate, risedronate, and ibandronate have consistently demonstrated considerable increases in bone mineral density (BMD) of the spine and hip and decreases in the biochemical markers of bone turnover together with substantial antifracture efficacy (CitationBlack et al 1996; CitationCummings et al 1998; CitationHarris et al 1999; CitationReginster et al 2000; CitationChesnut et al 2004). Additionally, the bisphosphonates are the only antiresorptive agents shown in a meta-analysis to significantly reduce the risk of nonvertebral fractures (CitationCranney et al 2002) and, in a prospective analysis, risedronate was shown to reduce the risk of hip fracture (CitationMcClung et al 2001). All three of these bisphosphonates have favorable safety profiles, which have been shown in clinical studies to be similar to placebo. However, long-term treatment with bisphosphonates is required for optimal and sustained benefit and although oral bisphosphonates have proven efficacy in women with postmenopausal osteoporosis in clinical trials, the therapeutic benefits in clinical practice are often compromised by patients’ low compliance to, and persistence with, their prescribed medication. Compliance describes the quality of intake of a given medication and considers the extent to which a dosing regimen and its associated instructions are followed. Compliance can often be quantified by a surrogate measure, the medication possession ratio, which is the number of days of available medication divided by the number of days of study follow-up. Persistence describes the length of time patients continue to take their medication, and is defined as the time from treatment initiation to treatment completion/discontinuation. Using the PHARMO Record Linkage System, which includes drug-dispensing records from community pharmacies serving more than 1 million community-dwelling patients in the Netherlands, CitationPenning-van Beest and colleagues (2004) showed that, overall, 1-year persistence with bisphosphonate therapy (daily or weekly) is low. Among 2124 new bisphosphonate users (alendronate daily or weekly, risedronate daily, etidronate daily), only 43% were persistent at 1 year. As few as 39% of patients may persist with weekly bisphosphonates at 1 year (CitationCowell et al 2005) and persistence at 2 years could be as low as 18%–23% (CitationHarris et al 2005; CitationSiris et al 2005).
Impact of poor therapeutic adherence
Adherence is a summary term that is determined by compliance and persistence of medication intake. Adherence is thus used to describe the extent and the quality of medication intake. Adherence to medication in postmenopausal osteoporosis is in line with the general finding of low persistence rates in other chronic diseases. Following an inspection of several reviews, the World Health Organization estimated that long-term adherence in chronic disease averages only 50% (CitationWHO 2003b). In a recent meta-analysis of the association between adherence to drug therapy and mortality, nonadherent patients with chronic disorders had a higher mortality rate than their adherent counterparts (CitationSimpson et al 2006). It seems that this is particularly true for diseases like postmenopausal osteoporosis that have few or no clinical symptoms, as the patient does not experience ill effects from the disease or the subsequent benefit from treatment.
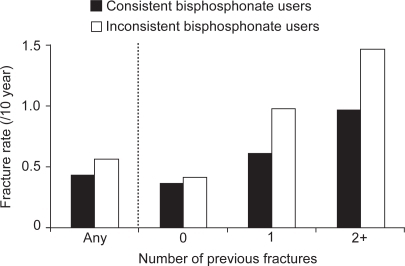
In the treatment of osteoporosis, nonadherence to bisphosphonate therapy negatively impacts upon treatment outcomes, for instance poor adherence is associated with a significantly higher rate of vertebral and nonvertebral fractures (see Figure ), which are in turn associated with a decreased quality of life (CitationCaro et al 2004; CitationSebaldt et al 2004; CitationHarris et al 2005; CitationSiris et al 2005). The antifracture efficacy of bisphosphonates has been demonstrated in clinical studies with 2–4 years of treatment, therefore if fewer than half of patients are adhering to therapy after just 1 year, it is unlikely that the same degree of antifracture efficacy will be achieved as has been shown in clinical trials. Nonadherent bisphosphonate use also increases the risk of hospitalization associated with osteoporotic fractures (CitationGoettsch et al 2005). Thus, as would be expected, nonadherence with bisphosphonate therapy correlates with reduced gains in BMD and lower reductions in the levels of bone turnover markers (CitationEastell et al 2003; CitationSebaldt et al 2004). As well as adversely affecting these primary treatment outcomes, nonadherence leads to an increased incidence of secondary complications associated with fractures, such as pain, nosocomial infections, and pulmonary thromboembolism, and hence to increased healthcare costs.
Figure 1 Fractures are increased as a result of suboptimal adherence: trend of a 33% greater fracture rate in inconsistenta versus consistent users (CitationSebaldt et al 2004).
Understanding the causes of poor adherence
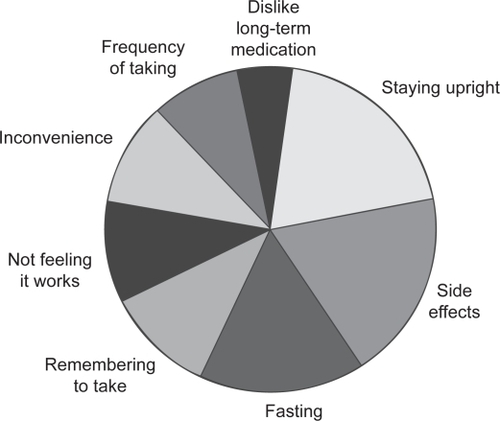
Given the impact of nonadherence to therapy on patient outcomes and healthcare resources, it is clearly important to improve adherence. However, to improve therapeutic adherence it is important to know why patients stop taking, or do not take adequate amounts of, their medication. Overall, the main reasons patients cite for not continuing to take their osteoporosis medication are the stringent dosing schedule, adverse events, not feeling that treatment is working, or not believing that they have a disease that needs treating (see Figure ) (CitationIOF 2005). The most common of the reasons cited above for nonadherence is that patients find the strict dosing instructions for bisphosphonates difficult to follow; fasting (overnight for at least 6 hours prior to taking the medication and 30–60 minutes after administration), and posture (staying upright for 30–60 minutes after taking the medication) requirements can be inconvenient and often not feasible in the daily routine. The strict requirements interfere not only with eating and drinking, but also with taking other medications, especially if these need to be taken with food. The next most common reason for discontinuation of therapy is that despite clinical studies reporting side-effect profiles that are similar to placebo, many patients stop taking treatment due to adverse events. The main complaints with oral bisphosphonates are upper gastrointestinal irritation, dyspepsia, nausea, upper abdominal pain, vomiting, and gastroesophageal reflux. Finally, as patients often have no symptoms until they suffer a fracture, they do not feel that treatment is worth taking or do not believe they have a disease that needs treatment. This means they may consider the pill burden and inconvenience of the dosing requirements to be unnecessary. It has been suggested that, compared with other chronic diseases, adherence in osteoporosis is compromised because measures of therapeutic outcome (such as increases in BMD and reductions in the level of biochemical markers of bone turnover) are not readily available, therefore patients are unable to monitor their response to medication and thus gain feedback regarding the benefits of their medication (CitationIOF 2005).
Figure 2 Reasons given by patients for not adhering to bisphosphonate therapy for osteoporosis (CitationIOF 2005).
Many physicians believe that the main reason for patients not continuing to take their osteoporosis medication is a lack of understanding of the benefits of treatment. However, in the IOF adherence survey (CitationIOF 2005), 71% of physicians questioned recognized that they did not know why their patients were discontinuing treatment. Almost half of the physicians surveyed believed that the best way to motivate patients to continue on treatment is to talk to them about risks and complications, however, the patients surveyed believed that it may be better to adopt a more positive approach that stresses the benefits of therapy.
Strategies to overcome the problem of nonadherence
On the whole, patients with osteoporosis want an effective and well-tolerated treatment, however, even with the proven efficacy and safety profiles of the bisphosphonates from clinical trials, patients still do not remain on treatment. Therefore other strategies are needed to improve therapeutic adherence. Improved communication between physicians and their patients may be one way to help. During the IMPACT (Improving the Measurements of Persistence on Actonel Treatment) study, patients were given verbal feedback regarding their bone turnover marker results (CitationDelmas et al 2003). The study showed that a significant improvement in persistence was achieved if patients were given a positive message regarding their response to treatment. It has also been shown that involving patients in treatment decisions and matching them with their preferences improves patient satisfaction, adherence, perception of health, and outcomes (CitationLopes et al 2001; CitationJanz et al 2004; CitationJahng et al 2005; CitationLin et al 2005). With regards to pharmacological intervention, patients have identified key product attributes as being efficacy, side effects, formulation, costs, drug interactions, dosing procedure, and dosing frequency. For the bisphosphonates, it is acknowledged that most of these attributes cannot be altered. However, one attribute that can be changed is the dosing frequency. Several studies have evaluated patient preferences for different dosing schedules and the impact this has had on therapeutic adherence.
Patient preference for an increased dosing interval: weekly dosing
The registration studies, which showed antifracture efficacy for the oral bisphosphonates, were all conducted using a daily regimen (CitationBlack et al 1996; CitationCummings et al 1998; CitationHarris et al 1999; CitationReginster et al 2000; CitationChesnut et al 2004). The comparable antifracture efficacy of weekly oral bisphosphonate regimens of alendronate and risedronate with their respective daily regimens has been inferred based on equivalent increases in BMD (a validated surrogate marker for antifracture efficacy), and decreases in bone turnover markers (CitationSchnitzer et al 2000; CitationBrown et al 2002). The weekly regimens of alendronate and risedronate are both licensed and are widely accepted as being at least as effective as the daily regimens with the added convenience of only one tablet a week. In several studies evaluating bisphosphonate regimen preference (using alendronate or risedronate), there was a strong preference for a weekly regimen versus daily dosing (see Table ). The weekly regimen was considered by the patients to be more convenient, would allow them to achieve better long-term compliance, and was the regimen that most said they would be willing to take for an extended period of time, ie, would improve their adherence (CitationSimon et al 2002; CitationBaroutsou et al 2004; CitationCramer et al 2004; CitationKendler et al 2004; CitationRecker et al 2004; CitationBartl et al 2006).
Table 1 Patients’ preference for weekly versus daily dosing of bisphosphonates
It has been demonstrated that patient preference for reduced bisphosphonate dosing frequency has impacted on therapeutic adherence. Studies that compared daily and weekly regimens found that a weekly regimen of alendronate or risedronate significantly increased rates of compliance versus a daily treatment (see Table ) (CitationCramer et al 2005; CitationRecker et al 2005; CitationBartl et al 2006). Patients receiving daily bisphosphonates filled prescriptions for only 33%–58% of their prescribed medication, while patients receiving a weekly regimen obtained more of their prescribed medication (46%–69%). Around half (41%–55%) of patients receiving a weekly regimen were highly compliant (at least 80% of prescribed medication taken) compared with only 23%–40% of patients taking a daily treatment. Preference for less frequently dosed bisphosphonate regimens also translates to improved therapeutic persistence (see Table ). Analyses of a number of health databases of patients in the clinical setting show that 1-year persistence increases with weekly bisphosphonate dosing regimens by 12%–29% versus daily dosing (CitationEttinger et al 2004; CitationPenning-van Beest et al 2004; CitationSunyecz et al 2004; CitationCramer et al 2005; CitationBartl et al 2006). However, fewer than half of patients receiving a weekly regimen persist with their therapy for 12 months so, even though adherence has been improved with weekly regimens versus daily, it is still suboptimal. Adherence, and therefore potentially quality of life, may be improved by further increasing the dosing interval, for instance from daily or weekly to monthly (CitationSimon et al 2005).
Table 2 Studies of compliance with weekly compared with daily bisphosphonate regimens
Table 3 Studies of persistence with weekly compared with daily bisphosphonate regimens
Increasing the dosing interval further: monthly dosing
Ibandronate is the first nitrogen-containing oral bisphosphonate for osteoporosis that can be administered in a monthly regimen, and it is anticipated that this regimen may have a positive impact on adherence, and therefore ultimately on fracture prevention. Similarly to the other currently licensed oral bisphosphonates for postmenopausal osteoporosis, daily ibandronate has well documented clinical efficacy (CitationRiis et al 2001; CitationChesnut et al 2004; CitationMiller et al 2005). When given in an intermittent schedule (dosing interval >2 months), oral ibandronate also provides significant vertebral antifracture efficacy (relative risk reduction 50%, p=0.0006 versus placebo), which represents the first prospective demonstration of antifracture efficacy with a bisphosphonate administered less frequently than daily (CitationChesnut et al 2004). It is important to note that when the treatment-free interval is increased, the cumulative bisphosphonate dose must also be increased. Daily and intermittent ibandronate of the same cumulative dose provided comparable efficacy, however, similar to studies of daily and weekly bisphosphonates, a small, yet consistent, efficacy advantage was seen in favor of the daily regimen. Hence, in studies to evaluate a monthly regimen, oral doses beyond the cumulative monthly dose provided by the daily regimen were explored to compensate for the greater between-dose interval. As with the weekly regimens of alendronate and risedronate, a comparable vertebral antifracture profile for the monthly regimen of ibandronate with the daily regimen is inferred based on increases in BMD at all bone sites that are at least equivalent to those observed with the daily regimen (CitationMiller et al 2005; CitationReginster et al 2006). Trial data from the MOBILE (Monthly Oral Ibandronate In Ladies) study showed that monthly ibandronate increased lumbar spine BMD and decreased markers of bone turnover and that the 150 mg monthly regimen was superior to the daily regimen (CitationMiller et al 2005; CitationReginster et al 2006). After both 1 and 2 years of the study, the monthly regimen showed a good tolerability profile, similar to that of the daily regimen (CitationChesnut et al 2004).
Patient preference for a monthly regimen
Two robust clinical studies with almost 700 patients using patient surveys have demonstrated a strong patient preference for a monthly versus a weekly oral bisphosphonate regimen (CitationEmkey et al 2005; CitationHadji et al 2006). The BALTO (Bonviva ALendronate Trial in Osteoporosis) studies evaluated patients’ preference for monthly oral ibandronate or weekly oral alendronate. BALTO I (CitationEmkey et al 2005) and BALTO II (CitationHadji et al 2006) comprised two separate studies of identical design; both were 6-month, randomized, multicenter, two-sequence, open-label, cross-over studies conducted in bisphosphonate-naïve or -lapsed women with postmenopausal osteoporosis as determined by the treating physician. BALTO I (n=342) was a US only study, whereas BALTO II (n=350) included centers in the USA and Europe. Postmenopausal women were randomized to receive either monthly oral ibandronate (150 mg) for 3 months followed by weekly oral alendronate (70 mg) for 12 weeks, or weekly oral alendronate (70 mg) for 12 weeks followed by monthly oral ibandronate (150 mg) for 3 months. All patients were informed that both drugs are indicated for the treatment of osteoporosis. Patient preference and opinions on convenience were assessed using a subject-completed questionnaire.
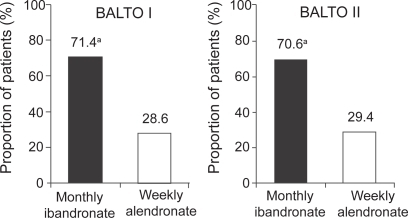
In BALTO I (CitationEmkey et al 2005), of those women who expressed a preference (92.6% of 298 participants), the majority (71.4%) preferred the monthly ibandronate regimen to the weekly alendronate regimen (p<0.0001; see Figure ). Similarly, in those patients expressing an opinion on convenience, the monthly regimen of ibandronate was assessed as being more convenient for patients than the weekly regimen of alendronate (74.6% vs 25.4%, respectively; p<0.0001; see Figure ). More women reported that the monthly regimen would be easier to follow for a long time (61% vs 25%), was better suited to their lifestyle (55% vs 21%), and was easier to tolerate (17% vs 4%) than the weekly regimen. In addition, patients indicated that there was a greater likelihood of long-term adherence and better tolerance of adverse events with the monthly regimen than the weekly regimen. The findings from BALTO I were confirmed by BALTO II. In BALTO II (CitationHadji et al 2006), of those patients expressing a preference (93.1%), the majority (70.6%) preferred the monthly ibandronate regimen to the weekly alendronate regimen (see Figure ). Again the difference in preference rate between weekly alendronate and monthly ibandronate was statistically significant (p<0.0001). Of the patients expressing an opinion on convenience, three-quarters found the monthly regimen more convenient than the weekly regimen (76.6% vs 23.4%, respectively; see Figure ). As in BALTO I, the most common reasons for preferring the monthly ibandronate regimen were ease of long-term adherence (81.5%) and better fit to lifestyle (75.4%).
Figure 3 The majority of patients expressing a preference prefer monthly bisphosphonate treatment to weekly (CitationEmkey et al 2005; CitationHadji et al 2006).
Figure 4 The majority of patients expressing an opinion perceived a monthly bisphosphonate regimen to be more convenient than a weekly regimen (CitationEmkey et al 2005; CitationHadji et al 2006).
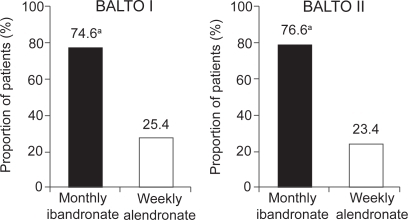
Note: Modified intent-to-treat populations = 298 (BALTO I), 321 (BALTO II); aPreference rate for monthly was significant in both studies (p<0.0001).

Patient preference for a medication may encourage therapeutic adherence, but further studies are needed to determine how well preference and convenience translate into prolonged adherence. The UK PERsistence Study of Ibandronate verSus alendronaTe (PERSIST) is the first trial to investigate persistence with 6 months of a monthly bisphosphonate regimen versus a weekly bisphosphonate regimen. Patients were randomized to receive either a monthly ibandronate regimen (plus a patient support program) or a weekly alendronate regimen (CitationCooper et al 2006). A patient support program is available to all patients who are prescribed monthly ibandronate in the UK, however, there is no equivalent support program available to patients who are prescribed weekly alendronate. Therefore, to reflect current UK practice, only patients randomized to the ibandronate arm were enrolled into the program. The 6-month data show that compared with alendronate there was a 47% relative improvement in the proportion of patients persisting with treatment in the ibandronate/patient support program group. This compares well with the reported relative improvements in 1-year persistence of 12%–29% with weekly versus daily dosing (CitationEttinger et al 2004; CitationPenning-van Beest et al 2004; CitationSunyecz et al 2004; CitationCramer et al 2005; CitationBartl et al 2006). Secondary endpoints, ie, proportion of patients remaining on treatment at study end and proportion of patients discontinuing from the study, were also in favor of the monthly regimen. The data from the UK PERSIST study suggest that this less frequent dosing schedule may provide improved adherence.
Conclusions
To achieve maximum treatment benefits for patients with osteoporosis, it is important that physicians discuss all options with their patients before a treatment choice is made. It has been shown that the majority of patients prefer weekly to daily therapy, although adherence with weekly regimens remains suboptimal. As adherence with daily or weekly bisphosphonates is suboptimal, patients and physicians feel there is a need for bisphosphonates with extended dosing intervals. Given the reported strong patient preference for a monthly regimen, and the impact of patient preference on adherence, it is anticipated that monthly ibandronate will offer patients an alternative convenient regimen that may improve adherence over weekly regimens and thereby enhance outcomes. The nitrogen-containing bisphosphonates are effective therapies for osteoporosis, but if patients are more likely to adhere to a monthly regimen than a daily or weekly regimen, then this may be the treatment of choice. An effective bisphosphonate that combines a good tolerability profile with a convenient dosing regimen would be beneficial for patients and it is important that physicians consider patient preference when prescribing the appropriate treatment for osteoporosis to ensure that the disease is effectively managed for the long-term benefit of the patient.
Acknowledgements
The author would like to thank Charlotte Kennerley (medical writer) for her editorial assistance.
References
- BaroutsouBBabiolakisDStamatiadouA2004Patient compliance and preference of alendronate once weekly administration in comparison with daily regimens for osteoporotic postmenopausal womenAnn Rheum Dis63Suppl 1455
- BartlRGoetteSHadjiP2006Persistence and compliance with daily- and weekly-administered bisphosphonates in German women with osteoporosisAnn Rheum Dis64Suppl 3364
- BlackDMCummingsSRKarpfDB1996Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research GroupLancet3481535418950879
- BrownJPKendlerDLMcClungMR2002The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalcif Tissue Int711031112085156
- CaroJJIshakKJHuybrechtsKF2004The impact of compliance with osteoporosis therapy on fracture rates in actual practiceOsteoporos Int151003815167989
- CenterJRNguyenTVSchneiderD1999Mortality after all major types of osteoporotic fracture in men and women: an observational studyLancet3538788210093980
- ChesnutCHSkagAChristiansenC2004Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res191241915231010
- ChrischillesEAButlerCDDavisCS1991A model of lifetime osteoporosis impactArch Intern Med1512026321929691
- CooperADrakeJBrankinE2006Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST studyInt J Clin Pract6089690516800837
- CooperCCampionGMeltonLJIII1992Hip fractures in the elderly: a world-wide projectionOsteoporos Int228591421796
- CowellWFulford-SmithAPoultneyS2005Adherence with bisphosphonate treatment for osteoporosis in UK patientsBone36Suppl 2S40910
- CramerJAAmonkarMHebbornA2004Assessing the relationship between bisphosphonate dosing regimen and treatment adherence among post-menopausal osteoporotic womenArthritis Rheum50SupplS294
- CramerJAAmonkarMMHebbornA2005Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosisCurr Med Res Opin2114536016197664
- CranneyAWellsGWillanA2002II. Meta-analysis of alendronate for the treatment of postmenopausal womenEndocr Rev235081612202465
- CummingsSRBlackDMThompsonDE1998Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention TrialJAMA2802077829875874
- CummingsSRMeltonLJ2002Epidemiology and outcomes of osteoporotic fracturesLancet3591761712049882
- DelmasPDVrijensBVan de LangerjitL2003Effect of reinforcement with bone turnover marker results on persistence with risedronate treatment in postmenopausal women with osteoporosis: improving the measurements of persistence on actonel treatment (IMPACT) studyCalcif Tissue Int72335
- EastellRGarneroPVrijensB2003Influence of patient compliance with risedronate therapy on bone turnover marker and bone mineral density response: the IMPACT studyCalcif Tissue Int72408
- EmkeyRKoltunWBeusterienK2005Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Bonviva Alendronate Trial in Osteoporosis (BALTO)Curr Med Res Opin21189590316368038
- EttingerMGallagherRAmonkarM2004Medication persistence is improved with less frequent dosing of bisphosphonates, but remains inadequateArthritis Rheum50SupplS513
- GoettschWGPenningFErkensJE2005Persistent bisphosphonate usage reduces the risk of hospitalizations for osteoporotic fracturesJ Bone Miner Res20Suppl 1S278
- HadjiPBenhamouCLDevasV2006Women with postmenopausal osteoporosis prefer once-monthly oral ibandronate to weekly oral alendronate: results of BALTO IIOsteoporos Int17Suppl 1S69
- HarrisSTSirisEAbbottT2005Reduced osteoporotic fracture risk in patients adherence to bisphosphonate therapyProgram & Abstracts of The Endocrine Society’s 87th Annual Meeting4–7 June 20053382
- HarrisSTWattsNBGenantHK1999Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupJAMA28213445210527181
- HodgsonSFWattsNBBilezikianJP2003American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003Endocr Pract95446414715483
- IOF2003Osteoporosis in the European community: action plan [online]. Accessed 1 September 2006. URL: http://www.osteofound.org/advocacy_policy/eu_policy_project/pdf/action_plan_03_e.pdf#search=%22Osteoporosis%20in%20the%20European%20community%3A%20action%20plan%22
- IOF2005IOF adherence gap report [online]. Accessed 1 September 2006. URL: http://www.osteofound.org/publications/pdf/adherence_gap_report.pdf
- JahngKHMartinLRGolinCE2005Preferences for medical collaboration: patient-physician congruence and patient outcomesPatient Educ Couns573081415893213
- JalavaTSarnaSPylkkanenL2003Association between vertebral fracture and increased mortality in osteoporotic patientsJ Bone Miner Res1812546012854835
- JanzNKWrenPACopelandLA2004Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decisionJ Clin Oncol223091815284259
- JohnellO1997The socioeconomic burden of fractures: today and in the 21st centuryAm J Med10320S5S9302894
- JohnellOKanisJAOdenA2004Mortality after osteoporotic fracturesOsteoporos Int15384214593451
- JohnellOOdenACaulinF2001Acute and long-term increase in fracture risk after hospitalization for vertebral fractureOsteoporos Int122071411315239
- KanisJAOdenAJohnellO2004Excess mortality after hospitalisation for vertebral fractureOsteoporos Int151081214598026
- KendlerDKungAWFuleihan GelH2004Patients with osteoporosis prefer once weekly to once daily dosing with alendronateMaturitas482435115207890
- LinPCampbellDGChaneyEE2005The influence of patient preference on depression treatment in primary careAnn Behav Med301647316173913
- LipsP2003Invest in your bones: quality of Life. Why prevent the first fracture? International Osteoporosis Foundation [online]. Accessed 1 September 2006. URL: http://www.osteofound.org/publications/pdf/quality_of_life.pdf#search=%22Invest%20in%20your%20bones%3A%20quality%20of%20Life.%20Why%20prevent%20the%20first%20fracture%3F%22
- LipsPCooperCAgnusdeiD1999Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for OsteoporosisOsteoporos Int101506010501796
- LopesPRozenbergSde GraafJ2001Aerodiol versus the transdermal route: perspectives for patient preferenceMaturitas38Suppl 1S31911390122
- MadhokRKerrHCapellHA2000Recent advances: rheumatologyBMJ321882511021871
- McClungMRGeusensPMillerPD2001Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study GroupN Engl J Med3443334011172164
- MeltonLJIII2000Who has osteoporosis? A conflict between clinical and public health perspectivesJ Bone Miner Res1523091411127196
- MeltonLJIII2003Adverse outcomes of osteoporotic fractures in the general populationJ Bone Miner Res1811394112817771
- MeltonLJIIIChrischillesEACooperC1992Perspective. How many women have osteoporosis?J Bone Miner Res71005101414493
- MillerPDMcClungMMacoveiL2005Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE studyJ Bone Miner Res2013152216007327
- NavesBaz-LopezJBGomezC2003The effects of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish populationOsteoporos Int14520412730754
- Penning-van BeestFGoettschWErkensJ2004Persistence with bisphosphonate therapy among post-menopausal osteoporotic women and the impact of dosing frequencyValue Health7724
- ReckerRRGallagherRAmonkarAA2004Medication persistence is better with weekly bisphosphonates, but it remains suboptimalJ Bone Miner Res19Suppl 1S172
- ReckerRRGallagherRMacCosbePE2005Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of womenMayo Clin Proc808566116007889
- ReginsterJYAdamiSLakatosP2006Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE studyAnn Rheum Dis656546116339289
- ReginsterJYMinneHWSorensenOH2000Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupOsteoporos Int11839110663363
- RiisBJIseJvon SteinT2001Ibandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosisJ Bone Miner Res161871811585352
- RossP1997Clinical consequences of vertebral fracturesAm J Med10330S42S9302895
- RussellRGRogersMJ1999Bisphosphonates: from the laboratory to the clinic and back againBone259710610423031
- SchnitzerTBoneHGCrepaldiG2000Therapeutic equivalence of alendronate 70mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study GroupAging (Milano)1211210746426
- SebaldtRJShaneLPhamB2004Longer-term effectiveness outcomes of non-compliance and non-persistence with daily-regimen bisphosphonate therapy in patients with osteoporosis treated in tertiary specialist careOsteoporos Int15Suppl 1S107
- SimonJABeusterienKMLeidyNK2005Women with postmenopausal osteoporosis express a preference for once-monthly versus once-weekly bisphosphonate treatmentFemale Patient30316
- SimonJALewieckiEMSmithME2002Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, cross-over studyClin Ther2418718612501880
- SimpsonSHEurichDTMajumdarSR2006A meta-analysis of the association between adherence to drug therapy and mortalityBMJ333151816790458
- SirisEHarrisSSilvermanS2005Adherence to bisphosphonates (BPs) is associated with reduced fracture risk in women with postmenopausal osteoporosis (PMO)Menopause12807
- SunyeczJAGallagherRSmithJC2004Weekly dosing with bisphosphonates has higher, but suboptimal days of therapy17th World Conference of Family Doctors (WONCA 2004)13–17 October 2004
- [WHO] World Health Organization2003bAdherence to long-term therapies: evidence for actionWorld Health Organization1211
- [WHO] World Health Organization2003aPrevention and management of osteoporosisWorld Health Organ Tech Rep Ser9211164