Abstract
Multiple myeloma (MM) is a B-cell malignancy characterized by clonal expansion of plasma cells within the bone marrow, the presence of a serum and/or urine monoclonal protein, lytic bone lesions, and anemia. On a cellular level, the disease is characterized by complex interactions between tumor cells and the surrounding bone marrow microenvironment. Understanding of the relationship between malignant plasma cells and the microenvironment has sparked ongoing efforts to develop targeted therapeutic agents for treatment of this disease. The successful development of the first-in-class small-molecule proteasome inhibitor bortezomib occurred as a result of these efforts. This review focuses on the rationale for bortezomib therapy in the treatment of patients with newly diagnosed and relapsed MM, important treatment-related side effects, and future directions for use of bortezomib and other, emerging proteasome inhibitors.
Introduction
Multiple myeloma (MM) is a hematologic malignancy of B-cell origin that constitutes approximately 1% of all malignant tumors and 10% to 15% of hematopoietic neoplasms.Citation1 In 2008, there were an estimated 19,920 new cases of MM in the United States and 10,690 deaths attributable to the disease.Citation2 It primarily occurs in older individuals, with an average age at diagnosis of 65,Citation3 and is associated with a number of important clinical manifestations including osteolytic bone lesions, renal failure, anemia, recurrent infections, neuropathy, and hypercalcemia. For over 40 years, corticosteroids and conventional chemotherapy provided the basis for MM therapy with such regimens as high-dose dexamethasone;Citation4,Citation5 melphalan and prednisone;Citation6 and vincristine, doxorubicin, and pulsed high-dose dexamethasone (VAD).Citation7–Citation11 High-dose therapy and autologous stem cell transplantation (ASCT) in MM was pioneered during the 1980sCitation12,Citation13 and has a well established role in the treatment of appropriately selected individuals with MM. Over the past decade, though, the therapy for MM has changed significantly with the introduction of the immunomodulatory drugs (IMiDs) thalidomide and lenalidomide as well as the proteasome inhibitor bortezomib. These agents target specific pathways within MM cells and the bone marrow microenvironment that have been identified and characterized through careful preclinical investigation. This review focuses specifically on the development and current applications of the first-in-class proteasome inhibitor bortezomib in the treatment of MM.
Mechanism of action
The ubiquitin-proteasome pathway plays an important role in intracellular protein homeostasis by regulating protein degradation. As such, it affects critical cellular processes such as cell cycle regulation, antigen processing, and apoptosis. Protein degradation occurs through a 2-step process in which proteins destined for removal first undergo ATP-dependent ubiquitination followed by a second step of proteolysis within the 26S proteasome, which consists of a proteolytic core, the 20S proteasome, surrounded by two 19S regulatory complexes.Citation14 The 20S proteasome possesses chymotrypsin-like, trypsin-like, and caspase-like catalytic activity. Proteasome inhibitors are classified as either reversible or irreversible and on the basis of their inhibition of chymotrypsin-like, trypsin-like, or caspase-like catalytic activity.
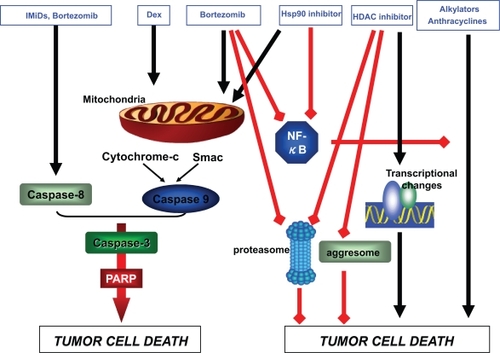
Bortezomib is a boronic acid dipeptide small molecule that reversibly inhibits the chymotrypsin-like activity of the 20S proteasome. The onset of bortezomib’s anti-MM activity is rapid, with apoptosis of MM cells occurring within several hours after exposure.Citation15 NF-κB is an important target of bortezomib within MM cells. Inhibition of NF-κB decreases adherence of MM cells to the bone marrow stromal cells, thus inhibiting paracrine-mediated growth of MM cells and enhancing susceptibility to therapeutic agents.Citation16 While inhibition of NF-κB provided the initial rationale for use of this agent in MM, it is now understood that bortezomib targets a number of other molecules and pathways within MM cells. For example, bortezomib-induced apoptosis of MM cells is associated with activation of caspase-8/9 and caspase-3.Citation15 Bortezomib also cleaves DNA repair enzymes, increasing the susceptibility of MM cells to classes of DNA-damaging agents such as alkylating agents and anthracyclines.Citation17,Citation18 In addition, the agent induces pro-apoptotic elements of the unfolded protein response such as PERK, ATF4, and CHOP/GADD153.Citation19,Citation20 Finally, IL-6- induced activation of ERK, STAT3, and AKT is inhibited by bortezomib via its ability to downregulate gp130.Citation21
In addition to these anti-tumor effects, bortezomib has important effects on the development and progression of MM-associated bone disease. Bone abnormalities such as osteoporosis, compression fractures, and lytic lesions are characteristic of MM and are present in approximately 80% of patients at the time of diagnosis.Citation22 MM cells secrete osteoclast activating factors such as RANKL, IL-3, macrophage inflammatory protein, and IL-6, while levels of the RANKL decoy receptor osteoprotegrin, which regulates osteoclast activity, are decreased in patients with the disease.Citation23 In addition, increased levels of osteoblast inhibitors dickhofp-1 (DKK1), IL-7, and IL-3 in MM diminish bone anabolism.Citation24 These events disrupt the balance of osteoblast and osteoclast function in favor of bone catabolism and contribute to the pathogenesis of MM-associated bone disease. Bortezomib antagonizes these processes in several ways. It decreases levels of RANKL and DKK1Citation25 and increases levels of alkaline phosphatase and osteocalcin, two markers of bone formation.Citation26 In addition, bortezomib appears to inhibit osteoclast differentiationCitation27 and augment osteoblast proliferation by inducing the differentiation of mesenchymal stem cells into osteoblasts.Citation28 The beneficial effects of bortezomib on bone disease in MM have also been demonstrated in vivo through studies utilizing murine models.Citation29,Citation30
Bortezomib-based treatment strategies
Relapsed MM
The significant anti-MM activity of bortezomib observed in preclinical studies provided the impetus for subsequent clinical development of the drug. Phase I and II studies involving patients with relapsed MM demonstrated a manageable toxicity profile and confirmed the activity of bortezomib in this setting.Citation31–Citation33 These efforts culminated in the international, multicenter phase III Assessment of Proteasome Inhibtion for Extending Remissions (APEX) trial, in which 669 patients with relapsed MM, more than 50% of whom had undergone two or more prior lines of therapy, were randomized to receive either bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each 21-day cycle for eight 3-week cycles, followed by treatment on days 1, 8, 15, and 22 for four 5-week cycles; or dexamethasone 40 mg on days 1–4, 9–12, 17–20 for four 5-week cycles, followed by treatment on days 1–4 for five 4-week cycles.Citation34 Bortezomib was superior to high-dose dexamethasone for overall response (OR) rate (38% vs 18%), complete response (CR) rate (6% vs 1%), median time to progression (TTP) (6.22 vs 3.49 months), and 1-year overall survival (OS) (80% vs 66%). Grade 3/4 treatment-related toxicities included thrombocytopenia (26%), neutropenia (14%), anemia (10%), peripheral neuropathy (7%), and diarrhea (7%). With extended follow up of APEX participants, the OR and CR rates among bortezomib-treated patients improved to 43% and 9%, respectively,Citation35 while the median OS was 29.8 months in the bortezomib arm compared to 23.7 months in the dexamethasone arm.
Demonstration in preclinical studies of synergy between bortezomib and other classes of agents such as corticosteroids, alkylating agents, and anthracyclines prompted evaluation of bortezomib-based combinations.Citation15,Citation17,Citation36 In the phase II Study of Uncontrolled Myeloma Management with proteasome Inhibition Therapy (SUMMIT) and Clinical Response and Efficacy Study of bortezomib in the Treatment of refractory myeloma (CREST) trials, patients with progressive disease after two cycles or stable disease after four cycles could receive oral dexamethasone 20 mg on the day of and day after bortezomib. In the SUMMIT trial, 13 (18%) of 78 patients with stable or progressive disease after several cycles of bortezomib monotherapy achieved a minimal or partial response with the combination.Citation32 Among CREST study participants, the OR rate among those who received the combination was 50%.Citation33
As predicted by preclinical models, combinations including bortezomib and anthracyclines have also been effective in relapsed and refractory MM. In a randomized, phase III trial involving 646 individuals with relapsed and refractory disease, 66% of whom had received two or more prior lines of therapy, treatment with bortezomib plus liposomal doxorubicin was superior to bortezomib alone in terms of median TTP (9.3 vs 6.5 months) and 15 month OS (76% vs 65%).Citation37 Although grade 3/4 toxicities such as anorexia, vomiting, thrombocytopenia, neutropenia, and hand-foot syndrome occurred more frequently with the doublet, cardiac toxicity was only minimally increased with the combination and rates of peripheral neuropathy (PN) were nearly equivalent. In a phase II study involving 64 heavily pretreated patients with relapsed and refractory MM, bortezomib, doxorubicin, and dexamethasone (PAD) produced a partial response (PR) or better in 67% and a very good partial response (VGPR) or better in 25%.Citation38 Frequent grade 3/4 toxicities included thrombocytopenia, neutropenia, infection, and peripheral neuropathy, and two patients experienced grade 3/4 congestive heart failure.
In addition to its sensitizing effect on corticosteroids and conventional classes of chemotherapeutic agents such as anthracyclines, bortezomib exhibits significant activity in combination with other novel agents in the treatment of relapsed and refractory MM. In a phase I/II study, bortezomib and thalidomide were administered to 85 patients with relapsed and refractory MM.Citation39 The dose range of bortezomib was 1.0–1.3 mg/m2 on days 1, 4, 8, and 11, while thalidomide was given starting with cycle two at doses of 50–200 mg/day. Dexamethasone 20 mg on the day of and day after bortezomib was added during cycle four for patients with less than a PR. A minor response (MR) or better occurred in 79% of study participants, while 63% achieved a PR or better. The most common grade 3/4 toxicities included thrombocytopenia and neutropenia. Although the cumulative incidence of PN with this combination has been approximately 60%, grade 3/4 PN has been infrequent and the neuropathy has, in many instances, been reversible.
Bortezomib plus lenalidomide, and dexamethasone (RVD) is also very active in relapsed and refractory MM. The rationale for this approach is derived from preclinical work demonstrating dual apoptotic signaling, with in vitro modeling suggesting the synergistic tumoricidal activity of bortezomib and lenalidomide.Citation40 In a phase II study, 64 patients with relapsed and refractory MM who had received 1–3 prior lines of therapy received bortezomib 1.0 mg/m2 days 1, 4, 8, and 11; lenalidomide 15 mg days 1–14, and dexamethasone 40 mg (cycles 1–4) or 20 mg (cycles 5–8) on days of, and after, bortezomib for up to eight 21-day cycles.Citation41 To date, the rate of MR or better in this study is 86%, with 24% of study participants achieving a CR/near CR (nCR) and 67% achieving a PR or better. Among patients who respond to therapy, the median duration of response (DOR) has been 21 weeks. Importantly, response rates have been equivalent among patients with standard risk features and those with high-risk disease characterized by advanced ISS stage and cytogenetic abnormalities. Toxicities have included grade 1–2 myelosuppression and two cases of deep vein thrombosis (DVT).
Newly diagnosed MM
Bortezomib has also proven effective in the treatment of patients with newly diagnosed MM on the basis of numerous clinical trials involving patients who are eligible for ASCT and those who are not. In a large, multicenter phase III trial involving 682 ASCT-ineligible patients with newly diagnosed MM, bortezomib plus melphalan and prednisone (VMP) was compared to MP alone.Citation42 All patients received melphalan 9 mg/m2 and prednisone 60 mg/m2 days 1–4 of each 6-week cycle, while bortezomib was administered in the VMP arm at 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1–4 and on days 1, 8, 22, and 29 during cycles 5–9. VMP was superior to MP in terms of the study’s primary endpoint of TTP (24 vs 16.6 months), as well as secondary endpoints CR rate (30% vs 4%) and DOR (19.9 vs 13.1 months). The hazard ratio for survival favored VMP to MP (0.61). Grade 3 toxicities were more common with VMP than MP (53% vs 44%), while grade 4 toxicities were equivalent (28% vs 27%). 13% of participants in the VMP arm experienced grade 3 PN, while one patient developed grade 4 PN.
A variety of bortezomib-based combinations have been utilized in the upfront treatment of individuals eligible for ASCT. In one of the largest of these trials, 480 patients with newly diagnosed MM were randomized to either VAD or bortezomib plus dexamethasone induction.Citation43 This was followed by a second randomization to two cycles of dexamethasone, cyclophosphamide, etoposide, platinum (DCEP), consolidation or not, prior to ASCT. In preliminary analysis, bortezomib plus dexamethasone was superior to VAD induction with respect to rates of VGPR or better (46.7% vs 18.6%) and CR/nCR (21.3% vs 8.3%), even among patients with an advanced ISS score and del(13). Importantly, the benefit of bortezomib-based induction in this trial persisted post-ASCT with respect to both VGPR or better (40.8% vs 28.8%) and CR/nCR (71.8% vs 51%). DCEP consolidation did not improve response rates in either treatment group. Although treatment-related PN occurred more frequently with bortezomib plus dexamethasone induction, the overall rate of therapy-related toxicities was equivalent. Stem cell collection was successful in 97% of patients who received bortezomib plus dexamethasone and 99% of those who received VAD.
In another large, phase III trial, 480 transplant-eligible patients with newly diagnosed MM were randomized to bortezomib plus thalidomide and dexamethasone (VTD) or thalidomide and dexamethasone (TD) alone. Patients in the TD arm received thalidomide 200 mg daily for days 1–63 with dexamethasone 40 mg daily on days 1–4 and 9–12 of each 21-day cycle. Patients in the VTD arm received the same dose/schedule of thalidomide, with bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle and dexamethasone 40 mg on the day of, and after, each dose of bortezomib.Citation44 Preliminary analysis presented at the 2008 American Society of Hematology (ASH) meeting demonstrated the superiority of VTD in comparison to TD with respect to OR rate (92% vs 78.5%), CR/ nCR rate (33% vs 12%), and VGPR or better (61% vs 30%). While the rate of ≥ grade 3 PN was higher in the VTD arm (9% vs 2.5%), the overall rate of serious adverse events was similar. A sufficient number of stem cells for up to two ASCT were collected in 91% of patients in the VTD group and 87% in the TD arm. Among patients who underwent ASCT, those who received VTD did better in terms of VGPR or better (75% vs 53%), CR/nCR (54% vs 29%), and CR rate (41% vs 20%). After 15 months of follow-up, progression-free survival (PFS) was superior among study participants who received VTD (93% vs 86%), while the 20-month overall survival rate was equivalent in the two arms.
In the phase III HOVON-65/GMMG-HD4 study, preliminary results of which were also presented at the 2008 ASH annual meeting, 833 transplant-eligible patients with newly diagnosed MM were randomized to either VAD or PAD induction followed by stem cell mobilization and either single or tandem ASCT.Citation45 This was followed by maintenance therapy with either thalidomide 50 mg daily (VAD arm) or bortezomib 1.3 mg/m2 every 2 weeks (PAD arm) for 2 years. In a preliminary analysis, PAD induction proved superior to VAD with respect to rates of OR (80% vs 64%), VGPR or better (41% vs 17%), and CR (5% vs 0%). The benefit of PAD over VAD induction persisted post-transplant, with superior OR (92% vs 77%) and CR (15% vs 4%) rates. In this initial analysis, bortezomib maintenance further improved the CR/nCR rate from 23% to 35%, suggesting that additional bortezomib-based therapy post-transplant deepens the overall response to therapy.
RVD is also highly effective as initial therapy for both transplant-eligible and transplant ineligible patients with newly diagnosed MM. In a phase I/II study of this combination, 68 patients have received the combination to date, 33 as part of the phase I dose-escalation phase and 35 in the phase II phase utilizing the maximum tolerated dose (MTD) of lenalidomide 25 mg and bortezomib 1.3 mg/m2.Citation46,Citation47 The OR rate is 100%, with 74% of patients achieving a VGPR or better and 44% achieving a CR/nCR. Among patients who received the MTD, the OR rate was 100%. It is notable that rates of response were not affected by such adverse prognostic features as del(13) and t(4; 14). Moreover, the regimen was well-tolerated, with low rates of both DVT/pulmonary embolism (5%) and grade ≥ 3 PN (3%).
Bortezomib-based therapy in unique patient populations
Coupled with advances in translational science, rapid drug development in MM has substantially expanded treatment options for patients. This is underscored by the most recent guidelines on MM therapy from the National Comprehensive Cancer Network (NCCN), which include multiple treatment regimens for both relapsed and refractory disease and newly diagnosed MM.Citation48 Various factors are considered in decisions on appropriate therapy for an individual patient, including the clinical/biological features of a patient’s disease, comorbid conditions, and mode of drug administration. Clinical experience with bortezomib indicates there are certain patients in whom the agent appears to confer unique benefits.
Bortezomib appears to overcome the poor prognosis associated with an elevated B2-microglobulinCitation49 and with chromosomal abnormalities such as 13q deletion and t(4; 14).Citation49–Citation52 The agent is thus an important component of therapy for individuals with high-risk MM on the basis of these prognostic factors. Bortezomib has also proven effective in the management of MM patients with renal dysfunction, a common manifestation of the disease noted at the time of diagnosis in approximately 30% of individuals.Citation22,Citation53 Based on the results of a case series involving MM patients with dialysis-requiring renal failure at the time of bortezomib therapy, the agent yields OR and CR rates in this population that are comparable to those seen among individuals without renal failure.Citation54 Moreover, four patients in this series became dialysis-independent as a result of bortezomib therapy. As discussed previously, bortezomib therapy is beneficial for individuals with significant disease-related bone disease due to its inhibitory effect on osteoclastogenesisCitation27 and stimulatory effect on osteoblast differentiation and proliferation.Citation28 Finally, MM patients with associated AL amyloidosis can be considered for bortezomib-based therapy on the basis of preliminary evidence of its efficacy in this setting. In a retrospective analysis of 20 patients with previously treated systemic AL amyloidosis who received bortezomib on a standard treatment schedule, 16 responded,Citation55 with amyloidotic organ function improved in 6 of the 16 patients who responded to therapy.
Treatment-related side effects
Optimization of therapeutic benefit with bortezomib use requires familiarity with, and appropriate management of, treatment-related side effects associated with the agent. Fatigue, diarrhea, PN, thrombocytopenia, and herpes zoster reactivation are among those that are monitored throughout the course of therapy.
Peripheral neuropathy
Initial symptoms associated with bortezomib-induced PN include pain involving the distal extremities along with sensory dysfunction resulting from small-fiber axonal injury. With progression of PN, proprioceptive loss, distal weakness of the upper and lower extremities, and suppression of deep tendon reflexes may occur.Citation56,Citation57 Among 256 patients enrolled in the phase II SUMMIT and CREST trials, 90 (35%) developed treatment-emergent PN or exacerbation of pre-existing PN.Citation58 The incidence of PN was dose-related, occurring more frequently at the 1.3 mg/m2 dose than 1.0 mg/m2, and peaked at cycle 5 with a cumulative dose of approximately 30 mg/m2. While the rate of bortezomib-associated PN was similar among patients with and without baseline PN, patients with pre-existing PN experienced more severe treatment-related symptoms. One or more courses of therapy was withheld as a result of treatment-associated PN in 19/90 (21%) patients, while dose reduction or discontinuation were required in 12% and 5%, respectively. Bortezomib-associated PN is reversible with treatment interruption in the majority of patients.Citation58,Citation59 Indeed, among SUMMIT and CREST study participants, symptoms improved to baseline in 71% of those who experienced ≥ grade 3 treatment-associated PN.
Various measures can be employed to prevent bortezomib-induced PN and manage symptoms when PN occurs. Alpha-lipoic acid, an organosulfur enzyme cofactor that possesses antioxidant properties and modulates glucose uptake, has been used for prevention of diabetic peripheral neuropathy and may be beneficial for MM patients who receive bortezomib, although this intervention requires further study in bortezomib-treated MM patients.Citation60 Similarly, acetyl-L-carnitine, an ammonium-containing compound derived from the amino acids methionine and lysine, has antioxidant and neurotrophic activity and appears to ameliorate chemotherapy-induced PN.Citation61 In addition, the regular application of thick emollients enriched with antioxidants and putative neurotransmitters, such as cocoa butter or topical menthol-containing preparations,Citation62 appears to benefit patients receiving bortezomib, perhaps by enhancing small fiber function and recovery. There is solid clinical rationale for these interventions, although prospective studies are needed to validate their efficacy in the context of bortezomib-based MM therapy.
Despite such strategies aimed at limiting the development or progression of bortezomib-induced PN, a significant number of MM patients require additional intervention. A prospective algorithm for bortezomib dose reductions as was derived from the SUMMIT and CREST trials and applied in the APEX study is recommended for individuals who receive the agent ().Citation34 Patients with pain or severe paresthesias may require symptom-directed pharmacotherapy. Options that have proven effective in this respect include the anticonvulsants gabapentin and pregabalin; serotonin-norepinephrine reuptake inhibitors such as duloxetine; tricyclic antidepressants amitriyptyline and desipramine; and opioids such as oxycodone, morphine sulfate, and hydrocodone.Citation63
Table 1 Dose modification guideline for bortezomib-related neuropathic pain and/or peripheral sensory or motor neuropathyCitation10
Thrombocytopenia
In the SUMMIT and CREST trials, therapy-associated thrombocytopenia occurred in 42% of study participants, and was the most frequently reported grade 3 adverse event.Citation64 Grade 3/4 thrombocytopenia was noted in 30% of study participants overall but importantly was uncommon (13%) in individuals with a baseline platelet count >200 × 109/L. Indeed, there was an inverse correlation between baseline platelet count and the incidence of grade 3/4 thrombocytopenia. Bleeding episodes associated with bortezomib-induced thrombocytopenia were infrequent, but can occur as a complication of therapy, albeit rarely. In one instance, gastrointestinal bleeding developed in the setting of grade 3 thrombocytopenia. In another, grade 1 epistaxis occurred in the context of grade 4 thrombocytopenia.
Although the mechanism by which bortezomib induces thrombocytopenia is unknown, murine studies suggest that it does involve a direct cytotoxic effect on megakaryocytes or alteration in thrombopoietin (TPO) levels.Citation64 During a standard 21-day treatment cycle, the platelet count typically follows a biphasic pattern, with a decline of on average 60% of the baseline platelet count during the 11-day period of bortezomib administration followed by recovery during the rest period.Citation64 The platelet count is thus monitored closely during treatment, with platelet transfusions if indicated and bortezomib dose reduction for high grade thrombocytopenia if it is persistent despite transfusion and if there is a concern regarding hemorrhage. The presence of baseline thrombocytopenia and concomitant use of agents known to cause myelosuppression – such as anthracyclines, alkylating drugs, and lenalidomide – necessitates caution.
Gastrointestinal side effects
Gastrointestinal (GI) side effects associated with bortezomib were common among participants in the APEX trial, with diarrhea and nausea occurring in 57%, constipation in 42%, vomiting in 35%, anorexia in 23%, and abdominal pain in 16%.Citation34 However, grade 3/4 bortezomib-associated GI toxicities were infrequent in this study. Pre-emptive supportive care measures are an important component of care in the management of MM patients receiving bortezomib. Corticosteroids, 5-HT3 receptor antagonists such as ondansetron and phenothiazines are effective antiemetics in this setting. Stool softeners, laxatives, and antidiarrheals are utilized in the management of constipation and/or diarrhea. Proton-pump inhibitors and/or H2-receptor blockers are employed in patients who, due to prolonged steroid use, are prone to gastritis or gastric/duodenal ulceration. At our institution, we have also documented GI dysmotility based on evaluation of gastric emptying in patients receiving bortezomib-based therapy. Promotility agents such as metoclopramide can be used in this situation, and attention to the avoidance of constipation is important. The International Myeloma Foundation (IMF) Nurse Leadership Board has also compiled comprehensive guidelines for the management of GI side effects in MM patients treated with novel therapies.Citation65
Herpes zoster reactivation
Herpes zoster virus (HZV) reactivation within nerve cell bodies results in a characteristic painful, vesicular, dermatomal rash. It most often occurs in immunocompromised individuals, who receive prolonged corticosteroid therapy or other immunosuppressive drugs, and among recipients of solid-organ or stem cell transplantation.Citation66 MM is associated with deficiency in both humoral and cellular immunity,Citation67 and as such predisposes affected individuals to infection. An increased incidence of HZV reactivation among MM patients has not been conclusively demonstrated but seems very likely based on available data. In the APEX trial, however, bortezomib therapy was associated with an increased incidence of herpes zoster reactivation as compared to high-dose dexamethasone (13% vs 5%, P = 0.0002).Citation34 The majority of these infections were grade 1/2 in severity, and the incidence of higher grade HZV reactivation was similar in the two treatment arms.Citation68 While the biological mechanism underlying the apparent association between bortezomib and HZV reactivation has not been elucidated, it does not appear related to a direct effect of bortezomib on T-cells,Citation69 mature lymphocytes,Citation70 or viral replication.Citation71 On the basis of evidence from the APEX study as well as our experience in treating patients with newly diagnosed disease, we routinely recommend antiviral prophylaxis using an agent such as acyclovir or valacyclovir.
Future directions
The successful use of bortezomib in MM has fueled interest in novel approaches to the application of this agent as well as in the development of new proteasome inhibitors. Combination therapy incorporating bortezomib together with emerging classes of drugs in MM are a key area of interest. As alluded to previously, the anti-MM mechanisms of bortezomib are synergistic with those of other drug classes (), and combination therapy improves response and survival rates in comparison to single-agent therapy. In particular, the high level of activity observed to date with bortezomib-based combinations such as VTD, PAD, and RVD has provided the impetus for new approaches to therapy incorporating emerging drugs in MM. For example, the combinations of bortezomib and the histone deacetylase (HDAC) inhibitor vorinostat,Citation72–Citation74 bortezomib and the heat shock protein 90 (Hsp90) inhibitor tanespimycin,Citation75 and bortezomib and the Akt pathway inhibitor perifosineCitation76 have been evaluated in early phase clinical trials involving patients with relapsed and/or refractory MM. These regimens have shown promising anti-MM activity, even among patients who previously were refractory to bortezomib, and the respective toxicity profiles have been favorable. As additional classes of drugs gain a foothold in MM therapy, it will be necessary to determine the efficacy of certain regimens, but also the sequence and schedule by which agents are administered.
Figure 1 Synergistic anti-MM activity of bortezomib in combination with other agents.
Abbreviations: Hsp90 inhibitor, heat shock protein 90 inhibitor; HDAC inhibitor, histone deacetylase inhibitor; IMiD, immunomodulatory drugs; MM, multiple myeloma; PARP, poly(ADP-ribose)polymerase; Smac, second mitochondria-derived activator of caspase.
Historically, ASCT in MM has served as a means by which to increase the depth of response achieved through induction therapy. Indeed, ASCT following induction has been a standard of care for younger MM patients eligible for the procedure and is associated with a PFS advantage as compared to induction therapy alone in this group of patients.Citation77 Whether induction regimens incorporating bortezomib and other novel agents, which are associated with unprecedented response rates in this disease, will improve the relative benefit of ASCT in MM is unknown. That response rates among patients who receive bortezomib-based induction improve further in the aftermath of ASCT suggests this may be true, but the issue needs to be definitively assessed in a randomized comparison of novel therapy-based induction with or without ASCT consolidation.
The use of bortezomib maintenance therapy following ASCT is also an area of considerable interest. The practice in MM of post-ASCT maintenance therapy using thalidomide has been established on the basis of results from four randomized clinical trials that suggest the approach improves PFS.Citation78–Citation80 Moreover, results from three of these trials indicate that thalidomide maintenance is associated with an OS benefit. Caution must be exercised with this approach, however, as there is evidence thalidomide maintenance after ASCT may be associated with shorter survival times following relapse.Citation78,Citation81 Whether post-ASCT maintenance with bortezomib, through continued inhibition of the proteasome, confers significant clinical benefit is unknown. This question is being addressed as part of the previously referenced phase III trial led by the HOVON group, in which transplant-eligible individuals with newly diagnosed MM receive either VAD or PAD induction followed by ASCT and subsequently by maintenance thalidomide (in the VAD arm) or bortezomib administered every other week (in the PAD arm).Citation45 As discussed previously, preliminary results indicate that bortezomib maintenance deepens the level of response to therapy. Updated analyses of this study are awaited with interest.
Finally, novel proteasome inhibitors are being developed with the aim of maintaining potent proteasome inhibition while modulating toxicity, specifically neurotoxicity, and altering bioavailability such that oral administration is possible. Two new proteasome inhibitors, carfilzomib (PR-171) and salinosporamide (NPI-0052), have to date undergone preclinical evaluation and are currently being assessed in early phase clinical trials involving MM patients with relapsed and/or refractory disease.Citation82–Citation84
Conclusions
As highlighted by this review, both bortezomib alone and in particular bortezomib-based combination therapy are important treatment options for individuals with newly diagnosed and relapsed MM. Bortezomib can be utilized safely in patients with renal dysfunction and has unique impact on bone disease frequently associated with MM. Moreover, the agent is effective in patients considered to have high-risk disease on the basis of chromosomal abnormalities and advanced ISS stage. The ability to safely and effectively partner bortezomib with other agents, both conventional and novel, is especially encouraging. In this context, treatment-associated toxicities associated with bortezomib are manageable with close monitoring and appropriate dose modifications as well as supportive care interventions. It is likely that with ongoing translational and clinical research efforts, patients with MM will, in the future, derive even greater benefit from bortezomib and the emerging second-generation proteasome inhibitors.
Disclosures
JP Laubach: Advisory Board Novartis Pharmaceuticals. CS Mitsiades: Consultant Millennium Pharmaceuticals, Novartis Pharmaceuticals, and Kosan Pharmaceuticals. RL Schlossman: Speakers Bureau Celgene Corporation and Millennium Pharmaceuticals. Nikhil Munshi: Speakers Bureau Celgene Corporation and Millennium Pharmaceuticals; Advisory Board Celgene Corporation, Millennium Pharmaceuticals, and Novartis Pharmaceuticals. IM Ghobrial: Speakers Bureau Celgene Corporation, Millennium Pharmaceuticals, and Novartis Pharmaceuticals; research support Celegene Corporation and Millennium Pharmaceuticals. KC Anderson: Advisory Board Celgene Corporation, Millennium Pharmaceuticals; consultant Celgene Corporation and Millennium Pharmaceuticals; research support Celgene Corporation and Millennium Pharmaceuticals. PG Richardson: Advisory Board Celgene Corporation, Millennium Pharmaceuticals; Speakers Bureau Celgene Corporation and Millennium Pharmaceuticals.
References
- McKennaRWKyleRAKuehlWMGroganTMHarrisNLCouplanRWPlasma Cell NeoplasmsSwedlowSHCampoEHarrisNLWHO Classification of tumors of haematopoietic and lymphoid tissuesLyonInternational Agency for Research on Cancer2008
- JemelASiegelRWardEHaoYXuJMurrayTCancer StatisticsCA Cancer J Clin2008582719618287387
- BarlogieBShaughnessyJEpsteinJPlasma Cell MyelomaLichtmanMABeutlerEKippsTJSeligsohnUKaushanskyKPrchalJTWilliams Hematology7th edNew YorkMcGraw-Hill2005 p.
- AlexanianRBarlogieBDixonDHigh dose glucocorticoid treatment of resistant myelomaAnn Intern Med198610518113717812
- GertzMAGartonJPGreippPRWitzigTEKyleRAA phase II study of high-dose methylprednisolone in refractory or relapsed multiple myelomaLeukemia1995912211521188609725
- OkenMMHarringtonDPAbramsonNKyleRAKnopseWGlickJHComparison of melphalan and prednisone with vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of multiple myeloma – Results of Eastern Cooperative Oncology Group Study E2479Cancer1997798156115679118039
- BarlogieBSmithLAlexanianREffective treatment of advanced multiple myeloma refractory to alkylating agentsN Engl J Med198431021135313566546971
- AndersonHScarffeJHRansonMVAD chemotherapy as remission induction for multiple myelomaBr J Cancer19957123263307841049
- GertzMAKalishLAKyleRAHahnRGTormeyDCOkenMMPhase III study comparing vincristine, doxorubicin (Adriamycin), and dexamethasone (VAD) chemotherapy with VAD plus recombinant interferon alfa-2 in refractory or relapsed multiple myeloma. An Eastern Cooperative Oncology Group studyAm J Clin Oncol19951864754808526187
- LokhorstHMMeuwissenOJBastEJDekkerAWVAD chemotherapy for refractory multiple myelomaBr J Haematol198971125302644970
- PhillipsJKSherlaw-JohnsonCPearceRA randomized study of MOD versus VAD in the treatment of relapsed and resistant multiple myelomaLeuk Lymphoma1995175–64654727549839
- BarlogieBHallRZanderADickeKAlexanianRHigh-dose melphalan with autologous bone marrow transplantation for multiple myelomaBlood1986675129813013516252
- BarlogieBAlexanianRDickeKAHigh-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myelomaBlood19877038698723304465
- MyungJKimKBCrewsCMThe ubiquitin-proteasome pathway and proteasome inhibitorsMed Res Rev200121424527311410931
- HideshimaTRichardsonPChauhanDThe proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cellsCancer Res20016173071307611306489
- HideshimaTChauhanDRichardsonPNF-kappa B as a therapeutic target in multiple myelomaJ Biol Chem200227719166391664711872748
- MitsiadesNMitsiadesCSRichardsonPGThe proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applicationsBlood200310162377238012424198
- HideshimaTMitsiadesCAkiyamaMMolecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341Blood200310141530153412393500
- ObengEACarlsonLMGutmanDMHarringtonWJJrLeeKPBoiseLHProteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cellsBlood2006107124907491616507771
- DongHChenLChenXDysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cellsLeuk Lymphoma200950697498419391038
- HideshimaTChauhanDHayashiTProteasome Inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myelomaOncogene200322528386839314627979
- KyleRAGertzMAWitzigTEReview of 1027 patients with newly diagnosed multiple myelomaMayo Clin Proc2003781213312528874
- LentzschSEhrlichLARoodmanGDPathophysiology of multiple myeloma bone diseaseHematol Oncol Clin North Am200721610351049viii17996587
- GiulianiNRizzoliVRoodmanGDMultiple myeloma bone disease: Pathophysiology of osteoblast inhibitionBlood2006108133992399616917004
- TerposEHeathDJRahemtullaABortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myelomaBr J Haematol2006135568869217107351
- TerposESezerOCroucherPDimopoulosMAMyeloma bone disease and proteasome inhibition therapiesBlood200711041098110417494860
- ZavrskiIKrebbelHWildemannBProteasome inhibitors abrogate osteoclast differentiation and osteoclast functionBiochem Biophys Res Commun2005333120020515936724
- MukherjeeSRajeNSchoonmakerJAPharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in miceJ Clin Invest2008118249150418219387
- DeleuSLemaireMArtsJBortezomib alone or in combination with the histone deacetylase inhibitor JNJ-26481585: effect on myeloma bone disease in the 5T2MM murine model of myelomaCancer Res200969135307531119531653
- PennisiALiXLingWKhanSZangariMYaccobySThe proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivoAm J Hematol200984161418980173
- OrlowskiRZStinchcombeTEMitchellBSPhase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignanciesJ Clin Oncol200220224420442712431963
- RichardsonPGBarlogieBBerensonJA phase 2 study of bortezomib in relapsed, refractory myelomaN Engl J Med2003348262609261712826635
- JagannathSBarlogieBBerensonJA phase 2 study of two doses of bortezomib in relapsed or refractory myelomaBr J Haematol2004127216517215461622
- RichardsonPGSonneveldPSchusterMWBortezomib or high-dose dexamethasone for relapsed multiple myelomaN Engl J Med2005352242487249815958804
- RichardsonPGSonneveldPSchusterMExtended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trialBlood2007110103557356017690257
- MaMHYangHHParkerKThe proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agentsClin Cancer Res2003931136114412631619
- OrlowskiRZNaglerASonneveldPRandomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progressionJ Clin Oncol200725253892390117679727
- PalumboAGayFBringhenSBortezomib, doxorubicin and dexamethasone in advanced multiple myelomaAnn Oncol20081961160116518326520
- Pineda-RomanMZangariMvan RheeFVTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myelomaLeukemia20082271419142718432260
- MitsiadesNMitsiadesCSPoulakiVMolecular sequelae of proteasome inhibition in human multiple myeloma cellsProc Natl Acad Sci U S A20029922143741437912391322
- RichardsonPJagannathSJakubowiakALenalidomide, bortezomib, and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma (MM): encouraging response rates and tolerabilitywith correlation of outcome and adverse cytogenetics in a phase II studyAmerican Society of Hematology Annual Meeting2008 Abstract 1742.
- San MiguelJFSchlagRKhuagevaNKBortezomib plus melphalan and prednisone for initial treatment of multiple myelomaN Engl J Med2008359990691718753647
- HarousseauJLMathiotCAttalMBortezomib/dexamethasone versus VAD as induction prior to autologous stem cell transplantation (ASCT) in previously untreated multiple myeloma (MM): updated data from IFM2005/01 trialAmerican Society of Clinical Oncology Annual Meeting2008 Abstract 8505.
- CavoMTacchettiPPatriarcaFSuperior complete response rate and progression-free survival after autologous transplantation with up-front velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly diagnosed multiple myelomaAmerican Society of Hematology, Annual Meeting2008 Abstract 158.
- SonneveldPVan der HoltBSchmidt-WolfIFirst analysis of HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, adriamycine, dexamethasone (PAD) vs VAD as induction treartment prior to high dose melphalan (HDM) in patients with newly diagnosed multiple myeloma (MM)American Society of Hematology Annual Meeting2008 Abstract 653.
- RichardsonPLonialSJakubowiakALenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: encouraging efficacy in high risk groups with updated results of a phase I/II studyAmerican Society of Hematology, Annual Meeting2008 Abstract 92.
- RichardsonPLonialSJakubowiakALenalidomide, bortezomib, and dexamethasone has notable activity in high-risk first-line myeloma. 2009 International Myeloma Workshop; 2009 Abstract 224.
- National Comprehensive Cancer NetworkNCCN clinical practice guidelines in oncology. Multiple Myeloma. v.2.2009 URL: www.nccn.org.
- RichardsonPGBarlogieBBerensonJClinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myelomaBlood200510692977298116020506
- JagannathSRichardsonPGSonneveldPBortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trialsLeukemia200721115115717096017
- ChangHTrieuYQiXXuWStewartKAReeceDBortezomib therapy response is independent of cytogenetic abnormalities in relapsed/refractory multiple myelomaLeuk Res200731677978216996589
- SagasterVLudwigHKaufmannHBortezomib in relapsed multiple myeloma: response rates and duration of response are independent of a chromosome 13q-deletionLeukemia200721116416817096015
- BladeJFernandez-LlamaPBoschFRenal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institutionArch Intern Med199815817188918939759684
- Chanan-KhanAAKaufmanJLMehtaJActivity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective studyBlood200710962604260617138816
- WechalekarADLachmannHJOfferMHawkinsPNGillmoreJDEfficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal diseaseHaematologica200893229529818245653
- ArgyriouAAIconomouGKalofonosHPBortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literatureBlood200811251593159918574024
- CavalettiGNobile-OrazioEBortezomib-induced peripheral neurotoxicity: still far from a painless gainHaematologica200792101308131018024368
- RichardsonPGBriembergHJagannathSFrequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomibJ Clin Oncol200624193113312016754936
- BadrosAGoloubevaODalalJSNeurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literatureCancer200711051042104917654660
- PackerLKraemerKRimbachGMolecular aspects of lipoic acid in the prevention of diabetes complicationsNutrition2001171088889511684397
- De GrandisDAcetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: a short reviewCNS Drugs200721Suppl 13943 discussion 5–617696592
- ColvinLAJohnsonPRMitchellRFleetwood-WalkerSMFallonMFrom bench to bedside: a case of rapid reversal of bortezomib-induced neuropathic pain by the TRPM8 activator, mentholJ Clin Oncol200826274519452018802169
- ClearyJFThe pharmacologic management of cancer painJ Palliat Med20071061369139418095817
- LonialSWallerEKRichardsonPGRisk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myelomaBlood20051063777378416099887
- SmithLCBertolottiPCurranKJenkinsBGastrointestinal side effects associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership BoardClin J Oncol Nurs200812Suppl 3375218490256
- ArvinAMVaricella-Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipientsBiol Blood Marrow Transplant20006321923010871147
- SchuttPBrandhorstDStellbergWPoserMEbelingPMullerSImmune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infectionsLeuk Lymphoma20064781570158216966269
- Chanan-KhanASonneveldPSchusterMWStadtmauerEAFaconTHarousseauJLAnalysis of herpes zoster events among bortezomib-treated patients in the phase III APEX studyJ Clin Oncol200826294784479018711175
- BlancoBPerez-SimonJASanchez-AbarcaLIBortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokinesBlood200610793575358316282346
- MasedaDMeisterSNeubertKHerrmannMVollREProteasome inhibition drastically but reversibly impairs murine lymphocyte developmentCell Death Differ200815360061218188168
- NeznanovNDragunskyEMChumakovKMDifferent effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replicationPLoS ONE200834e188718382670
- RichardsonPMitsiadesCColsonKPhase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myelomaLeuk Lymphoma200849350250718297527
- WeberDBadrosAZJagannathSVorinostat plus bortezomib for the treatment of relapsed/refractory multiple Mmyeloma: early clinical experienceBlood2008102322
- BadrosAPhilipSNiesvizkyRPhase I trial of suberoylanilide hydroxamic acid (SAHA) + bortezomib (bort) in relapsed multiple myeloma (MM) patientsAmerican Society of Hematology Annual Meeting2007 Abstract 1168
- RichardsonPGChanan-KhanALonialSTanespimycin (T) + bortezomib (bz) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulationAmerican Society of Hematology Annual MeetingBlood2007110 Abstract 1165.
- RichardsonPWolfJJakubowiakAPhase I/II results of a multicenter trial of perifosine (KRX-0401) + bortezomib in patients with relapsed or relapsed / refractory multiple myeloma who were previously relapsed from or refractory to bortezomibBlood2008102321
- KorethJCutlerCSDjulbegovicBHigh-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trialsBiol Blood Marrow Transplant200713218319617241924
- BarlogieBTricotGAnaissieEThalidomide and hematopoietic-cell transplantation for multiple myelomaN Engl J Med2006354101021103016525139
- AttalMHarousseauJLLeyvrazSMaintenance therapy with thalidomide improves survival in patients with multiple myelomaBlood2006108103289329416873668
- SpencerAPrinceHMRobertsAWConsolidation Therapy With Low-Dose Thalidomide and Prednisolone Prolongs the Survival of Multiple Myeloma Patients Undergoing a Single Autologous Stem-Cell Transplantation ProcedureJ Clin Oncol200927111788179319273705
- MorganGJJacksonGHDaviesFEMaintenance thalidomide may improve progression free but not overall survival; results from the myeloma IX maintenance randomisationAmerican Society of Hematology Annual MeetingBlood2008112 Abstract 656.17890457
- RichardsonPHofmeisterCCZimmermanTMPhase 1 clinical trial of NPI-0052, a novel proteasome inhibitor in patients with multiple myelomaBlood2008112 Abstract 2770.17890457
- VijRWangMOrlowskiRInitial results of PX-171-004, an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients with relapsed myeloma (MM)American Society of Hematology, Annual MeetingBlood2008112 Abstract 865.17890457
- JagannathSVijRStewartAKInitial results of PX-171-003, an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients with relapsed and refractory multiple myeloma (MM)Blood2008112 Abstract 864.17890457