Abstract
Osteoporosis is a chronic condition that generally requires long-term therapy for fracture risk reduction to become apparent. Although the bisphosphonates have made a major contribution to how clinicians manage osteoporosis, compliance with therapy has generally been less in the real-world setting than seen in clinical trials. Less-frequently administered dosage regimens or nonoral routes may enhance compliance and so maximize the therapeutic benefit of bisphosphonates. Ibandronate is a nitrogen-containing bisphosphonate, whose high potency allows it to be administered orally or intravenously with extended dosing intervals. This paper will review the role of intravenous ibandronate in the treatment of postmenopausal osteoporosis.
Introduction
The bisphosphonates have made a major contribution to how clinicians manage osteoporosis and are regarded as first-line therapy. Their chemical structure comprises of two phosphate groups linked through a central carbon atom, with the various members of the class distinguished by the two side chains that bind to it. Two classes of bisphosphonates are distinguished on the basis of their side chains; those that contain a nitrogen atom (eg, alendronate, risedronate, ibandronate, and zoledronate) (), and those that do not (eg, etidronate and clodronate). Oral bisphosphonates are associated with poor adherence in the long term, possibly due to inconvenient dosing (CitationBauss and Russell 2004). Therefore, less-frequent, intermittent dosing may offer benefits for the patient and reduce fracture risk.
Figure 1 Generic chemical structure of a bisphosphonate and of ibandronate, risedronate, alendronate and zoledronate. In all cases R1 resembles a hydroxyl group while R2 is either an alkylamine (ibandronate and alendronate) or an acrylamine (risedronate and zoledronate). Copyright © 2004. Reproduced with permission from Springer Science and Business Media. Bauss F, Russell RG. 2004. Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int, 15:423–33.
Ibandronate is a nitrogen-containing bisphosphonate with a strong affinity for bone that potently inhibits osteoclast mediated bone resorption. Oral ibandronate is rapidly absorbed (tmax <1 hour), with low bioavailability (0.63%) that is further reduced in the presence of food by up to 90% (CitationBarrett et al 2004). Ibandronate has a wide therapeutic index and is not metabolized and therefore has a low potential for drug interactions (CitationBarrett et al 2004). Due to its metabolic stability, ibandronate is eliminated from the blood by partitioning into bone (40%–50%) and through renal clearance (approximately 60 mL/minute). The renal clearance of ibandronate is linearly related to creatinine clearance. The sequestration of ibandronate in bone (volume of distribution >90 L) results in a multiphasic elimination (t1/2 range of approximately 10–60 hours), characterized by the slow release of ibandronate from the bone compartment. The potency of ibandronate and its sequestration into bone allows it to be administered orally or intravenously, the latter as a bolus injection over 15–30 seconds, both with extended dosing intervals (CitationSchimmer and Bauss 2003). This review will focus on the intravenous (iv) administration of ibandronate and review its preclinical and clinical data.
Preclinical and nonosteoporotic clinical studies
Although some other currently available bisphosphonates can be administered by iv injection, slow infusions are generally required due to renal safety concerns. Indeed, acute renal failure has been observed with some bisphosphonates given by rapid iv administration or at high doses (CitationBounameaux et al 1983; CitationDumon et al 1991; CitationO’Sullivan et al 1994). However, unlike other bisphosphonates, ibandronate can be administered by an iv bolus injection without adversely affecting renal function. CitationPfister and colleagues (2003), have compared the potential for subclinical renal damage to accumulate to clinically relevant levels when minimally nephrotoxic doses of ibandronate (1 mg/kg) or zoledronate (1 mg/kg or 3 mg/kg) were given intermittently to rats, with a between-dose interval of 3 weeks or as a single dose by iv injection over 25 weeks. A single dose and intermittent dosing of ibandronate resulted in a similar incidence (one of six and two of six rats, respectively) and severity score (1.0 for both) of proximal tubular degeneration and single cell necrosis. Importantly, no accumulation of histopathological renal damage was observed. In contrast, intermittent dosing of zoledronate induced a higher incidence (six of six rats) and severity score (3.0) of histological renal damage compared with single dosing (four of six rats and 1.3 respectively).
The renal safety of ibandronate has also been demonstrated in human studies in the oncology setting with much higher doses than those used in osteoporosis (CitationPecherstorfer and Diel 2004). In clinical trials of patients with malignant hypercalcemia or metastatic bone disease due to breast cancer, 2 hour infusions of ibandronate at doses of up to 6 mg had a low potential for renal events (CitationPecherstorfer et al 2003), with a renal tolerability profile similar to placebo (CitationBody et al 2003, Citation2006; CitationLyubimova et al 2003). In pilot studies, repeated daily infusions of ibandronate (4 mg infused over 2 hours for 4 consecutive days or 6 mg infused over 1 hour for 3 consecutive days) for severe metastatic bone pain were not associated with renal toxicity (CitationPecherstorfer and Diel 2004). Studies of healthy volunteers and patients with metastatic bone disease related to breast cancer or multiple myeloma also confirmed the safety of single 15 minute infusions of 6 mg ibandronate (CitationNeugebauer et al 2001; CitationBody et al 2004). In addition, the risk of renal dysfunction was not increased with single and rapid bolus injections of 2 mg or 3 mg ibandronate in patients with skeletal metastases (CitationBody et al 2003; CitationPecherstorfer and Diel 2004).
Intravenous ibandronate has been shown to protect against ovariectomy-related bone loss in animal studies. In rats, iv ibandronate inhibited bone resorption and increased bone mass in the tibial metaphysis without affecting mineralization (CitationMuhlbauer et al 1991). A study of 66 female cynomolgus monkeys, aged 9 years and older, was performed in which the monkeys were ovariectomized or sham operated. Intravenous bolus injections of ibandronate at doses of 10 μg/kg, 30 μg/kg, or 150 μg/kg or placebo were administered at 30 day intervals (corresponding to intervals of 3 months in humans), starting from the time of ovariectomy and followed for 16 months (CitationSmith et al 2003). Ovariectomy significantly decreased bone mass at the lumbar spine, proximal femur, femoral neck and radius, and increased bone turnover in a time-dependent manner. Ibandronate bolus injections administered at 30 μg/kg every 30 days prevented ovariectomy-induced osteopenia. Ovariectomy-induced increases in bone turnover (as determined by activation frequency, bone formation rate, and biochemical markers of bone turnover) were suppressed on treatment and bone mass, architecture (assessed by histomorphometry) and bone strength were preserved at clinically relevant sites (CitationSmith et al 2003). Strength was assessed by compression testing to failure of the first lumbar vertebra and the femur load to failure. Treatment with high-dose, iv bolus injections of ibandronate (150 μg/kg/dose) further increased bone mass and improved bone strength at both the spine and femoral neck, without adversely affecting bone quality. In contrast, treatment with a 10 μg/kg/dose only partially prevented the ovariectomy induced effects.
Clinical trials with iv ibandronate in osteoporosis
With regard to iv ibandronate, CitationThiébaud and colleagues (1997) first investigated its efficacy in postmenopausal osteoporosis in a dose-ranging, randomized, placebo-controlled study of 125 women (mean age, 64 years). The groups comprised placebo or ibandronate at doses of 0.25 mg, 0.5 mg, 1 mg, or 2 mg administered every 3 months and all patients received calcium supplementation. Lumbar spine bone mineral density (BMD) showed a small nonsignificant increase in the placebo group by 12 months (+0.85%). For ibandronate the increases in lumbar spine BMD were 2.4%, 3.5%, 3.7%, and 5.2% after 12 months in a dose-dependent order. There was no significant difference between ibandronate groups, but the increases were statistically significantly different from placebo for the 0.5 mg (p < 0.006), 1 mg (p < 0.004), and 2 mg (p < 0.001) groups and there was a clear trend favoring the 2 mg dose at 12 months (). There were no significant changes with ibandronate in femoral neck BMD compared with placebo, but total hip and trochanter BMD showed dose-dependent significant changes. The increases were 1.8% and 2.9% for total hip and 2.7% and 4.2% for the trochanter in the 1 mg and 2 mg groups, respectively. Urinary excretion of C-telopeptide and N-telopeptide decreased after 1 month in all ibandronate groups in a dose-dependent fashion (mean change: −16% placebo, −33% 0.25 mg, −41% 0.5 mg, −57% 1 mg, and −66% 2 mg), but there was a gradual increase towards baseline thereafter. However, for the 2 mg dose, by 3 months (just prior to the next injection), there was a persistent and significant reduction in these markers of bone resorption compared with placebo of about 40% (p < 0.003) (). It should be noted that the study did not extend beyond 12 months and the power of the study to show differences between groups was modest with only approximately 25 subjects in each group. Nevertheless, based upon these data, approval was given for a phase III fracture trial using the 0.5 mg and 1 mg doses over 3 years, but not the 2 mg dose. Importantly, subsequent studies discussed below confirm a superior BMD and marker response for the 2 mg dose, especially beyond 12 months.
Figure 2 Changes (% ± SEM) in lumbar spine BMD (L2–L4) before and 3, 6, 9, and 12 months after iv injection of ibandronate or placebo. The arrows indicate the time of iv injection of study drug. Copyright © 1997. Reproduced with permission from Elsevier. Thiébaud D, Burckhardt P, Kriegbaum H, et al. 1997. Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med, 103:298–307.
Abbreviations: BMD, bone mineral density; iv, intravenous; SEM, standard error of mean.
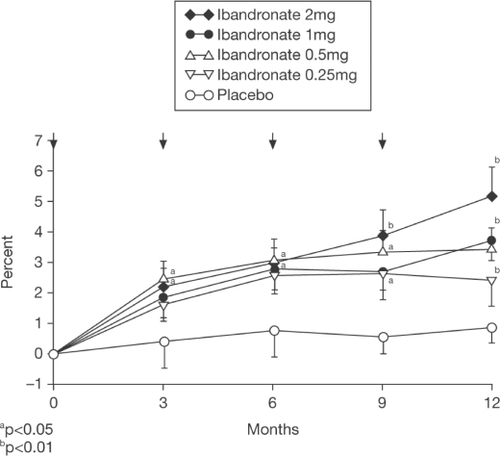
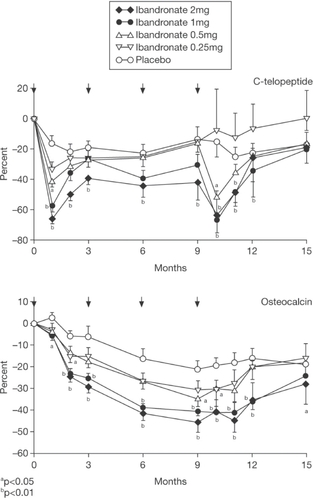
Figure 3 Changes (% ± SEM) in C-telopeptide in 2-hour fasting morning urine (upper panel) and serum osteocalcin (lower panel) before and 1, 2, 3, 6, 9, 10, 11, 12, and 15 months after iv injection of ibandronate or placebo. The arrows indicate the time of iv injection of study drug Copyright © 1997. Reproduced with permission from Elsevier. Thiébaud D, Burckhardt P, Kriegbaum H, et al. 1997. Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med, 103:298–307.
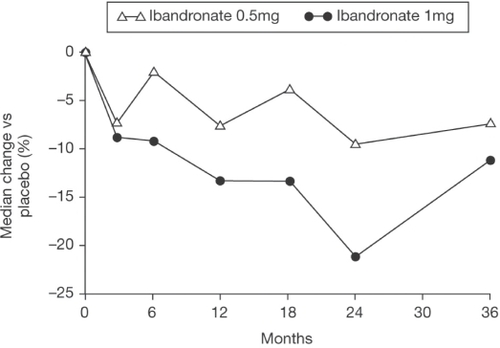
The subsequent phase III fracture trial was a double-blind, placebo-controlled, randomized study in 2862 women (aged 55–76 years) with postmenopausal osteoporosis defined by one to four prevalent vertebral fractures and a lumbar spine T-score between −2.0 and −5 in at least one vertebral body (CitationRecker et al 2004). The latter criterion is different to most other studies in postmenopausal osteoporosis which have usually required a T-score below −2 or −2.5 in the integrated lumbar spine BMD (ie, L1–L4 or L2–L4). All participants received daily vitamin D (400 IU) and calcium (500 mg) supplementation. At the two doses studied (0.5 mg and 1 mg quarterly), iv ibandronate produced dose-dependent, but comparatively small, increases in lumbar spine BMD (2.9% and 4.0%, respectively) over 3 years compared with placebo. At the total hip the increases were also modest at 2.3% and 3.6% compared with placebo, respectively. Similarly, decreases in biochemical markers of bone resorption, measured just before the next dose, were modest at 41.4% and 45.0% from baseline respectively and were 7.2% and 10.8% relative to placebo for serum concentration of the C-terminal collagen I telopeptide (sCTX) and importantly, as in the Thiébaud study (CitationThiébaud et al 1997), showed gradual recovery towards placebo values by 3 months ().
Figure 4 Median change versus placebo (%) in CTX/creatinine excretion with 1 mg, 0.5 mg ibandronate and placebo iv injections given once every 3 months. Copyright © 2004. Reproduced with permission from Elsevier. Recker RR, Stakkes-tad J, Chesnut C, et al. 2004. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone, 34:890–9.
The primary study endpoint was the incidence of new morphometric vertebral fractures at 3 years. Although a trend towards a reduction in the incidence of new vertebral fractures was observed in the active treatment arms compared with placebo (10.7%, 8.7%, and 9.2% in the placebo, 0.5 mg, and 1 mg groups, respectively), the magnitude of fracture reduction was insufficient to achieve statistical significance (CitationRecker et al 2004). In the per-protocol (PP) analysis, the fracture risk reduction approached statistical significance for the 1 mg dose (26% reduction, p = 0.0549). A similar, nonsignificant trend was seen in the incidence of nonvertebral and hip fractures but the study was not powered to address these endpoints.
It was concluded that optimal fracture efficacy most likely required more substantial increases in BMD, as well as more pronounced suppression of bone turnover and that this required higher iv doses of ibandronate. The results of a subsequent phase II/III study, IRIS (Intermittent Regimen Intravenous Ibandronate Study), discussed below support this hypothesis.
A number of other studies of iv ibandronate were performed around this time. CitationStakkestad and colleagues (2003) studied the dose response and efficacy of iv ibandronate in a different population to previous studies, namely early postmenopausal women without osteoporosis, ie, this was a prevention study. Six-hundred and twenty-nine women (mean age 54.75 years) within 10 years of their menopause (mean duration 4.3 years) were enrolled into this multicenter, double-blind, placebo-controlled trial. Patients were stratified according to time since menopause (less than or more than 3 years) and baseline lumbar spine BMD (normal or osteopenic) and were randomly allocated to receive iv ibandronate 0.5 mg, 1 mg or 2 mg, or placebo every 3 months as well as daily calcium supplementation. The study was intended to run for 2 years but stopped after 1 year when the results of the phase 3 fracture study (CitationRecker et al 2004) showed suboptimal efficacy with the 0.5 mg and 1 mg doses. The 12-month data showed a dose-dependent gain in lumbar spine BMD from baseline of 2.5%, 1.8%, and 1.0% in the groups receiving 2 mg, 1 mg, and 0.5 mg ibandronate, respectively, compared with a loss of BMD of 0.4% in the women in the placebo group (p = 0.0001 for each ibandronate dose vs placebo). The largest BMD gains occurred in women with osteopenia receiving 2 mg ibandronate. Similarly, at the hip, all three doses of ibandronate produced significantly better gains in BMD than placebo (p < 0.05), with the greatest gains in the women with osteopenia receiving the 2 mg dose. Intravenous ibandronate concomitantly and dose-dependently suppressed markers of bone turnover in comparison with placebo. sCTX and urinary CTX/creatinine were reduced by 42% and 50% after 12 months for patients receiving 2 mg ibandronate every 3 months compared with 9% and 7% for patients receiving placebo plus calcium, respectively. Myalgia was reported in 22% of patients receiving the 2 mg ibandronate dose compared with 4% in the placebo group.
CitationRinge and colleagues (2003) investigated the use of iv ibandronate in patients with glucocorticoid-induced osteoporosis in an open-label study. A total of 115 participants, all receiving at least 7.5 mg prednisone daily, were assigned to receive daily calcium supplements (500 mg) plus either quarterly ibandronate (2 mg) injections or daily oral alfacalcidol (1 μg) for 3 years. Intravenous ibandronate produced significantly greater increases in mean BMD at the lumbar spine (13.3% vs 2.6%, respectively; p < 0.001), and femoral neck (5.2% vs 1.9%, respectively; p < 0.001) than daily oral alfacalcidol, at 3 years, relative to baseline. It is worth noting that these increases in BMD are substantially greater than might be expected based upon previous studies in postmenopausal osteoporosis, but no explanation was provided. Biochemical markers of bone turnover were not measured. Despite the study not being powered for fracture, at 3 years the incidence of patients with new vertebral fractures was significantly lower in the patients receiving ibandronate relative to those taking alfacalcidol (8.6% vs 22.8%, respectively; p = 0.043). Patients treated with iv ibandronate also experienced less back pain (p < 0.001) and height loss (p = 0.001) than those receiving oral alfacalcidol. Despite the use of corticosteroids in all subjects, which might be expected to modify expression of side effects, a higher incidence of myalgia/arthralgia was observed in the ibandronate group (7.0% and 13.8%, respectively), although this adverse event was generally mild, temporary, and manageable, and was mostly seen in the first 6 months of treatment. It was concluded that intermittent iv ibandronate injections are efficacious and well tolerated in glucocorticoid-treated patients.
The above two studies had BMD as their primary endpoint but other studies have addressed the effects of iv ibandronate primarily on bone turnover. For example, CitationChristiansen and colleagues (2003) studied bone turnover in 73 healthy postmenopausal women following iv ibandronate. The study had three groups, either no treatment or an iv injection of 1 mg or 2 mg ibandronate on days 0 and 84, with follow-up to 168 days. Bone turnover was assessed by sCTX and osteocalcin at 19 consecutive timepoints. Mean sCTX decreased rapidly reaching a nadir 7 days after ibandronate. Maximal changes from baseline in the 1 mg and 2 mg ibandronate groups were −81% and −90%, respectively (p < 0.001). Two weeks after drug administration, sCTX started to rise in both treatment groups, reaching −16% and −20% below baseline by day 84, ie, immediately before the second administration. In contrast, osteocalcin showed a slower but progressive decrease over time reaching a nadir at −35% inhibition after 5 months. The authors interpreted this study as indicating that the relatively short inhibition of bone resorption in comparison with bone formation may explain its lack of efficacy in the phase III trial (CitationRecker et al 2004), although the physiological relevance of the relative changes in formation versus resorption markers remains unclear. Moreover, it is worth noting that although the study was randomized, it was not double-blind, had relatively low power (sample size 23 or 25 in each group) and only relatively short follow-up (6 months).
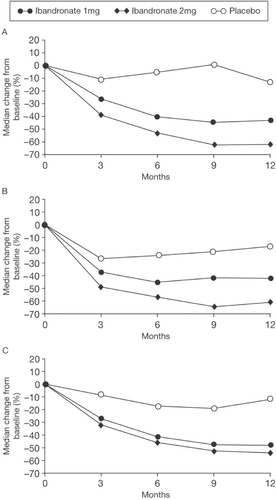
Returning to BMD endpoints, the IRIS study examined the dose-response relationship with intermittent iv ibandronate injections in 520 postmenopausal osteoporotic women (aged 55–75 years, >5 years since menopause) (CitationAdami et al 2004). Participants were randomized to receive either quarterly 2 mg (n = 261) or 1 mg (n = 131) ibandronate or placebo (n = 128) iv injections. After 1 year, ibandronate therapy produced significant and dose-dependent increases in lumbar spine and hip BMD and decreases in biochemical markers of bone turnover. Lumbar spine BMD increased by 2.8% and 5.0% in the 1 mg and 2 mg groups, respectively and decreased by 0.04% in the placebo group (). Similarly, total hip BMD increased by 2.2%, 2.9%, and 0.6%, in the corresponding groups, respectively. These BMD data are remarkably similar to that reported in the Thiébaud study (CitationThiébaud et al 1997). Serum and urinary CTX, reflecting bone resorption, decreased by 62.5% and 61%, respectively, with the 2 mg dose, and by 43.5% and 42%, respectively, with the 1 mg dose (). It was noted that the IRIS study reported a greater magnitude of suppression of biomarkers than in the CitationThiébaud (1997) or CitationChristiansen (2003) studies. An explanation for these differences included the reporting of mean values in the latter two studies as opposed to median values and the lack of use of calcium supplementation in the CitationChristiansen study (2003). It was concluded that the IRIS study findings demonstrate that the 2 mg dose provided significantly greater efficacy, using these surrogates, than the 1 mg dose. Intravenous ibandronate was well tolerated with a similar incidence of adverse events to placebo and no indication of renal toxicity. In summary, a clear dose-response relationship was again observed and this study showed that the 2 mg ibandronate regimen provided significantly greater BMD increases and significantly greater suppression of bone resorption markers than the 1 mg dose used in the previous phase 3 fracture prevention study.
Figure 5 Mean (SD) change (%) from baseline in lumbar spine (L1–L4) BMD; ITT analysis. Copyright © 2004. Reprinted with permission from Elsevier. Adami S, Felsenberg D, Christiansen C, et al. 2004. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone, 34:881–9.
Abbreviations: BMD, bone mineral density; ITT, intention-to-treat; SD, standard deviation.
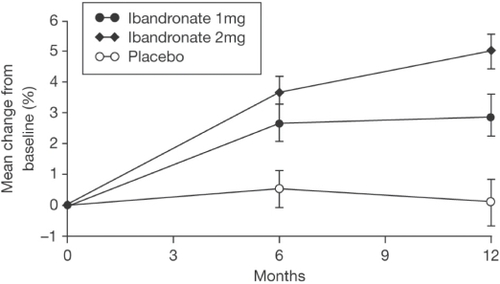
Figure 6 Median change (%) from baseline in serum CTX (A), urinary CTX/creati-nine (B), and serum osteocalcin (C); ITT analysis. Copyright © 2004. Reprinted with permission from Elsevier. Adami S, Felsenberg D, Christiansen C, et al. 2004. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone, 34:881–9.
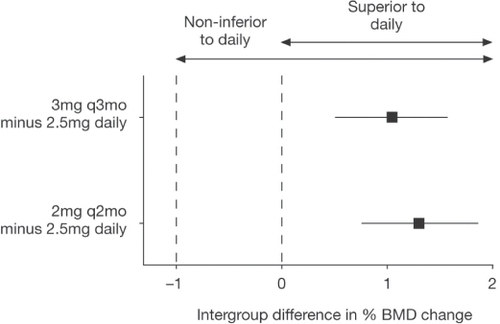
As noted above, ibandronate has been studied in phase III trials with daily and intermittent oral and iv formulations. Although this review deals primarily with iv ibandronate, it is important to note that in a study of 2946 women with postmenopausal osteoporosis, oral ibandronate, either continuously (2.5 mg/day) or intermittently (20 mg given every other day for the first 24 days, followed by 9 weeks without active drug) produced changes in BMD comparable with those found with oral alendronate or risedronate. In the primary endpoint of this study, oral ibandronate reduced vertebral fracture risk at 3 years by 62% with daily therapy and 50% with intermittent therapy (CitationChesnut et al 2004). A subsequent randomized, double-blind, double-dummy, phase III, noninferiority study (DIVA) was performed with two regimens of intermittent iv injections of ibandronate (2 mg every 2 months and 3 mg every 3 months) compared with 2.5 mg daily oral ibandronate. The latter was chosen as a comparator because of its proven antifracture efficacy (CitationChesnut et al 2004). The cumulative dose over 12 months with both ibandronate regimens was the same, ie, 12 mg, but study of a lower dose with shorter dosing interval (2 mg every 2 months) as well as the 3-monthly dose was to address the issue of recovery of bone turnover markers observed in the previous CitationThiébaud (1997) and CitationRecker (2004) studies. The DIVA study group comprised 1395 women (aged 55–80 years) who were at least 5 years postmenopausal, with osteoporosis (defined as a lumbar spine BMD T-score <–2.5). Participants also received daily calcium (500 mg) and vitamin D (400 IU). The primary endpoint was change from baseline in lumbar spine BMD at 12 months. Changes in hip BMD and in the level of sCTX were also measured, as were safety and tolerability. At 12 months, mean lumbar spine BMD increased by 5.1% in 353 patients receiving 2 mg ibandronate every 2 months, 4.8% in 365 patients receiving 3 mg ibandronate every 3 months and 3.8% in 377 patients receiving 2.5 mg daily oral ibandronate () (CitationDelmas et al 2006). This was a noninferiority study following the same design as the bridging studies for weekly alendronate and risedronate (CitationSchnitzer et al 2000; CitationBrown et al 2002). Both of the ibandronate regimens were not only noninferior, but also superior (p < 0.001) to the daily oral regimen () (CitationDelmas et al 2006). Hip BMD increased at all sites and was greater in the groups receiving iv ibandronate than in the group receiving daily oral ibandronate. Large decreases in the sCTX level were observed in all arms of the study and were similar in all three groups at 6 and 12 months (). Neither of the iv regimens affected renal function, but there was a higher incidence of flu-like illness in the iv ibandronate groups (5.1% and 4.9% in the 2-monthly and 3-monthly groups) versus 1.1% in the daily oral group but this mostly occurred at the time of the first injection.
Table 1 Change (%) from baseline in sCTX levels in the PP population (CitationDelmas et al 2006)
Figure 7 Mean change from baseline in lumbar spine and proximal femur BMD after 1 year in the PP population. Bars show the 95% CI (CitationDelmas et al 2006).
Abbreviations: BMD, bone mineral density; CI, confidence interval; PP, per-protocol; q2mo, every 2 months; q3mo, every 3 months.
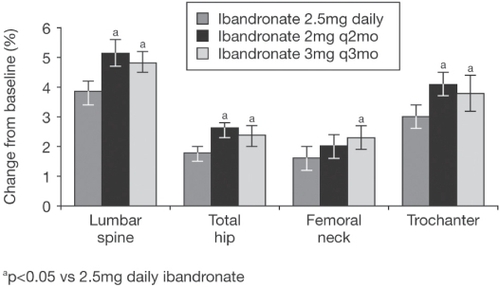
Figure 8 Noninferiority analysis of mean change (%) from baseline in lumbar spine (L2–L4) BMD after 1 year in the PP population. Squares and horizontal lines show the mean difference (and 95% CI) between each group receiving iv medication and the group receiving oral medication (expressed as iv minus oral) (CitationDelmas et al 2006).
The 2 year results of the DIVA study confirm that as assessed by BMD and markers, iv ibandronate is at least as effective as the oral regimen of 2.5 mg daily, which has proven antifracture efficacy (CitationEisman et al 2006). Since 3-monthly dosing is likely to be more convenient than 2-monthly dosing, 3 mg quarterly is the dosing regimen approved for use in the US and Europe.
Conclusion
Intravenous ibandronate at a quarterly dose of 3 mg is effective in normalizing bone turnover and increasing BMD at clinically relevant sites in women with postmenopausal osteoporosis with effects on these surrogate markers that are equivalent to or superior to those seen with daily oral therapy which has proven antifracture efficacy. Although iv administration is associated with a higher incidence of flu-like illness, this is usually mild and settles after the first injection. Administration of iv ibandronate may avoid the problems of poor adherence in those subjects intolerant of oral bisphosphonates and provides a further option for treatment of osteoporosis.
References
- AdamiSFelsenbergDChristiansenC2004Efficacy and safety of ibandronate given by intravenous injection once every 3 monthsBone34881915121020
- BarrettJWorthEBaussF2004Ibandronate: a clinical pharmacological and pharmacokinetic updateJ Clin Pharmacol449516515317823
- BaussFRussellRG2004Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosingOsteoporos Int154233315205712
- BodyJJDielIJLichinitserMR2003Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastasesAnn Oncol14139940512954579
- BodyJJDielIJTripathyD2006Intravenous ibandronate does not affect time to renal function deterioration in patients with skeletal metastases from breast cancer: phase III trial resultsEur J Cancer Care15299302
- BodyJJLichinitserMAndreevaN2004Safety of an intravenous (i.v.) dose of ibandronate followed by daily oral dosing in metastatic bone disease: Results of an open-label studyJ Clin Oncol2260S
- BounameauxHMSchifferliJMontaniJP1983Renal failure associated with intravenous diphosphonatesLancet14716131186
- BrownJPKendlerDLMcClungMR2002The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalcif Tissue Int711031112085156
- ChesnutCHSkagAChristiansenC2004Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res191241915231010
- ChristiansenCTankoLBWarmingL2003Dose dependent effects on bone resorption and formation of intermittently administered intravenous ibandronateOsteoporos Int146091312830369
- DelmasPDAdamiSStrugalaC2006Intravenous ibandronate injections in postmenopausal osteoporosis: 1-year findings from the DIVA studyArthritis Rheum5418384616729277
- DumonJCMagritteABodyJJ1991Efficacy and safety of the bisphosphonate tiludronate for the treatment of tumor-associated hypercalcemiaBone Miner15257661773138
- EismanJGarcia-HernandezPOrtiz-LunaG2006Intermittent intravenous ibandronate injections are an effective treatment option in postmenopausal osteoporosis: 2-year results from DIVAOsteoporos Int17Suppl. 2S212[abstract P316SA].
- LyubimovaNVKushlinskyNELichinitserM2003Long-term treatment with intravenous ibandronate does not effect renal function in breast cancer patients with metastatic bone diseaseSupport Care Cancer11416[abstract A–107].
- MühlbauerRCBaussFSchenkR1991BM 21.0955, a potent new bisphosphonate to inhibit bone resorptionJ Bone Miner Res61003111838661
- NeugebauerGKoehlerWAkinkunmiL2001Influence of peak ibandronic acid concentrations after 6 mg i.v. administration with shortened infusion time (15 and 30 minutes) on renal safety in manProc Am Soc Clin Oncol20122A[abstract 486].
- O’SullivanTLAkbariACadnapaphornchaiP1994Acute renal failure associated with the administration of parenteral etidronateRen Fail16767737899588
- PecherstorferMDielIJ2004Rapid administration of ibandronate does not affect renal functioning: evidence from clinical studies in metastatic bone disease and hypercalcaemia of malignancySupport Care Cancer128778115372222
- PecherstorferMSteinhauerEURizzoliR2003Efficacy and safety of ibandronate in the treatment of hypercalcemia of malignancy: a randomized multicentric comparison to pamidronateSuppor Care Cancer1153947
- PfisterTAtzpodienEBaussF2003The renal effects of minimally nephrotoxic doses of ibandronate and zoledronate following single and intermittent intravenous administration in ratsToxicology1911596712965119
- ReckerRRStakkestadJChesnutC2004Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosisBone34890915121021
- RingeJDDorstAFaberH2003Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative studyOsteoporos Int14801714610641
- SchimmerRCBaussF2003Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studiesClin Ther25193412637110
- SchnitzerTBoneHGCrepaldiG2000Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study GroupAging (Milano)1211210746426
- SmithSYReckerRRHannanM2003Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeysBone32455512584035
- StakkestadJABenevolenskayaLIStepanJJ2003Intravenous ibandronate injections given every three months: a new treatment option to prevent bone loss in postmenopausal womenAnn Rheum Dis629697512972476
- ThiébaudDBurckhardtPKriegbaumH1997Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosisAm J Med1032983079382122