Abstract
Erlotinib hydrochloride (Tarceva®) is a member of a class of small molecule inhibitors that targets the tyrosine kinase domain of the epidermal growth factor receptor (EGFR), with anti-tumor activity in preclinical models. Erlotinib represents a new-generation of agents known as “targeted therapies” designed to act upon cancer cells by interfering with aberrant specific activated pathways needed for tumor growth, angiogenesis and cell survival. Since its approval in November 2004 for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after the failure of at least one prior chemotherapy regimen and with a view to improving patients’ outcomes and prevent symptoms, the scientific community has evaluated the potential role of erlotinib in other scenarios such as in maintenance therapy and, in first-line setting for a selected population based on biological markers of response such as mutations of the EGFR. The convenient once-a-day pill administration and the good toxicity profile of erlotinib make it a reasonable candidate for testing in this context. This report provides a review of the role of erlotinib therapy in advanced NSCLC. It summarizes current data and perspectives of erlotinib in upfront treatment and maintenance for advanced NSCLC as well as looking at candidate biomarkers of response to these new targeted-agents.
Introduction
Erlotinib is a class of the newly named “targeted therapies”, designed to inhibit the epidermal growth factor receptor (EGFR) (). The small molecule was designed to bind to the ATP pocket of the intracellular tyrosine kinase domain of the EGFR, inhibiting the phosphorylation and thereby blocking the initiation of the intracellular cascade of transduction signals.Citation1,Citation2 The EGFR is part of a well-known member of the TK receptors family, with key functions in regulating proliferation, apoptosis, angiogenesis and metastasis, necessary to sustain cancer cells’ growth and progression in various solid tumors such as non-small-cell lung cancer (NSCLC).Citation3,Citation4 Erlotinib is indicated for the treatment of all subgroups of advanced NSCLC after failure of at least one prior chemotherapy regimen and for the treatment of patients with metastatic pancreatic cancer in combination with gemcitabine.Citation5,Citation6 This oral tyrosine kinase inhibitor (TKI) is also recommended in third-line treatment of advanced NSCLC after second-line chemotherapy failure.Citation7 More recently erlotinib has gained another indication and has been approved as maintenance treatment for patients with locally advanced or metastatic NSCLC whose disease has not progressed after 4 cycles of platinum-based first-line chemotherapy. Erlotinib, administered once a day orally, is very convenient for patients and associated toxicities are mild, the most common being skin rash and diarrhea (9% and 6% grade 3/4 respectively).Citation6
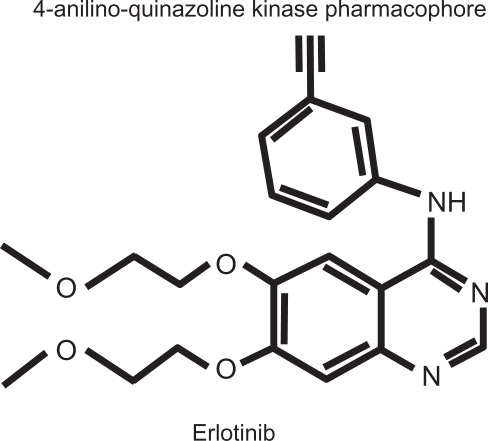
Figure 1 Erlotinib hydrochloride molecule: N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; C22H23N3O4.HCl; MW 429.90.
Lung cancer is the leading cause of cancer-related mortality; Citation8 the overwhelming majority of lung cancers, almost 80%, belong to the “non-small” major histotype subgroup and about 50% of the patients are presented with extensive disease at the time of diagnosis. During the last 2 decades, we have moved from the situation in which there was believed to be no effective treatment for this distinctive aggressive disease to one in which new targeted agents have been developed along with innovative biomarkers used to identify individuals that are more likely to benefit from these therapies. This shift started in the 1980s with the first randomized trials that demonstrated the benefit of cisplatin-based chemotherapy in terms of survival, quality of life (QoL) and relieved symptoms in advanced NSCLC patients.Citation9 Subsequently, the introduction of third-generation cytotoxic drugs to the platinum agents (known as doublets), including paclitaxel, docetaxel, gemcitabine and vinorelbine, gave rise to higher response rates (RRs) and longer overall survival (OS).Citation10 This led to platinum doublets becoming standard in first-line advanced NSCLC treatment. With these new generation agents in advanced NSCLC, we can predict RRs of 20% to 30%, with a median survival of 8 to 12 months and a 1-year survival rate of 30% to 40%Citation10. Since then, other trials have demonstrated the efficacy of new cytotoxic agents such as pemetrexed, a thymidylate synthase inhibitor,Citation11 and new targeted agents used in first-line setting combined with chemotherapy, such as bevacizumab an anti-vascular endothelial growth factor (VEGF) inhibitor or cetuximab, an EGFR inhibitor.Citation12–Citation14 Hence, the choice of optimal treatment for advanced NSCLC is no longer limited to the different platinum-based doublets.Citation15 Likewise, in the second-line setting, which represents a small part of the population (around 30%–50% according to different phase III trials) due to clinical deterioration,Citation11,Citation12,Citation14,Citation16 prognostic improvement has been also achieved: first with the approval of single agent, docetaxel, which proved to be superior to placebo in OS and symptoms controlCitation17 and more recently with the approval of pemetrexed and erlotinib,Citation18,Citation19 drugs with a more favorable toxicity profile than docetaxel. However, some questions remains to be answered, such as what is be best duration of the second-line treatment and which is the optimal time to introduce them. Recently, fresh information has been gathered on the treatment of advanced NSCLC. For the first time in NSCLC, there is evidence of distinct sensitivity to chemotherapy depending on the histological subtype.Citation11,Citation19,Citation20 It has been observed that in patients with squamous histology the efficacy of pemetrexed-platinum combinations is limited compared to gemcitabine combinations, whereas in non-squamous histology groups there is a benefit in OS with the addition of pemetrexed, leading the introduction of pemetrexed in first-line setting of advanced NSCLC with the indication restricted to the non-squamous subtypes.Citation11,Citation15 Second, the identification of molecular markers to guide the selection of specifically targeted types of therapy, ensuring better efficacy without unnecessary side effects. The most remarkable advance in this field has been the recognition of aberrant activation of the EGFR as a marker of response to TKI such as erlotinib.Citation21–Citation23
This review focuses specifically on erlotinib, a small molecule inhibitor of the EGFR tyrosine kinase, as part of the treatment for advanced NSCLC in two new scenarios: in the first-line setting, and as maintenance therapy continued beyond a first-line induction chemotherapy regimen. An overview of the pharmacogenomic properties of the drug in NSCLC, as well as candidate biomarkers for identifying subgroups of patients that benefit from erlotinib treatment, is also reviewed.
Pharmacogenomic properties of erlotinib in NSCLC
The EGFR family of TKs, referred to as the HER/ErbB family, consists of 4 members-EGFR (HER1/ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4)-that regulate many developmental, metabolic and physiological processes. The intracellular TK activity of EGFR is increased as a consequence of the binding of various ligands, which include EGF, transforming growth factor-α, amphiregulin, epiregulin and others, leading to the homodimerization of 2 EGFRs or the heterodimerization of EGFR with other family and non-family members including HER2, HER3 and insulin growth factor receptor 1 (IGFR-1R).Citation24,Citation25
The activation of TK receptor leads to the autophosphorylation of the intracellular domain of the EGFR, and the phosphotyrosine residues that are formed act as a docking site for various adapter molecules, resulting in the activation of the Ras/mitogen-activated protein kinase (MAPK) pathway, the PI3K/Akt pathway and signal transducers and activators of transcription signaling pathways.Citation26 In tumor cells, the TK activity of EGFR may be deregulated by various oncogenic mechanisms, including EGFR gene mutation, increased gene copy number and EGFR protein overexpression.Citation27 Improper activation of EGFR TK results in increased malignant cell survival, proliferation, invasion and metastasis.Citation28
Mutations in the EGFR have long been known to cause a constitutive, growth factor-independent activation of the EGFR downstream pathways and are frequently found in malignant diseases including brain tumorsCitation29 and NSCLC.Citation21–Citation23 The presence of these mutations in NSCLC correlates with responsiveness to reversible and irreversible TKIs. In a notable retrospective study, Shigematsu et al reported the genomic analysis of more than 2000 NSCLCs; EGFR mutations were found to be more common in adenocarcinoma (30%) than in lung cancers of other histologies (2%), and more frequent in lung cancer from never- (45%) than ever-smokers (7%).Citation30
A number of distinct alterations have been identified including point mutations within the nucleotide-binding loop in exon 18, small deletions in exon 19 or insertions in exon 20, as well as point mutations in the activation loop in exon 21. Structurally, these mutations cluster around the active site cleft of the TK domain. The two most frequent mutations are the exon 19 deletion that removes residues 746–750 of the expressed protein (48.2%) and the exon 21 point substitution that replaces leucine 858 with arginine – L858R (42.7%).Citation31,Citation32 The L858R substitution is the single most common mutation, and it lies in the activation loop (A-loop) of the kinase. Other point mutations are observed in glycine 719, although less frequently; Gly719 is found in the adjacent phosphate-binding P-loop of the kinase, and is substituted with serine, cysteine or alanine. The L858R and G719S point mutations, as well as the exon 19 deletions and exon 20 insertions, can transform both, fibroblasts and lung epithelial cells, in the absence of exogenous epidermal growth factor.Citation33–Citation36
Interestingly, the clinical correlation between the presence of specific mutations and therapeutic response to TKIs is mirrored in cell lines and EGFR-transfected cells. Cells bearing the mutant EGFR are in general more sensitive to TKIs than cells expressing the wild-type kinase. In particular, the L858R mutant is 10- to 100-fold more sensitive to erlotinib and gefitinib than the wild-type kinaseCitation22,Citation35,Citation37,Citation38 and significantly more sensitive than the G719S mutant.Citation39 At the same time, there are exceptions to this rule, for example the fact that the exon 20 mutants are highly resistant to both gefitinib and erlotinib further underscores the dependence of inhibitor responses on specific mutations.Citation40
Erlotinib structure is based on the 4-anilino-quinazoline kinase pharmacophore. Crystallography studies suggests that selective inhibitors of the EGFR bind to the ATP-binding pocket, with the aniline head group fitting into the selectivity pocket of EGFR.Citation41,Citation42 When examined in an in vitro enzyme analysis, erlotinib has shown comparable binding affinities (Ki) values against wild-type (3.86 nmol/L) and L858R mutant EGFR (4.76 nmol/L) and no significant differences in activity were found across an enzyme panel of more than 200 isolated targets (predominantly kinases).Citation43 In the same way, erlotinib showed a high correlation in growth inhibitory activity across a panel of 34 NSCLC cell lines (Pearson’s r = 0.975), including 3 cell lines harboring activating EGFR mutations. Similar activity was observed in the assessment of pharmacodynamic biomarkers of erlotinib activity that showed a high dose response relationship because of the inhibition of pEGFR, cell proliferation measured by inhibition of BrdU uptake, and apoptosis (annexin V labeling).Citation44
Erlotinib in first-line treatment of advanced NSCLC
Currently, screening for common EGFR mutations in patients with NSCLC can be performed in the clinical setting to predict which patients will respond to EGFR TKIs.Citation45,Citation46 A seminal work recently published by the Spanish Lung Cancer Group (SLCG) demonstrates the feasibility of large-scale screening of EGFR mutations and analyzed the association between this condition and clinical outcomes to erlotinib therapy.Citation46 From the analysis of more than 2000 NSCLC cases, mutations in the EGFR were found in 350 patients (16.6%). The mutations were detected more frequently in women, never-smokers, and in patients with adenocarcinoma (30%, 37.7% and 17.3%, respectively). Erlotinib was administered to 217 patients, of whom 113 received the TKI as first-line therapy and 104 as second- or third-line therapy. EGFR exon 19 deletions were detected in 135 cases, and the L858R point mutation in 82 tumors. The RR with erlotinib was 70.6%, 12.2% presented complete responses and a better outcome was associated with the exon 19 deletion than with the L858R mutation (odds ratio 3.08; P = 0.001).Citation46 This registry also reveals a median progression-free survival (PFS) of 14 months, a period that was similar between patients receiving first or second-line therapy. There were no significant differences in PFS according to performance status (PS), age, first vs second or third-line therapy, or smoking history. Median OS was 27 months and the multivariate analysis found that PS 1, male sex, the presence of the L858R mutation, brain metastases, and bronchioloalveolar adenocarcinoma were associated with poor prognosis.Citation46
Until now, there have been no published randomized trials of EGFR TKIs vs chemotherapy as first-line therapy for NSCLC patients from Western countries; however, 2 integrative studies of phase II trials support the findings of the SLCG and promote the role of erlotinib as first-line therapy for patients with NSCLC carrying EGFR mutations. Jackman et al pooled the data of 5 first-line phase II trials designed to prove the role of erlotinib or gefitinib monotherapy in patients in whom EGFR mutations were assessed.Citation47 However, patients were customized in only one study to receive gefitinib based on the presence of this genetic condition.Citation48 A total of 317 chemotherapy-naïve patients were treated with erlotinib or gefitinib, and tumor specimens from 223 of these patients were tested for EGFR mutations. Tumors from 84 selected patients were found to harbor a sensitizing EGFR mutation. Eighty-one percent of EGFR-mutant patients were women, 89% had adenocarcinoma, and 58% had no smoking history. Of the 84 patients harboring a sensitizing EGFR mutation treated with erlotinib or gefitinib, 67% achieved an objective response, with a median PFS of 11.8 months and a median OS of 23.9 months.Citation47 In contrast, for 83 patients with wild-type EGFR and wild-type Kras, the RR was 5%, the PFS was 3.1 months, and the OS was 11.8 months. Finally, in 41 patients with wild-type EGFR and mutated Kras, RR was 0%, PFS was 3.3 months, and OS was 13 months. Outcomes of the 84 patients with EGFR mutations were also compared according to the EGFR directed therapy; 56 and 28 patients received erlotinib and gefitinib, respectively. There were no significant differences in RR (erlotinib 70% and gefitinib 60%; P = 0.47), median PFS (erlotinib 13 months and gefitinib 11.4 months; P = 0.49), or OS (erlotinib 28.7 months and gefitinib 20.8 months; P = 0.10).Citation47
Following the same perspective Paz-Ares et al added the information from twelve trials of erlotinib (n = 365), 39 of gefitinib (n = 1069) and 9 trials that assessed the role of chemotherapy (375 patients) as first-line therapy for patients with EGFR mutations.Citation49 In the weighted pooled analysis, the overall median PFS was 13.2 months with erlotinib, 9.8 months with gefitinib, and 5.9 months with chemotherapy. Using a 2-sided permutation analysis, erlotinib and gefitinib produced a longer median PFS vs chemotherapy, both individually (P = 0.000 and P = 0.002, respectively) and as a combined group (EGFR TKI vs chemotherapy, P = 0.000).Citation49
More information is available from patients with EGFR mutations treated with TKIs in Asia; recently, Mok et al reported the final data of the IPASS study (Iressa Pan-Asia Study) that found a significant interaction between treatment and EGFR mutation with respect to PFS (P < 0.001).Citation50 This outcome was significantly longer among patients receiving gefitinib than among those receiving carboplatin/paclitaxel in the mutation-positive subgroup (hazard ratio [HR] 0.48; 95% confidence interval [CI] 0.36 to 0.64; P < 0.001) and significantly shorter among patients receiving gefitinib than among those receiving chemotherapy in the mutation-negative group (HR 2.85; 95% CI 2.05 to 3.98; P < 0.001). Similarly, results in the subgroup with unknown EGFR-mutation status were similar to those for the overall population.Citation50 These data were confirmed by a homologous multicenter phase II study which included 30 chemotherapy-naïve patients with poor PS who had EGFR mutations and received gefitinib alone. The overall RR was 66% (90% CI 51% to 80%), and the disease control rate was 90%. PS improvement rate was 79% (P < 0.00005) and the median PFS, median OS, and 1-year survival rate were 6.5 months, 17.8 months, and 63%, respectively.Citation51
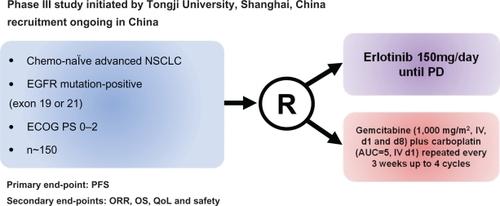
Morita et al evaluated and integrated 7 published phase II trials of gefitinib as a single first-line therapy for NSCLC patients with EGFR mutations treated in Asia and performed a pooled analysis based on individual data.Citation52 A total of 148 patients were included; 69% were women, 71% were never-smokers, and 97% have adenocarcinoma. The RR was significantly higher (79.3 vs 24.6%; P < 0.001), and PFS was longer (10.7 vs 6 months; P < 0.001) for patients receiving the TKI than in those receiving chemotherapy, whereas there was no significant difference in OS between the two groups of patients (27.7 vs 25.7 months).Citation52 Interestingly, the Cox regression analysis revealed that PFS after gefitinib treatment was significantly longer in the chemotherapy-naïve patients than those who had received previous chemotherapy. This result highlights the fact that first-line chemotherapy could have a detrimental effect on the later use of EGFR TKIs in NSCLC patients harboring EGFR mutations.Citation53 Currently there are 2 ongoing phase III trials, which aim to give more insights into the role of the erlotinib in the first-line setting of patients harboring EGFR mutations ( and ): the EURTAC trial (NCT00446225),Citation54 from European countries, and the OPTIMAL trial (NCT00874419) in Asia.Citation55 Both trials randomized chemotherapy-naïve NSCLC EGFR-mutant patients to receive erlotinib vs chemotherapy, PFS being the primary end-point in both studies.
Figure 2 Design of the phase III trial of erlotinib in first-line advanced NSCLC with EGFR mutations in Europe: the EURTACC trial.Citation54
Abbreviations: ECOG, Eastern Oncology Cooperative Group; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PD, progressive disease; QoL, quality of life.
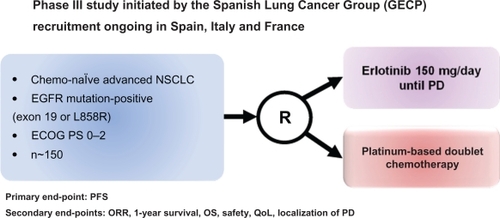
Figure 3 Design of the phase III trial of erlotinib in first line advanced NSCLC with EGFR mutations in Asian population: the OPTIMAL trial.Citation55
Abbreviations: ECOG, Eastern Oncology Cooperative Group; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; PD, progressive disease; PFS, progression-free survival; ORR, overall response rate; OS, overall survival; QoL, quality of life.
Role of erlotinib as maintenance therapy in advanced NSCLC
Erlotinib was the first new class of drugs against a specific molecular target directed to a TK that demonstrate single-agent activity in advanced NSCLC patients. Erlotinib was first evaluated in a randomized, double-blind, placebo-controlled trial with 731 patients with advanced NSCLC who had previously received one or more prior chemotherapy regimens (BR.21).Citation18 Patients were randomized to receive either oral erlotinib 150 mg/daily or a placebo. The primary end-point was OS that favor the group of patients treated with erlotinib (6.7 months vs 4.7 months; P < 0.001) with an adjusted HR of 0.70 (95% CI 0.58 to 0.85). The benefit in survival was consistent among the different patient subgroups based on sex, histology and smoking habits. However, patients never-smokers got more benefit than did smokers (HR 0.42 and 0.87, respectively).Citation56 RR and PFS were also significantly higher (P < 0.001) with erlotinib than with the placebo group (8.9% vs 1% and 2.2 vs 1.8 months respectively) and although the side effects were higher in the erlotinib arm, most adverse events were mild or moderate.Citation18 On the basis of data from the BR21 trial,Citation18 erlotinib was approved by the US Food and Drug Administration (FDA) in November 2004 for the treatment of all subtypes of advanced NSCLC after failure of at least one prior chemotherapy regimen.Citation6 At the moment, together with docetaxel and pemetrexed, erlotinib remains the standard of treatment in second-line advanced NSCLC. Nonetheless, one question remains unanswered; it is not yet known which of the three drugs approved in second-line treatment should be selected, unless the criterion of toxicity profile is applied. A randomized phase III biomarker validation study of second-line therapy of erlotinib versus pemetrexed in patients with advanced NSCLC (NCCTG-N0723) aims to shed some light on this issue.Citation57
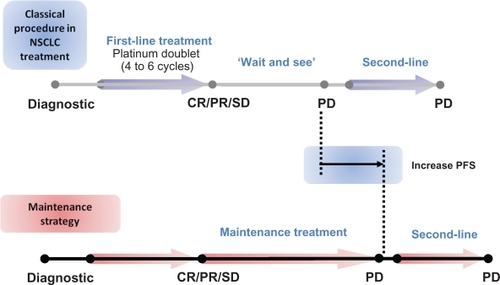
Currently, second-line treatment in advanced NSCLC is indicated if there is a relapse or disease progression after the first-line platinum-based combination and most patients who are treated with first-line chemotherapy will experience disease progression within 3 or 4 months.Citation10 The term “maintenance” therapy is usually used when one of the drugs used in the upfront combination treatment is maintained beyond the initial 4 to 6 cycles of chemotherapy, generally as first-line treatment, until disease progression or unacceptable toxicity (). The continuous administration of therapy after the recommended 4 to 6 treatment cycles is used with patients for whom the treatment has been demonstrated to be effective, ie, patients with a stabilization or tumor response with the up-front chemotherapy, which accounts around 75% of the treated population. There is some semantic controversy surrounding the use of the word “maintenance” and the switch to another non-cross resistant drug early after first-line therapy without progression is also referred to as “early second-line” or “consolidation” therapy. In advanced NSCLC, there has been a renewed interest in assessing both the duration of the up-front treatment as well as when to start the second-line treatment. It is worth mentioning that in advanced NSCLC, there is no standard approach to follow-up the tumor growth and consequently there is concern about our ability to detect disease progression before it impairs patient’s condition. When lung cancer patients experience tumor growth their physical condition promptly deteriorates due to the distinctive aggressive behavior of this disease, restricting the options for further treatments.Citation11,Citation12,Citation14,Citation16 Therefore the rationale of the maintenance therapy approach is to improve the outcomes, by maintaining the initial responses to the platinum-doublet therapy, as well as the QoL, by delaying cancer-related symptoms.
Figure 4 Current strategies to treat advanced NSCLC patients.
Abbreviations: NSCLC, non-small-cell lung cancer; PD, progressive disease; CR, complete response; PR, partial response; SD, stable disease.
Several old trials investigated the role of longer platinum-based combinations with old cytotoxic agents and failed to demonstrate a survival advantage.Citation58–Citation63 The idea of “the more chemotherapy the better” was finally laid to rest with a recent meta-analysis which corroborated the absence of a survival advantage in advanced NSCLC with the continuation of the same platinum-based chemotherapy beyond the standard 4 to 6 cycles in first-line setting.Citation64 The meta-analysis performed with PFS as end-point (n = 1907) showed the advantage in PFS with extending chemotherapy duration, more evident when a third-generation agent was used,Citation64 but it is worth noting that QoL analysis was not assessed in all the trials and in the few in which it was, a trend to worsening QoL, in terms of chemotherapy-related side effects, was observed with more chemotherapy.Citation62–Citation64 However, one has to bear in mind that toxicity profiles of the drugs used in these trials were worse than the ones related to new third-generation cytotoxic and targeted agents. Consequently the use of third-generation cytotoxic agents or targeted therapies with a better toxicity profile and more convenient administration are attracting interest, as they could avoid the development of cancer-related symptoms by controlling tumor growth without the undesirable toxicities associated with the long-term use of cytotoxic agents. Moreover, the use of molecularly targeted therapies (such as erlotinib) as maintenance raise the possibility of a more specifically molecular-guided tumor approach based on the distinctive biological features of each tumor.
The maintenance approach with targeted therapies was initially considered in trials that valued the role of the concurrent addition of new targeted agents to chemotherapy as a way of seeing whether they could improve outcomes in advanced NSCLC patients.Citation16,Citation65–Citation67 In these trials maintenance with the targeted agent after induction was allowed until unacceptable toxicity or disease progression set in, on the grounds that they would delay disease progression with a good tolerability profile (). The TRIBUTE trial,Citation65 a randomized, double-blind, phase III trial, assigned chemotherapy-naïve advanced NSCLC patients with good PS to receive orally erlotinib 150 mg/daily or a placebo combined with up to 6 cycles of carboplatin plus paclitaxel. In the Tarceva Lung Cancer Investigation Trial (TALENT)Citation16 patients received 150 mg of oral erlotinib daily or a placebo, combined with up to 6 gemcitabine platinum-based combinations. Both trials failed to demonstrate their main objective and provided no evidence of a survival advantage with erlotinib added to the cisplatin combination, Citation16,Citation65 or for maintenance with erlotinib. The same holds true for the gefitinib trials in first line with chemotherapy.Citation66,Citation67 However in the TALENT trial, although the proportion of patients with objective responses was similar for the erlotinib and placebo arm (31.5% vs 29.9%, respectively), the duration of the response (though not the median time to symptomatic progression) in the experimental arm with erlotinib was small but significantly greater (median 25.4 vs 23.9 weeks; HR 0.77; P = 0.045).Citation16 The FAST-ACT (First Asian Sequential Tarceva and Chemotherapy Trial) is a first-line randomized phase II trial of an intermittent erlotinib (days 15 to 28) and gemcitabine platinum-based combination (GC) in a majority of the Asian population.Citation68 In this trial, responding patients were also permitted to continue with erlotinib or placebo until there was disease progression or unacceptable toxicity. The trial did not achieve its main objective to improve the non-progression rate at 8 weeks with the “pulsed” administration of erlotinib (80.3% GC-erlotinib vs 76.9% GC-placebo, P = 0.5143) and there were no significant differences in the RR between groups (35.5% GC-erlotinib vs 24.4% GC-placebo, P = 0.12). However, there was a significant (P = 0.0002) 53% improvement in PFS (29.4 vs 23.4 weeks; HR 0.47) which favors the erlotinib plus chemotherapy group.Citation68 Although the majority of patients included in the trial were Asian, the observed PFS benefit was consistent across all the predefined clinical subgroups, including smokers, males and non-adenocarcinoma tumor patients.
Table 1 Randomized trials of maintenance therapy with erlotinib
Other trials with other targeted agents such as bevacizumab or cetuximab,Citation12–Citation14 allowed the continuation of the targeted agent after completion of the combination chemotherapy and the targeted agent. However these trials were not initially designed to evaluate the efficacy of maintenance with the targeted agent, and there is no valid control arm for the maintenance part of the trials. The data from these trials indicate that continuation with the targeted agent is feasible, but they do not provide data about the incremental benefit of continuing the targeted agent beyond completion of the initial treatment. Therefore the question of whether maintenance with cetuximab or bevacizumab is helpful in this setting remains unanswered until well-designed phase III trials are carried out.
The concept of consolidation, using a non-cross agent treatment started before tumor progression, also referred to as an “early second-line”, has been investigated in several trials.Citation20,Citation69 The prompt use of a different agent to the ones used for the induction treatment has some potential advantages. Some authorsCitation70–Citation72 suggest that the prompt switch to another non-cross resistant agent may reduce the risk of development resistant clones that increases over time. Therefore, those patients shown to benefit from the upfront treatment with a platinum doublet would be candidates for a maintenance approach. Consolidation with chemotherapy has recently been evaluated; Fidias et alCitation69 could not demonstrate a statistically significant advantage in OS for patients treated immediately with docetaxel, started after completion of the first-line treatment with carboplatin and gemcitabine, compared to the conventional initiation after disease progression. There were no differences in QoL between the two groups and a highly significant benefit in terms of PFS (5.7 vs 2.7 months; HR 0.71) and a trend towards better survival (12.3 vs 9.7 months; P = 0.08) was observed for the randomly assigned patients in the immediate docetaxel arm as an “early second line”. However when patients not receiving docetaxel late second line were excluded (n = 98), OS was identical for both groups (12.5 months). More recently the JMEN trial,Citation20 a randomized phase III trial, has released results showing, for the first time, an advantage in terms of OS for a maintenance strategy in NSCLC. The trial assigned patients to receive pemetrexed versus placebo as maintenance after the induction with 4 cycles of a platinum combination that did not include pemetrexed. The study achieved its end-point and demonstrated a highly significant (P < 0.0001) benefit in terms of PFS (4.3 vs 2.6 months; HR 0.50) and OS (13.4 vs 10.6 months; HR 0.79, P = 0.012) with the immediate initiation of pemetrexed.Citation20 However it is worth mentioning that the trial was not designed to test the superiority in OS of pemetrexed over placebo and only 18% of patients in the placebo arm ever received pemetrexed as systemic post-discontinuation therapy. Interestingly, the subset analysis by histology suggested that squamous tumors do not benefit from maintenance with pemetrexed in terms of PFS and OS.Citation20
The use of targeted agents has also been evaluated in consolidation. Unlike cytotoxic agents such as intravenous chemotherapy, these targeted treatments may be more appealing in the maintenance setting and thus are generally well tolerated and have a more convenient oral administration for patients. Moreover, treatment with targeted therapies may overcome the undesirable cumulative toxicity caused by cytotoxic agents administered in first-line setting. On the other hand, the cytostatic rather than cytotoxic properties of the new targeted drugs makes them a potential candidate for use in patients who have already shown a response after the initial chemotherapy by maintaining the tumor responses and delaying the progression event. In 2009, the results of 2 major phase III studies, ATLAS and SATURN, which evaluated the role of the TKI erlotinib as single-agent maintenance therapy in advanced NSCLC either with of without bevacizumab, an anti-VEGF receptor, were released in the annual American Society of Clinical Oncology meeting.Citation73,Citation74 These trials offered the possibility of continuing an active treatment with erlotinib after completing chemotherapy in order to delay disease progression and symptom deterioration. SATURN (Sequential Tarceva in Unresectable NSCLC)Citation73 is a randomized, placebo-controlled phase III trial in which erlotinib was evaluated as first-line maintenance therapy in advanced NSCLC patients whose disease had not progressed after 4 cycles of a platinum-doublet treatment. In this trial patients received erlotinib (150 mg/day) or placebo until disease progression or unacceptable toxicity, if disease control (stable disease, partial response or complete response) was documented after the 4 initial cycles of chemotherapy (). Tissue samples of the randomized patients were collected at baseline. The primary end-points were to determine whether the administration of erlotinib, as maintenance after the standard platinum-based chemotherapy, increases PFS in all patients and in those whose tumors express EGFR by inmunohistochemistry (IHQ). Secondary end-points included determining OS throughout the population and in patients according to protein expression of EGFR, as well as the analysis of other different biomarkers (EGFR gene copy number by fluorescent in situ hybridization – FISH, EGFR and Kras mutations with intron 1 CA-repeat polymorphism at EGFR by sequencing). Among the 889 patients randomized, out of a total of 1949, PFS (assessed by an investigator and an independent review and defined as the length of time from randomization to disease progression or death from any cause) was significantly prolonged with erlotinib versus placebo (HR 0.71; 95% CI 0.62 to 0.82; P < 0.0001). There was a 41% improvement in PFS but the absolute differences were less than we might have hoped (12.3 vs 11.1 weeks). The percentage of patients without progression at 3 and 6 months were 53% vs 40% and 31% vs 17% for the erlotinib and the placebo group respectively. In patients whose tumors expressed EGFR by IHQ (n = 618), the absolute benefit for erlotinib was 45% (HR 0.69; 95% CI 0.58 to 0.82; P < 0.0001). Although the advantage in PFS was observed for all the subgroups of population analyzed (HR 0.71), mean PFS was significantly increased by 1 month (3.75 vs 4.76 months; HR 0.72) in women, people with Asian ethnicity and never-smokers. It is worth mentioning that although the patients included in the trial had already responded to the induction chemotherapy, maintenance with erlotinib achieved an overall RR of 12% vs 5% in the placebo group (P = 0.0006) and the disease control rate after 12 weeks of treatment, in the erlotinib group, was almost double that of the placebo arm (40.8% vs 27.4%, P = 0.0001). In general, erlotinib was well tolerated; the withdrawals from the study due to treatment-related adverse events were mild without differences in both groups and the major treatment-related toxicities were the anticipated grade I/II rash and diarrhea. Nevertheless, the assessment of QoL by the FACT-L (Functional Assessment of Cancer Therapy-Lung questionnaire) provided no evidence of a significant benefit, or impairment, in QoL with the active treatment (HR 0.96, 95% CI 0.79 to 1.16; P = 0.6530), and only pain and analgesic use seemed to be significantly delayed with the erlotinib manteinance.Citation73
Figure 5 Design of erlotinib maintenance phase III trials in advanced NSCLC treatment (SATURNCitation73 and ATLASCitation74).
Abbreviations: NSCLC, non-small cell lung cancer; non-PD, complete responses, partial responses, stable disease; PD, progressive disease; PFS, progression-free survival.
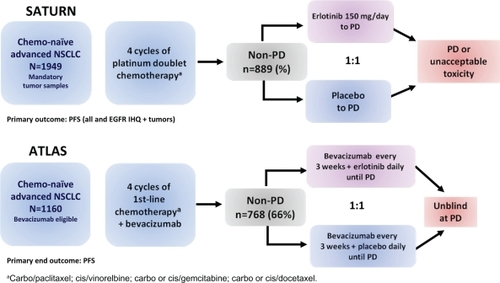
Later in the year, the much anticipated final results on survival were released at the 13th World Conference of Lung Cancer and the 34th ESMO congress.Citation75,Citation76 Maintenance with erlotinib significantly increased OS vs placebo in the intention-to-treat advanced NSCLC patients (HR 0.81, 95% CI 0.70 to 0.95; P = 0.0088), but as observed for PFS, the absolute differences in OS with erlotinib were minor (12.3 vs 11.1 months). However, OS differences for non-squamous disease treated with erlotinib appeared more worthwhile (13.7 vs 10.5 months, HR 0.79; P = 0.01). Among subgroup analysis, the greatest advantage in OS was observed for women (HR 0.64), people with Asian ethnicity (HR 0.66), never-smokers (HR 0.69) and adenocarcinoma tumors (HR 0.77), although all the subgroups of population analyzed gain a survival benefit from erlotinib maintenance. Interestingly, the magnitude of the benefit was greater in patients with stable disease following first-line chemotherapy than in those achieving a complete or partial response. Erlotinib, compared with placebo, gave patients with stable disease a 39% improvement in OS and a 2.3-month improvement in median survival (11.9 months vs 9.6 months; HR 0.72; P = 0.0019). Based on these results the European Commission (EMEA) has approved erlotinib as monotherapy for maintenance treatment in patients with locally advanced or metastatic NSCLC with stable disease following 4 cycles of standard platinum-based first-line chemotherapy.
The initial planned biomarker assessment revealed that the benefit on PFS was observed in all patients subgroups regardless EGFR or Kras mutation status.Citation77 Some groups benefited disproportionally from erlotinib such as EGFR positive by IHQ (HR 0.69) or by FISH (HR 0.68) and wild type Kras (HR 0.70). On the other hand, the strongest benefit in PFS was observed for the 49 patients with mutation at EGFR exon 19 or 21 (HR = 0.10; 95% CI 0.04 to 0.25; P < 0.0001), whereas EGFR wild type tumors seemed to benefit more from the erlotinib treatment (HR 0.77) compared to EGFR mutated tumors (HR 0.83).Citation77 These confusing results might be explained by the high level (67%) of subsequent EGFR tyrosine kinase inhibitor use in the placebo arm after progression in the EGFR mutated group, by the immature data on OS or by the unrepresentatively small sample in the subset analysis (n = 49). Meanwhile, multivariate analyses are needed for biomarker analysis as many of these biomarker-selected groups may overlap.
The randomized, double-blind, placebo-controlled, phase IIIB ATLAS (Adjuvant Tamoxifen Longer Against Shorter) trial, comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy with bevacizumab for the treatment of locally advanced, recurrent, or metastatic NSCLC, is the second important trial designed to assess the role of maintenance with bevacizumab with or without erlotinibCitation74 (). Patients were initially treated with 4 cycles of bevacizumab in combination with the investigators’ choice of platinum-based chemotherapy regimens. If their cancer did not progress and they did not experience significant toxicity, patients were then randomized (n = 768) to receive maintenance therapy with bevacizumab (15 mg/kg) plus erlotinib (150 mg/day) or placebo until disease progression. Patients with treated brain metastases, those receiving anticoagulation treatment with low-molecular-weight heparin and peripheral squamous tumors were also eligible for the study. The primary objective of the study was PFS started from the beginning of the maintenance phase after initial treatment with chemotherapy and bevacizumab, and the secondary included safety assessment and OS. The ATLAS study was prematurely stopped on the recommendation of an independent data safety monitoring board after a pre-planned interim efficacy analysis showed the study met the primary end-point and that combining erlotinib and bevacizumab significantly extended the time patients live without disease progression compared to bevacizumab plus placebo.Citation78 The preliminary safety analysis also gave evidence of a safe toxicity profile with adverse events consistent with other previous studies of bevacizumab and erlotinib and no new safety signals were observed. The median PFS was 4.8 months for bevacizumab and erlotinib vs 3.7 months for bevacizumab without erlotinib (HR 0.72, 95% CI 0.59 to 0.88; P = 0.0012). The percentage of patients without progression at 3 months was 67% for the erlotinib group vs 53% for the placebo group and at 6 months was 40% and 28% respectively.Citation74 The results on OS are expected for the first half of 2010. The trial included a prospective analysis of several biomarkers,Citation79 EGFR IHQ, gene copy number by FISH and EGFR and Kras mutations by sequencing. The results suggest that EGFR FISH positive (HR 0.66), EGFR mutated (HR 0.44) and Kras wild type (HR 0.67) patients, could derive the greatest improvement in PFS with bevacizumab and erlotinib.Citation79
Resistance to erlotinib in EGFR mutant patients and future therapeutic strategies
In NSCLC patients carrying EGFR mutations the treatment with first generation TKIs provides dramatic clinical and radiological responses; overall, EGFR mutations carriers have a RR around 75%, compared with a RR lower than 10% for patients with wild type EGFR.Citation80 Furthermore, patients with EGFR mutations have been shown to have longer progression-free and OS.Citation46 Despite this encouraging data, almost all cases invariably develop “acquired” resistance to TKIs,Citation40 which means the progression of the tumor that had previously responded to the treatment. About 43% to 50% of cases with acquired resistance to reversible EGFR TKIs can be accounted for by a secondary mutation, the gatekeeper mutation T790M located in exon 20 of the EGFR kinase domain.Citation81 This acquired alteration increases the ATP binding affinity of EGFR approximately 10-fold in the presence or absence of a TKI allowing ATP to competitively displace gefitinib and erlotinib from EGFR.Citation82 At least 10 other activating mutations (less common single amino acid substitutions such as D761Y, L747S, and T854A) have been reported within the kinase domain and the novel E884K mutation has been associated with resistance to gefitinib and erlotinib.Citation83
Balak et al noted that given the proportion of patients with acquired resistance, whose tumors contain T790M, malignant cells remain dependent on mutant EGFR for survival in at least half of patients.Citation84 On the other hand, the T790M mutation can also be detected in pretreatment specimens (38%), a condition that was associated with a short PFS (7.7 months vs 16.5 months in those without the mutation; HR for progression for the T790M allele, 11.5; P < 0.001).Citation85 All these aspects provide a rationale for developing second generation of irreversible TKIs, such as HKI-272, EKB-569, CI-1033 and BIBW2992, that bind covalently with the catalytic pocket of the TK receptor providing a sustained blockade against tumors harboring the T790 mutation.Citation86 Currently some phase II clinical trials with second-line TKIs, suggest a RR as high as 50% in some small cohorts, although only stable disease at best has been documented in patients with known T790M mutations.Citation87–Citation89
Another 20% of cases of acquired TKI resistance involve amplification of the MET proto-oncogen.Citation90 In this resistant tumors, amplification of MET activates PI3K-AKT signaling through erbB3.Citation90 IGFR-1R is another potential mechanism of escape to the therapeutic effect of TK reversible inhibitors.Citation91 Heterodimerization of the EGFR/IGFR-1R stimulates downstream pathways such as PI3K/AKT and MAPK, resulting in mammalian target of rapamycin (mTOR)-mediated protein synthesis of EGFR in NSCLC cells.Citation92,Citation93 These preclinical studies support the rational for clinical trials designs with combined treatment of a reversible TKI along with MET kinase, IGFR-1R or mTOR inhibitors. This approach could further improve the current results obtained with a single-agent in a subgroup of NSCLC patients with EGFR mutations and acquired resistance.Citation94
As reversible EGFR TKIs are now established as standard first-line therapy for patients with lung cancer with EGFR mutation, thoracic oncologists expect to see more patients with acquired resistance.Citation50 To simplify the definition of acquired resistance, recently Jackman et al proposed 4 easy and largely clinical criteria relevant to clinicians in their regular practice and to researchers in their design of studies.Citation95 The first criterion relates to patients who had previously received treatment with single-agent EGFR TKI; the second, that the tumor meet one or other of the following items: (A) that it harbors an EGFR mutation known to be associated with drug sensitivity; or (B) the patients do present clinical benefit from treatment with EGFR TKI (ie, either documented partial or complete response (RECIST or WHO), or a significant and durable (>6 months) stable disease after initiation TKIs); thirdly, there must be some evidence of systemic progression of disease while on continuous treatment with gefitinib or erlotinib within the last 30 days; and finally, that there is no intervening systemic therapy between cessation of the TKI and initiation of new therapy.Citation95 However, there is a small subgroup of EGFR mutant patients with primary, poorly understood resistance (6.5% to 10%) who never respond to first-line TKI therapy.Citation96
Conclusions
As highlighted by this review, erlotinib is an oral TKI with demonstrated activity in NSCLC. Thus far, erlotinib indications in NSCLC include all subgroups of locally advanced or metastatic NSCLC after failure of at least one prior chemotherapy regimen. In this context, erlotinib treatment improves OS and patient QoL. Erlotinib was clinically developed in parallel with the recent recognition of EGFR as a marker of response to TKIs. There is no doubt that NSCLC patients harboring EGFR mutations have a biologically different entity that requires personalized treatment strategies, including the use of TKIs instead of the unselective chemotherapy. Therefore, erlotinib clinical research has moved from second to first line, and at present, ongoing phase III trials aim to find out if erlotinib can be considered a new standard option in first-line intervention for patients harboring EGFR mutations. On the other hand, maintenance approach has recently received great attention as a suitable option in advanced NSCLC. Based on the results of the pivotal SATURN phase III trial, erlotinib as monotherapy has just been approved for patients with advanced NSCLC with non-progressive (FDA) or stable disease (EMEA), after first-line platinum-based initial chemotherapy. However, there still has been little movement toward accepting maintenance chemotherapy after first-line treatment, and many detractors cite the negligible survival benefit as the key reason. More convincing evidence for this approach could come from a prospective trial comparing the maintenance strategy with the same drug administered soon after first-line chemotherapy completion or after a demonstrated disease progression as a second line. Nevertheless, the concept of giving patients an oral, generally well-tolerated therapy such as erlotinib may be more appealing in the maintenance setting than more standard IV chemotherapy. To date, the SATURN trial, designed to test the role of erlotinib as maintenance treatment after initial chemotherapy, is the only randomized trial to demonstrate a significant survival advantage of maintenance with the use of a targeted agent. Unfortunately, most of the absolute advantages reported represent a restricted progress in the treatment of advanced NSCLC. Efforts have to be focused on identifying molecular predictive markers for a selective, rather than indiscriminate, treatment in NSCLC.
Disclosures
The authors have no conflicts of interest to disclose.
References
- MoyerJDBarbacciEGIwataKKInduction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinaseCancer Res199757483848489354447
- PollackVASavageDMBakerDAInhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic miceJ Pharmacol Exp Ther199929173974810525095
- DanceyJSausvilleEAIssues and progress with protein kinase inhibitors for cancer treatmentNat Rev Drug Discov2003229631312669029
- SalomonDSBrandtRCiardielloFNormannoNEpidermal growth factor-related peptides and their receptors in human malignanciesCrit Rev Oncol Hematol1995191832327612182
- EPARs for authorised medicinal products for human use, EMEA wwwemaeuropaeu/humandocs/Humans/EPAR/tarceva/tarcevahtm Accessed March 2, 2010.
- US Food and Drug Administration, FDA www.accessdatafdagov/Scripts/cder/DrugsatFDA/indexcfm?fuseaction=SearchDrugDetails Accessed March 2, 2010.
- VincentMDOptimizing the management of advanced non-small-cell lung cancer: a personal viewCurr Oncol20091692119672420
- JemalASiegelRWardECancer statistics, 2008CA Cancer J Clin200858719618287387
- BunnPAJrKellyKNew chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directionsClin Cancer Res19984108711009607565
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med2002346929811784875
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol2008263543355118506025
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med20063552542255017167137
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAilJ Clin Oncol2009271227123419188680
- PirkerRPereiraJRSzczesnaACetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trialLancet20093731525153119410716
- American Society of Clinical Oncology Clinical Practice Guidelines Update on Chemotherapy for Stage IV Non-Small Cell Lung Cancer www.ascoorg/guidelines/nsclc. Accessed March 2, 2010.
- GatzemeierUPluzanskaASzczesnaAPhase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation TrialJ Clin Oncol2007251545155217442998
- ShepherdFADanceyJRamlauRProspective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapyJ Clin Oncol2000182095210310811675
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med200535312313216014882
- HannaNShepherdFAFossellaFVRandomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapyJ Clin Oncol2004221589159715117980
- CiuleanuTBrodowiczTZielinskiCMaintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 studyLancet20093741432144019767093
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med20043502129213915118073
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A2004101133061331115329413
- PaezJGJannePALeeJCEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience20043041497150015118125
- GazdarAFActivating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitorsOncogene200928Suppl 1S24S3119680293
- BazleyLAGullickWJThe epidermal growth factor receptor familyEndocr Relat Cancer200512Suppl 1S17S2716113093
- KumarAPetriETHalmosBBoggonTJStructure and clinical relevance of the epidermal growth factor receptor in human cancerJ Clin Oncol2008261742175118375904
- CiardielloFTortoraGEGFR antagonists in cancer treatmentN Engl J Med20083581160117418337605
- GazdarAFMinnaJDDeregulated EGFR signaling during lung cancer progression: mutations, amplicons, and autocrine loopsCancer Prev Res (Phila Pa)20081156160
- EkstrandAJSugawaNJamesCDCollinsVPAmplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tailsProc Natl Acad Sci U S A199289430943131584765
- ShigematsuHGazdarAFSomatic mutations of epidermal growth factor receptor signaling pathway in lung cancersInt J Cancer200611825726216231326
- MitsudomiTKosakaTYatabeYBiological and clinical implications of EGFR mutations in lung cancerInt J Clin Oncol20061119019816850125
- TanakaTMatsuokaMSutaniAFrequency of and variables associated with the EGFR mutation and its subtypesInt J Cancer201012665165519609951
- SordellaRBellDWHaberDASettlemanJGefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathwaysScience20043051163116715284455
- AraoTFukumotoHTakedaMTamuraTSaijoNNishioKSmall in-frame deletion in the epidermal growth factor receptor as a target for ZD6474Cancer Res2004649101910415604279
- GreulichHChenTHFengWOncogenic transformation by inhibitor-sensitive and -resistant EGFR mutantsPLoS Med20052e31316187797
- JannePAJohnsonBEEffect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with nonsmall cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitorsClin Cancer Res2006124416s4420s16857820
- MukoharaTEngelmanJAHannaNHDifferential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutationsJ Natl Cancer Inst2005971185119416106023
- JackmanDMYeapBYSequistLVExon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinibClin Cancer Res2006123908391416818686
- JiangJGreulichHJannePASellersWRMeyersonMGriffinJDEpidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progressionCancer Res2005658968897416204070
- HammermanPSJannePAJohnsonBEResistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancerClin Cancer Res2009157502750920008850
- YunCHBoggonTJLiYStructures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivityCancer Cell20071121722717349580
- RukazenkovYSpeakeGMarshallGEpidermal growth factor receptor tyrosine kinase inhibitors: similar but different?Anticancer Drugs20092085686619657272
- SpeakeGAndertonJAchesonKA pharmacological comparison of gefitinib and erlotinib97th American Association of Cancer Research Annual MeetingWashington, DC, USA2006 poster 3784.
- YuzaYGlattKAJiangJAllele-dependent variation in the relative cellular potency of distinct EGFR inhibitorsCancer Biol Ther2007666166717495523
- SequistLVJoshiVAJannePAEpidermal growth factor receptor mutation testing in the care of lung cancer patientsClin Cancer Res2006124403s4408s16857818
- RosellRMoranTQueraltCScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med200936195896719692684
- JackmanDMMillerVACioffrediLAImpact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trialsClin Cancer Res2009155267527319671843
- SequistLVMartinsRGSpigelDFirst-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutationsJ Clin Oncol2008262442244918458038
- Paz-AresLSoulieresDMelezinekIClinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysisJ Cell Mol Med2009128 [Epub ahead of print]
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med200936194795719692680
- InoueAKobayashiKUsuiKFirst-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapyJ Clin Oncol2009271394140019224850
- MoritaSOkamotoIKobayashiKCombined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutationsClin Cancer Res2009154493449819531624
- RosellRViteriSMolinaMABenllochSTaronMEpidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancerCurr Opin Oncol20092211212019949333
- Phase III study (Tarceva®) vs chemotherapy to treat advanced nonsmall cell lung cancer (NSCLC) in patients with mutations in the TK domain of EGFR (NCT00446225) http://clinicaltrialsgov/. Accessed March 2, 2010.
- Erlotinib versus gemcitabine/carboplatin in chemo-naive stage IIIB/IV non-small cell lung cancer patients with epidermal growth factor receptor (EGFR) exon 19 or 21 mutation (ML20981)(NCT00874419) http://clinicaltrialsgov/ Accessed March 2, 2010.
- ClarkGMZborowskiDMSantabarbaraPSmoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study BR.21Clin Lung Cancer2006738939416800964
- Pemetrexed or erlotinib as second-line therapy in treating patients with advanced non-small cell lung cancer (NCCTG-N0723) http://clinicaltrialsgov/. Accessed March 2, 2010.
- von PlessenCBergmanBAndresenOPalliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancerBr J Cancer20069596697317047644
- SocinskiMASchellMJPetermanAPhase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancerJ Clin Oncol2002201335134311870177
- BuccheriGFFerrignoDCurcioAVolaFRossoAContinuation of chemotherapy versus supportive care alone in patients with inoperable non-small cell lung cancer and stable disease after two or three cycles of MACC. Results of a randomized prospective studyCancer1989634284322536288
- BrodowiczTKrzakowskiMZwitterMCisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trialLung Cancer20065215516316569462
- ParkJOKimSWAhnJSPhase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancerJ Clin Oncol2007255233523918024869
- SmithIEO’BrienMETalbotDCDuration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatinJ Clin Oncol2001191336134311230476
- SoonYYStocklerMRAskieLMBoyerMJDuration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trialsJ Clin Oncol2009273277328319470938
- HerbstRSPragerDHermannRTRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol2005235892589916043829
- GiacconeGHerbstRSManegoldCGefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1J Clin Oncol20042277778414990632
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2J Clin Oncol20042278579414990633
- MokTSWuYLYuCJRandomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancerJ Clin Oncol2009275080508719738125
- FidiasPMDakhilSRLyssAPPhase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancerJ Clin Oncol20092759159819075278
- MokTSRamalingamSSMaintenance therapy in nonsmall-cell lung cancer: a new treatment paradigmCancer20091155143515419658185
- JalalSIAdemuyiwaFOHannaNHThe role of maintenance chemotherapy in advanced nonsmall cell lung cancerCurr Opin Oncol20092111011519532011
- GoldieJHColdmanAJGudauskasGARationale for the use of alternating non-cross-resistant chemotherapyCancer Treat Rep1982664394497060033
- CappuzzoFCiuleanuTStelmakhLSATURN: A double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLCJ Clin Oncol200927Suppl 158001
- MillerVO’ConnorPSohCA randomized, double-blind, placebo-controlled, phase IIIB trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastasic non-small cell lung cancerJ Clin Oncol200927Suppl 15LBA8002
- CappuzzoFCoudertBWierzbickiREfficacy and safety of erlotiniib as first-line maintenance in NSCLC following nonprogression with chemotherapy: results from the phase III SATURN study (abstr A2.1)13th World Conference on On Lung CancerSan Francisco http://www.2009worldlungcancer.org/. Accessed March 2, 2010.
- CappuzzoFCoudertBWierzbickiROverall survival analyses from the SATURN phase III placebo-controlled study of erlotinib as first-line maintenance therapy in advanced non-small-cell lung cancer (NSCLC). (abstr 22LBA)ECCO 15–34th ESMO Multidisciplinary CongressBerlin http://www.ecco-org.eu/Conferences-and-Events/ECCO-15-ESMO-34/Abstracts-online/page.aspx/1729. Accessed March 2, 2010.
- BruggerWKimJHansenOMolecular markers and clinical outcome with erlotinib: results from the phase III placebo-controlled SATURN study of maintenance therapy for advanced NSCLC (abstr B9.1)13th World Conference on On Lung CancerSan Francisco Available from: http://www.2009worldlungcancer.org/. Accessed March 2, 2010.
- GenetechPress Release ATLAS study http://www.genecom/gene/news/press-releases/displaydo?method=detail&id=11827 Accessed March 2, 2010.
- JohnsonBMillerVAmlerLBiomarker evaluation in the randomized, double-blind, placebo-controlled, phase IIIb ATLAS trial, comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy with B for the treatment of locally-advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) (abstr 8LBA)ECCO 15–34th ESMO Multidisciplinary CongressBerlin http://www.eccoorg.eu/Conferences-and-Events/ECCO-15-ESMO-34/Abstracts-online/page.aspx/1729. Accessed March 2, 2010.
- CostaDBKobayashiSTenenDGHubermanMSPooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancersLung Cancer2007589510317610986
- EngelmanJASettlemanJAcquired resistance to tyrosine kinase inhibitors during cancer therapyCurr Opin Genet Dev200818737918325754
- YunCHMengwasserKETomsAVThe T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATPProc Natl Acad Sci U S A20081052070207518227510
- PaoWMillerVAEpidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directionsJ Clin Oncol2005232556256815767641
- BalakMNGongYRielyGJNovel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitorsClin Cancer Res2006126494650117085664
- MaheswaranSSequistLVNagrathSDetection of mutations in EGFR in circulating lung-cancer cellsN Engl J Med200835936637718596266
- KwakELSordellaRBellDWIrreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinibProc Natl Acad Sci U S A20051027665767015897464
- WongKKFracassoPMBukowskiRMA phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumorsClin Cancer Res2009152552255819318484
- JannePSchellensHEngelmanJPreliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLCJ Clin Oncol200826Suppl 158027
- MillerVWakeleeHLaraPActivity and tolerance of XL647 in NSCLC patients with acquired resistance to EGFR-TKIs: Preliminary results of a phase II trialJ Clin Oncol200826Suppl 158028
- EngelmanJAZejnullahuKMitsudomiTMET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signalingScience20073161039104317463250
- GuixMFaberACWangSEAcquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteinsJ Clin Invest20081182609261918568074
- MorgilloFWooJKKimESHongWKLeeHYHeterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinibCancer Res200666101001011117047074
- O’ReillyKERojoFSheQBmTOR inhibition induces upstream receptor tyrosine kinase signaling and activates AktCancer Res2006661500150816452206
- RosellRViteriSMolinaMABenllochSTaronMEpidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancerCurr Opin Oncol20102211212019949333
- JackmanDPaoWRielyGJClinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancerJ Clin Oncol20102835736019949011
- MokTSLiving with imperfectionJ Clin Oncol20102819119219949000