Abstract
Medical castration using gonadotropin-releasing hormone (GnRH) receptor agonists currently provides the mainstay of androgen deprivation therapy for prostate cancer. Although effective, these agents only reduce testosterone levels after a delay of 14 to 21 days; they also cause an initial surge in testosterone that can stimulate the cancer and lead to exacerbation of symptoms (“clinical flare”) in patients with advanced disease. Phase III trial data for the recently approved GnRH receptor blocker, degarelix, demonstrated that it is as effective and well tolerated as GnRH agonists. However, it has a pharmacological profile more closely matching orchiectomy, with an immediate onset of action and faster testosterone and PSA suppression, without a testosterone surge or microsurges following repeated injections. As a consequence, with this GnRH blocker, there is no risk of clinical flare and no need for concomitant antiandrogen flare protection. Degarelix therefore provides a useful addition to the hormonal armamentarium for prostate cancer and offers a valuable new treatment option for patients with hormone-sensitive advanced disease. Here, we review key preclinical and clinical data for degarelix, and look at patient-focused perspectives in the management of prostate cancer.
Introduction
The key role of testosterone in the growth of prostate cancer was first demonstrated back in 1941 by Huggins and Hodges;Citation1 since then, androgen deprivation therapy (ADT) has provided the mainstay of treatment for patients with hormone-sensitive advanced prostate cancer. Because of its documented links with therapeutic efficacy, testosterone suppression is now an accepted endpoint in prostate cancer clinical trials and has been used as a surrogate endpoint during the approval of several hormonal treatments.Citation2 Prostate-specific antigen (PSA) is currently widely-used in prostate cancer screening and this marker can also be helpful in monitoring treatment response, disease recurrence and potentially in providing evidence of disease progression during prostate cancer ADT.Citation3,Citation4 Testosterone production is regulated by two key hormones: gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH). GnRH is secreted from the hypothalamus in a pulsatile manner and binds to high-affinity cell surface receptors in the pituitary gland, activating a chain of events that lead to synthesis and secretion of LH and follicle-stimulating hormone (FSH).Citation5 LH stimulates testosterone production and secretion by the Leydig cells of the testes. Bilateral orchiectomy was the first ADT approach used in prostate cancer therapy and rapidly reduces circulating testosterone levels.Citation6 Although effective, its irreversibility and associated adverse psychological effects are often unacceptable to patients and so, with the advent of newer pharmacological approaches to ADT, orchiectomy is now used much less frequently.
GnRH receptor agonists were first introduced in the 1980s and are currently the most commonly used ADT in prostate cancer.Citation7 These agents act on the hypothalamic–pituitary–gonadal (HPG) axis via a negative feedback mechanism.Citation8 Binding of a GnRH receptor agonist to the GnRH receptor initially triggers the secretion of LH, which in turn causes a surge in testosterone production lasting 1 to 2 weeks.Citation9,Citation10 This may stimulate prostate cancer cells, and in patients with advanced disease, exacerbate clinical symptoms such as skeletal pain, ureteral obstruction and spinal cord compression, which may lead to paralysis and, in rare cases, death.Citation11,Citation12 The clinical effects of flare can be limited by concomitant antiandrogen treatment (eg, flutamide or bicalutamide),Citation13 which acts to inhibit the stimulatory effect of the testosterone surge by blocking testosterone binding to androgen receptors in prostate cancer cells. However, this strategy is not always effective and antiandrogens are also associated with additional side effects.Citation14,Citation15
Chronic administration of GnRH receptor agonists eventually leads to suppression of LH, which results in a reduction of testosterone release. However, GnRH agonists may also induce “microsurges” of LH and testosterone after each re-injection.Citation16,Citation17 Overall, 22.6% of patients in the Zinner et al studyCitation17 had a testosterone surge on at least one repeat injection; this comprised 18.6% of patients receiving goserelin 10.8 mg and 27.9% of those receiving goserelin 3.6 mg depots. No symptoms of clinical flare were reported at the time of surge for any of the patients experiencing a testosterone surge in this study. In a separate study looking at the potential for agonistic stimulation during leuprolide treatment, 5.9% and 2.9% of patients receiving leuprolide monthly depot had testosterone increases to ≥0.5 ng/mL after the second and third injection, respectively.Citation16 In a further study of 73 patients receiving GnRH agonist treatment, overall, 56.2% of patients had breakthrough increases in testosterone of ≥20 ng/mL, with increases >50 ng/mL occurring in 24.7% of patients.Citation18 In contrast to the results of Zinner et al, a significant association was shown between testosterone increases and clinical outcome in terms of PSA progression, demonstrating the importance of keeping testosterone levels low during ADT. The effects were most notable the higher the testosterone breakthrough threshold breached. Further analysis of data from this trial showed that patients who maintained their testosterone levels consistently below 32 ng/mL had significantly longer PSA progression-free survival compared with those having any breakthrough increases above this threshold (137 months vs 88 months, respectively; P < 0.03).Citation18 A more recent report of data from 129 men with previously untreated prostate cancer and bone metastases demonstrated that risk of death significantly correlated (P < 0.05) with the 6-month serum testosterone level achieved during goserelin treatment.Citation19 The authors concluded that based on their results, lowering testosterone levels as much as possible should be the goal of ADT in patients with metastatic prostate cancer.
Other pharmacological endocrine options for prostate cancer include the use of estrogens, antiandrogen monotherapy, and complete androgen blockade using an antiandrogen plus a GnRH receptor agonist.Citation7 However, these approaches are used infrequently in practice due to concerns about efficacy and/or side effects, which can include cardiotoxicity, gynecomastia, breast pain and liver toxicity.Citation7 The need exists, then, for additional effective and well-tolerated treatment options for patients with advanced, hormone-sensitive prostate cancer.
Degarelix: a new GnRH receptor blocker
The testosterone surge and clinical flare associated with GnRH agonists led to research into new GnRH analogues that blocked the GnRH receptor directly, thus obviating these agonist-associated problems. GnRH receptor antagonists (blockers) are a new class of endocrine therapy that bind directly to the GnRH receptor, rapidly blocking the release of both LH and FSH, and thereby reducing testosterone secretion ().Citation20–Citation25 In contrast to the agonists, GnRH antagonists do not cause an initial stimulation of LH production, and therefore do not cause testosterone surge or clinical flare.Citation12 Abarelix was the first GnRH antagonist to be licensed for prostate cancer treatment; however, this agent was associated with immediate-onset systemic allergic reactions resulting from histamine release, and so is currently marketed only in Germany.Citation26
Figure 1 Mode of action of GnRH receptor antagonists.Citation58 Reproduced with permission from Anderson J. Degarelix: a novel gonadotropin-releasing hormone blocker for the treatment of prostate cancer. Future Oncol. 2009;5(4):433–443.Citation58 Copyright © 2009 Future Medicine Ltd.
Abbreviations: GnRH, gonadotrophin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
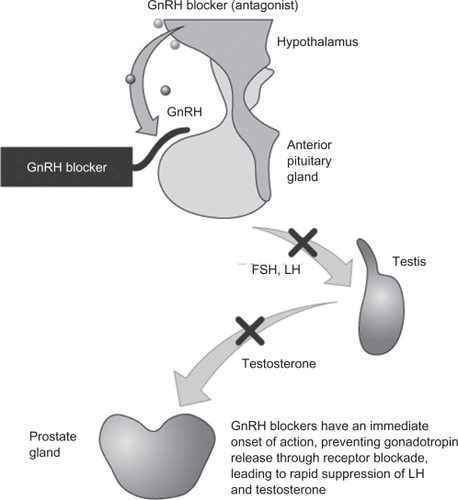
Degarelix is a new GnRH receptor blocker that has recently been approved for the treatment of men with advanced, hormone-sensitive prostate cancer.Citation27 It acts by immediate and competitive blockade of GnRH receptors in the pituitary; like other GnRH antagonists, degarelix does not cause an initial stimulation of LH production via the HPG axis, and therefore does not cause testosterone surge or clinical flare.Citation12
Pharmacology and pharmacokinetics of degarelix
Degarelix (Ac-D-2Nal-D-4Cpa-D-3Pal-Ser-4Aph (L-hydrorootyl)-D-4Aph (carbamoyl)-Leu-Ilys-Pro-D-Ala-NH2) is a fully synthetic, linear decapeptide amide analogue of natural GnRH that contains seven unnatural amino acids, five of which are D-amino acids.Citation28
Data from preclinical studies
The pharmacology of degarelix was initially assessed in various preclinical studies. In vitro radioligand binding assays demonstrated that degarelix has a high affinity to cloned human GnRH receptors expressed on COS-I cells, with a Ki value of 1.68 ± 0.12 nM.Citation29 Degarelix demonstrated similar functional antagonism to three other GnRH antagonists (azaline B, cetrorelix and ganirelix) and showed no significant affinity towards other tested receptors. Data from in vitro metabolism studies suggest that degarelix is unlikely to be associated with any clinically significant drug–drug interactions.Citation29
The in vivo effect of degarelix on tumor size was investigated in three experimental models of hormone-dependent prostate cancer. Antitumor effects were observed in androgen-dependent human prostate tumors (PAC120) in nude mice and androgen-dependent rat prostate tumors (Dunning R-3327H) when degarelix was administered at a dose of 2 mg/kg every 2 weeks or once a month, respectively. At the tested doses, degarelix reduced tumor volume with a similar efficacy to surgical castration.Citation29 Degarelix had no effect on the growth of the androgen-independent human prostate tumor PC3.
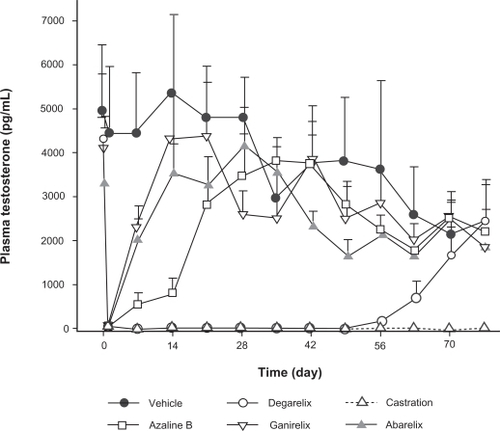
The pharmacological profile of subcutaneous degarelix was originally assessed in rats and monkeys.Citation25 Single subcutaneous injections in rats produced dose-dependent reductions in LH and testosterone levels and the duration of LH suppression was found to increase with dose. Degarelix fully suppressed LH and testosterone levels for more than 40 days after a single 2 mg/kg subcutaneous injection in castrated and intact rats () as well as in ovariectomized rhesus monkeys. The testosterone suppression profile during degarelix treatment more closely matched that of orchiectomy compared with the other GnRH antagonists tested. Furthermore, degarelix showed a longer duration of action than abarelix, ganirelix, cetrorelix, and azaline B and demonstrated only weak histamine-releasing properties in vitro. A more recent study has confirmed that cetrorelix and abarelix are potent activators of human skin mast cells and that ganirelix is a less potent activator in terms of inducing histamine release.Citation30 In the same study, degarelix had the lowest histamine-releasing potential, suggesting that it is the least likely agent to cause histamine-related immediate-onset allergic reactions.
Figure 2 Mean (n = 8; ± SEM) testosterone levels in the intact rat induced by degarelix, abarelix, azaline B, and ganirelix, administered at a dose of 2 mg/kg in 5% mannitol, compared with surgical castration. Reproduced with permission from Broqua P, Riviere PJ, Conn PM, Rivier JE, Aubert ML, Junien JL. Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther. 2002;301:95–102.Citation25 Copyright © 2002 American Society for Pharmacology & Experimental Therapeutics.
The pharmacological profile of degarelix was also assessed in male beagle dogs.Citation31 In this study, administration conditions were varied with respect to route, dose, concentration and volume. The plasma concentration–time profile of subcutaneous or intramuscular degarelix was best described by a two-compartment model, with two input functions to describe the rapid initial increase in the plasma levels, and the prolonged absorption profile of degarelix. Intramuscular administration led to more rapid absorption of degarelix. The relative fraction absorbed varied with the concentration of the dosing solution; the absorbed fraction was reduced by ∼50% when the concentration was increased from 1.25 to 40 mg/mL. The initial rate of absorption was also dependent on concentration, with slower absorption at higher concentrations. After subcutaneous administration, degarelix immediately forms a gel “depot” at the injection site, leading to sustained release of the drug into the circulation. Release from the depot was found to be dependent on the degarelix dose and the administration volume.Citation32
Reconstitution and administration of degarelix
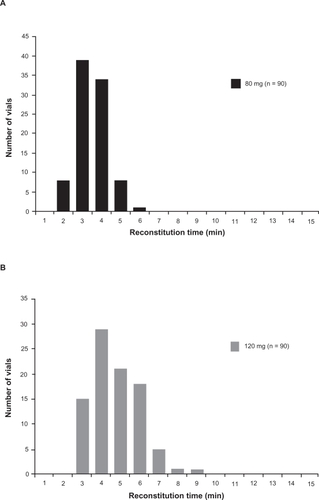
Reconstitution of the lyophilized degarelix powder involves addition of sterile water to the product vial followed by swirling to minimize production of foam. Recently, a study was performed to assess the time taken for reconstitution of two degarelix doses. It was found that average reconstitution times were 2.96 and 4.25 minutes for 80 and 120 mg degarelix doses, respectively (Ferring Pharmaceuticals, data on file). Virtually all (99%) of the 80 mg vials were reconstituted within 5 minutes compared with 72% of vials for the 120 mg dose (99% of which were reconstituted within 8 minutes) (). The average concentration of degarelix in the reconstituted samples was 19.9 mg/mL for the 80 mg dose and 39.3 mg/mL for the 120 mg dose, confirming that the reconstitution process in all vials was adequate.
Figure 3 Reconstitution time distribution profiles for degarelix doses of 80 mg (Panel A) and 120 mg (Panel B) (three batches combined).
Degarelix is administered via subcutaneous injection into the abdominal subcutaneous fat surrounding the umbilicus.Citation33 The 240 mg starting dose is given as two 3 mL injections (final degarelix concentration of 40 mg/mL) and the 80 mg maintenance dose is given as a single 4 mL injection (final degarelix concentration of 20 mg/mL).
Data from clinical studies
The pharmacokinetics of degarelix were initially assessed in a phase I study in 36 healthy male volunteers.Citation34 In this study, single doses of degarelix (1.5, 6.0, 15 or 30 μg/kg) were administered via 45-minute intravenous infusions, or a single dose of 20 mg was given intramuscularly or subcutaneously. As was seen in the early preclinical studies, degarelix naturally formed a depot at high concentrations (mg/mL) after both subcutaneous and intramuscular injection, which resulted in a longer half-life compared with intravenous injection. The pharmacokinetics of degarelix after subcutaneous injection were also found to be dose- and concentration-dependent, and bioavailability was estimated at 30% to 40% following both subcutaneous and intramuscular administration. Similar levels of LH, FSH and testosterone suppression were seen when degarelix was administered via the intravenous, subcutaneous or intramuscular routes.
Two further studies in prostate cancer patients assessed the effects of a single dose of degarelix, 120 to 320 mg given in various concentrations in the range of 12 to 27 mg/mL or subcutaneous degarelix administered in single initiation doses of 200 or 240 mg with maintenance doses of 80, 120 or 160 mg. In a combined analysis of these studies, the effects of degarelix were again shown to be dose- and concentration-dependent; higher doses resulted in superior testosterone suppression, but lower concentrations of a given dose yielded better responses.Citation35
In early single- and multiple-dose degarelix studies,Citation29 the mean terminal half-life was between 23 and 61 days with marked variability between patients and studies. Cmax and AUC were influenced by dose, number of injections and concentration and Tmax ranged between 34 and 62 hours. Steady-state plasma levels occurred after 5 to 6 months with the degarelix 240/80 mg regimen. Additional pharmacokinetic data relating to the subcutaneous administration of degarelix 240 mg at a concentration of 40 mg/mL were gained from the pivotal phase III trial (CS21).Citation29 In this trial, the AUC0–28 days was 635 (602 to 668) days * ng/mL. Cmax was 66.0 (61.0 to 71.0) ng/mL and occurred at a Tmax of 40 (37 to 42) hours. Mean trough values were 11 to 12 ng/mL after the starting dose and 11 to 16 ng/mL after maintenance dosing of 80 mg/month (at a concentration of 20 mg/mL). In humans, degarelix is subject to common peptiditic degradation during passage through the hepatobiliary system and is mainly excreted as peptide fragments in the feces, with approximately 20% excreted unchanged in the urine.
Dose-finding studies of degarelix
Study designs and patients
Two phase II, degarelix dose-finding studies of similar overall design were performed in Europe (CS12)Citation36 and North America (CS14)Citation37 Both were open-label, randomized, parallel-group, 1-year studies including adult male patients with histologically confirmed prostate cancer (all stages), in whom hormonal treatment was indicated. Patients were randomized to treatment with different degarelix dose regimens in these two studies. In CS12, 189 patients were randomized to one of six degarelix treatment groups: starter doses of either 200 or 240 mg followed by monthly maintenance doses of 80, 120, or 160 mg, all given via subcutaneous injection.Citation36 In CS14, 127 patients were randomized to one of two degarelix treatment groups: starter dose of 200 mg followed by monthly maintenance doses of either 60 or 80 mg, once again all given via subcutaneous injection.Citation37
Efficacy data
In the European study, 88% and 92% of patients in the 200 and 240 mg starter-dose groups, respectively, achieved testosterone levels ≤0.5 ng/mL by day 3, and there was no evidence of testosterone surge.Citation36 The proportion of patients achieving castrate testosterone levels after 1 month of treatment was significantly higher for patients treated with a degarelix starter dose of 240 mg compared with those receiving a starter dose of 200 mg (95% and 86%; P = 0.048). The proportion of patients with castrate levels from month 1 until the end of the study also increased with increasing maintenance dose (). In this study, inadequate testosterone suppression resulted in withdrawal of 8.5% of patients, with 50% of all withdrawals occurring in the lowest dose group (200/80 mg). Median time to a 50% reduction in PSA was 14 days for all groups and the median time to a 90% reduction was 56 days for all groups except those receiving degarelix 200/80 mg, which took an average of 84 days. After 12 months of treatment, the median PSA reduction from baseline was 97% to 98%; overall, 7% of patients experienced PSA progression.Citation36
Table 1 Percentage of patients with serum testosterone levels ≤0.5 ng/mL (responders) during monthly measurements from day 28 through to day 364 in phase II and III degarelix studiesCitation36–Citation38,Citation58
In the North American study, 89% of patients overall achieved testosterone levels ≤0.5 ng/mL by day 3 and there was no significant difference between treatment groups and no evidence of testosterone surge.Citation37 Somewhat surprisingly, after 1 month of treatment, there was a trend for improved testosterone control in the 60 mg maintenance dose group compared with the 80 mg group, with 93% and 83% of patients treated with these doses, respectively, achieving castrate testosterone levels (P = 0.073). The proportion of patients with castrate levels at month 1 who also had castrate levels at the 1-year study visit was 93% and 98% in the 60 and 80 mg maintenance dose groups, respectively (). However, when early failures due to an inadequate starter dose were excluded, a maintenance dose of degarelix 80 mg appeared most effective in maintaining castrate testosterone levels to 1 year (98% vs 93%, for 80 mg vs 60 mg, respectively). Similar to the European study, the median time to a 50% reduction in PSA was 14 days and the median time to 90% reduction was 56 days, in both treatment groups. After 12 months of treatment, the median PSA reduction from baseline was 96%; overall, 7% of patients experienced PSA progression.Citation37
Safety and tolerability data from the phase II dose-finding studies
In both studies, degarelix treatment was well tolerated, with most adverse events being mild to moderate in intensity and related to the known effects of androgen deprivation; there were no immediate-onset systemic allergic reactions. Overall, 7%Citation36 and 5%Citation37 of patients in these trials withdrew due to adverse events.
Efficacy and safety conclusions from the phase II dose-finding studies
Data from these trials suggested that degarelix 240 mg appeared to be the optimal starter dose for evaluation in future studies, as this resulted in castrate testosterone levels in >90% of patients within 3 days.Citation36 In contrast, inadequate suppression occurred with the use of a 200 mg starter dose used in the North American study.Citation37 Two maintenance doses (80 mg and 160 mg) were also identified for future evaluation as no dose-dependent adverse events were noted in either study. In these studies, degarelix demonstrated effects on testosterone and PSA levels similar to those observed with orchiectomy.
Phase III comparative study
Study design and patients
A 1-year, multicenter, randomized, open-label, parallel-group, phase III trial (CS21) was conducted, which was designed to demonstrate the statistical noninferiority of degarelix versus the GnRH receptor agonist leuprolide.Citation38 In this trial, patients with histologically confirmed prostate cancer (all stages), for whom ADT was indicated, were randomized to one of three treatment groups: degarelix (administered subcutaneously) at a starter dose of 240 mg followed by monthly maintenance doses of either 80 mg (240/80 group; n = 207) or 160 mg (240/160 group; n = 202), or monthly intramuscular injections of leuprolide depot 7.5 mg (n = 201). In the leuprolide group, antiandrogens could also be given as flare protection at the investigator’s discretion.
Efficacy data
Primary analyses
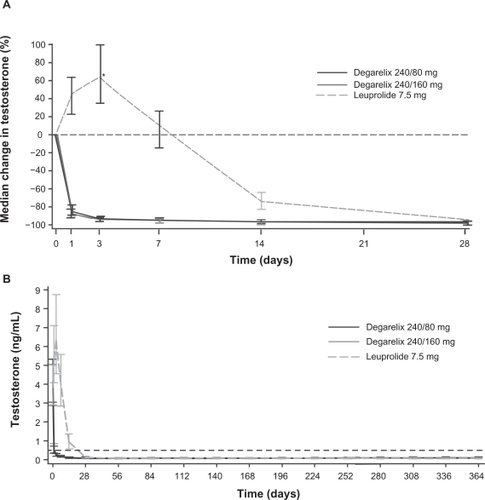
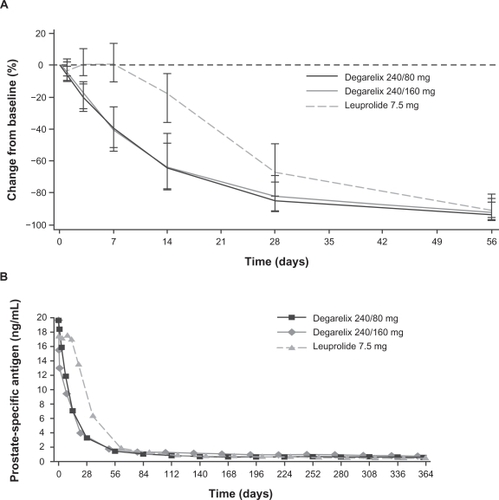
The primary analyses of data from the CS21 trial demonstrated that both degarelix doses were noninferior to leuprolide for the primary endpoint (testosterone response: serum testosterone ≤0.5 ng/mL at all monthly measurements between days 28 and 364; ).Citation38 A treatment response was achieved by 97.2%, 98.3% and 96.4% of patients in the degarelix 240/80 mg, 240/160 mg and leuprolide groups, respectively. Degarelix resulted in a more rapid treatment response than leuprolide and median testosterone levels were significantly lower in the degarelix groups by day 3 (P < 0.001; ). At this time point, >95% of patients in the degarelix groups had testosterone levels ≤0.5 ng/mL. In contrast, no patients receiving leuprolide had castrate levels at day 3; indeed, patients in this group showed a median increase in testosterone of 65% compared with baseline levels at this time point and median levels were >0.5 ng/mL until day 28. Both degarelix doses were as effective as leuprolide at suppressing testosterone levels from day 28 to study end (). No patient receiving degarelix experienced testosterone surge (≥15% increase in testosterone level vs baseline during the first 2 weeks) or microsurges (testosterone increase >0.25 ng/mL on days 255 or 259, 3 or 7 days after the ninth injection). In contrast, 144 patients (81%) in the leuprolide group experienced a testosterone surge, including 17 of the 23 patients (74%) who received concomitant bicalutamide. Eight leuprolide patients (4%) had microsurges, including four patients (2%) whose testosterone increased >0.5 ng/mL at the time points evaluated. Overall, patients in the leuprolide group showed a significant mean 0.05 ng/mL increase (P < 0.001) in testosterone levels on days 255/259 compared with day 252, whereas patients in the degarelix group showed a slight decrease.
Figure 4 Median testosterone levels with degarelix and leuprolide. Panel A depicts the first month of treatment; Panel B shows data from across the 1-year treatment period.Citation38 Reproduced with permission from Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538.Citation38 Copyright © 2008 Blackwell Publishing Ltd.
*P < 0.001 degarelix (both doses) versus leuprolide.
In the degarelix groups, median LH and FSH levels decreased rapidly and remained suppressed until the end of the study, whereas LH and FSH levels showed an initial increase for patients in the leuprolide group, and FSH levels did not fall to the same extent as they did in the degarelix arms. In line with the testosterone results, PSA levels also declined more rapidly in the degarelix 240/80 and 240/160 mg arms, and at days 14 (64% and 65% vs 18%) and 28 (85% and 83% vs 68%), significantly greater suppression was observed compared with leuprolide (P < 0.001; ). Beyond day 28, PSA levels remained effectively suppressed in all treatment groups until the end of the 1-year study (). Overall, PSA failure (a PSA increase of ≥50% from nadir and ≥5ng/mL on two consecutive occasions at least 2 weeks apart) occurred in 8.9% of patients in the degarelix 240/80 mg group, 14.2% of those in the degarelix 240/160 mg group, and 14.1% of those in the leuprolide 7.5 mg group.Citation38 In a subsequent analysis of PSA progression-free survival, a statistically lower (P = 0.0495; log-rank test) risk of PSA failure or death was found for patients randomized to degarelix 240/80 mg compared with patients randomized to leuprolide (ITT population).Citation39 Overall survival at 1 year did not differ significantly between degarelix 240/80 mg (probability of death by Day 364: 2.6%; 95% CI 1.1–6.2) and leuprolide groups (4.9%; 95% CI 2.6–9.3).
Figure 5 Median percentage change from baseline in PSA levels with degarelix and leuprolide. Panel A depicts the first month of treatment; Panel B shows data from across the 1-year treatment period. Reproduced with permission from Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538.Citation38 Copyright © 2008 Blackwell Publishing Ltd.
Post-hoc subgroup analyses
Exploratory post-hoc subgroup analyses assessing the impact of baseline testosterone, disease stage and PSA level on activity were subsequently performed on data from the CS21 trial for the degarelix 240/80 mg group (the approved dose) versus the leuprolide group only. The effects of both treatments on total serum alkaline phosphatase (S-ALP) levels were also investigated. S-ALP is a recognized marker of metastatic bone disease in patients with prostate cancer.Citation40 In both treatment arms, higher baseline testosterone led to slower achievement of castrate testosterone levels; however, achievement of castrate levels occurred significantly faster with degarelix than with leuprolide for all testosterone subgroups.Citation41 Reduction in PSA levels was also significantly faster with degarelix than leuprolide in all testosterone subgroups (P < 0.0001). In the degarelix group, a rapid reduction in PSA began on day 1, irrespective of baseline testosterone level. In contrast, there was a small increase in PSA on days 3 and 7 in the leuprolide group, which was somewhat larger in the subgroup of patients with baseline testosterone ≥5 ng/mL where the subsequent fall in PSA was also the slowest. In this subgroup of patients, the same level of PSA suppression was achieved for those receiving leuprolide and degarelix, but only after a delay of approximately 2 months in the leuprolide group.Citation41
In additional analyses of the PSA data, it was found that PSA failures occurred mainly in patients with metastatic disease at baseline or PSA levels >50 ng/mL; no PSA failures occurred in those with baseline PSA ≤ 20 ng/mL.Citation39 Patients with metastatic disease or PSA levels > 20 ng/mL at baseline experienced numerically fewer PSA failures with degarelix compared with leuprolide. Patients with metastatic disease or those with PSA levels >50 ng/mL at baseline also experienced greater reductions in S-ALP levels with degarelix than leuprolide.Citation42 Patients in the degarelix group maintained S-ALP suppression throughout the 1-year study and did not display signs of therapy failure, as indicated by the late rises in S-ALP levels that were observed in patients receiving leuprolide.
Safety and tolerability data
In the CS21 trial, both degarelix and leuprolide were well tolerated, with most adverse events being mild or moderate in intensity.Citation38 The most common adverse events observed during degarelix treatment were related to the known effects of androgen deprivation (hot flashes, weight increases) or the mode of administration (injection-site reactions; ). There was a significantly higher incidence of injection-site reactions and chills with subcutaneous degarelix treatment than with the intramuscular leuprolide injection (40% vs <1%; P < 0.001). Injection-site events occurred predominantly after the first dose, with their incidence declining during maintenance treatment. Overall, 33% of 409 starter dose injections and 4% of 2244 and 2208 maintenance dose injections (240/80 and 240/160 mg groups, respectively) were associated with injection-site events. Only five patients (1%) receiving degarelix discontinued treatment due to an injection-site reaction. Significantly more degarelix patients experienced chills (4% vs 0%; P < 0.01), whereas the incidence of disease-related adverse events such as arthralgia (4% vs 9%; P < 0.05) and urinary tract infection (3% vs 9%; P < 0.01) were significantly higher with leuprolide. In an exploratory analysis of tolerability data, musculoskeletal and connective tissue disorders were found to be more common in patients receiving leuprolide than degarelix, irrespective of disease stage.Citation43 Disease-related symptoms such as arthralgia and back pain occurred more frequently in leuprolide patients with metastatic disease than in degarelix patients with metastatic disease.
Table 2 Incidence and intensity of adverse events during degarelix and leuprolide treatment (incidence of ≥5% in any group)
As observed in the phase II dose-finding studies, no immediate-onset systemic allergic reactions were reported during degarelix treatment.Citation38 A similar proportion of patients in each group also experienced alanine aminotransferase/aspartate aminotransferase changes, and there were no statistically significant differences between groups in vital signs, body weight or QT interval.
Efficacy and safety conclusions from the phase III trial
Data from this pivotal phase III trial demonstrated that degarelix was an effective and well-tolerated treatment for patients requiring ADT for prostate cancer. Both degarelix doses (240/80 and 240/160 mg) were as effective as leuprolide in terms of treatment response. Due to its immediate onset of action, FSH, LH, testosterone, and PSA levels fell more rapidly with degarelix than with leuprolide. Unlike the GnRH agonist leuprolide, degarelix did not induce a surge (or microsurges) in testosterone and therefore there is no requirement for concomitant antiandrogen flare protection. The safety profile of degarelix, although displaying hormonal side effects in line with ADT, differed from that of leuprolide, mainly with respect to disease-related events. The higher incidence of injection-site events with degarelix predominantly occurred after the first injection and may be due to the mode of administration and the injection volume. Based on the results of this trial, degarelix 240/80 mg was approved in December 2008 by the FDA for the treatment of patients in the USA with advanced prostate cancer. This was followed in February 2009 by EMEA approval of the same degarelix dose for the treatment of adult male patients with advanced hormone-dependent prostate cancer.
Patient-focused perspectives
Because of their mode of action, all hormonal therapies are associated with side effects that can affect physical, sexual and psychological functioning of men with prostate cancer and may have an adverse impact on a patient’s quality of life. While it remains the “gold standard” ADT, orchiectomy is irreversible and associated with psychological drawbacks, and has therefore now largely been replaced by medical castration, as patients generally prefer this option.Citation44 GnRH agonists are currently the most commonly used form of medical ADT, as treatment with these agents is associated with improved quality of life compared with orchiectomy.Citation44 Nonetheless, the clinical flare associated with GnRH agonists can worsen disease symptomsCitation12 and deleteriously impact upon the quality of life of some patients with advanced disease. Co-administration of antiandrogens may reduce or eliminate flare, but is associated with an additional side effect burden and a higher cost.
Sexual dysfunction (eg, loss of libido and erectile dysfunction) is a common consequence of all types of prostate cancer therapy.Citation45,Citation46 Erectile dysfunction can be effectively treated in many men with the use of phosphodiesterase 5 inhibitors such as sildenafil or tadalafil.Citation46 There is also much interest in the use of intermittent ADT regimens in prostate cancer, as this can reduce the incidence of sexual side effects during off-treatment phases, thereby improving quality of life. This type of treatment may be particularly suitable for younger patients with less aggressive disease.Citation47 Many clinical trials are currently assessing the efficacy and safety of intermittent ADT, including several studies assessing the potential role of degarelix 240/80 mg in such regimens.
Hot flashes are another common side effect of hormonal therapy, being experienced by most men undergoing ADT.Citation46 These can be quite debilitating and may have a substantial impact on quality of life of some men. Transdermal estrogenCitation48 or megestrol acetateCitation49 can improve hot flashes, but are associated with gynecomastia and breast/nipple pain and sometimes also weight gain. Antidepressants such as paroxetine and venlafaxine have been shown to be effective in reducing hot flashes in women with breast cancer, and several small studies have suggested that these agents may also be beneficial in men with prostate cancer.Citation50,Citation51 As depression and emotional lability can also be a consequence of hormonal therapy,Citation45 antidepressants may also have other positive effects on the patient. Gynecomastia and breast pain/tenderness occur commonly in men undergoing antiandrogen therapy and are also associated to a lesser extent with orchiectomy and GnRH agonist treatment.Citation46 Prophylactic breast irradiationCitation52 or tamoxifen treatmentCitation53 can be effective in reducing the incidence and severity of breast pain and gynecomastia, but are both associated with an additional side-effect burden.
ADT increases markers of bone turnover, and several reports have demonstrated that ADT with GnRH receptor agonists results in a progressive decrease in bone mineral density, with the risk of fracture and/or development of osteoporosis increasing with duration of treatment.Citation46 Patients most at risk of adverse skeletal effects appear to be those with osteopenia at the start of treatment.Citation45 Studies on the skeletal consequences of treatment with GnRH receptor blockers such as degarelix are currently lacking, although initial analyses of the effect of degarelix on S-ALP levels suggest improved suppression with this agent compared with leuprolide.Citation42 Increased S-ALP levels can also reflect bone metastases progressionCitation54 and therefore improved S-ALP suppression (as well as fewer PSA failure events) may be suggestive of better disease control with degarelix. Various agents are available for preventing bone loss in prostate cancer patients undergoing ADT, including bisphosphonates such as pamidronate and zoledronic acid.Citation55,Citation56 Lifestyle changes such as stopping smoking and increased resistance weight training in addition to calcium and vitamin D supplementation may also be helpful.Citation45,Citation46 GnRH receptor agonist treatment also results in body composition changes that are accompanied by adverse metabolic consequences such as increased plasma cholesterol, triglycerides, glucose and insulin levels, which in turn increase risk of cardiovascular disease and diabetes.Citation46
Regular patient follow-up may permit early diagnosis and treatment of any treatment-emergent side effects and may therefore also improve outcomes. The monthly injection schedule of degarelix is convenient and also enables the patient to receive regular contact and support from their healthcare provider. This has been shown to be especially important during the early stages of treatment and may also positively contribute to the patient’s coping strategies and quality of life.Citation57 Less regular contact may be preferable to patients later in their treatment course, when any emergent adverse events are already being effectively managed; studies assessing the efficacy and tolerability of a degarelix 3-monthly dosing schedule are currently ongoing.
As prostate cancer is more common in older men, patients often have and are being treated for comorbid conditions in addition to receiving ADT. As well as making it difficult to assess any quality-of-life changes specifically associated with ADT, this could potentially complicate their management. Therefore, the potential for any drug–drug interactions also needs to be examined before initiating therapy. Preclinical studies suggest that degarelix has a mild pharmacokinetic interaction profile and is therefore unlikely to be the subject or cause of clinically significant drug–drug interactions.Citation29
Summary and conclusions
Orchiectomy is unacceptable to many prostate cancer patients, and so medical castration using GnRH receptor agonists now provides the mainstay of ADT for prostate cancer. The recently approved GnRH receptor blocker, degarelix, is as effective and well-tolerated as GnRH agonists but has the advantage of an immediate onset of action and faster testosterone and PSA suppression, without a surge or microsurges. The pharmacological effects of degarelix therefore more closely resemble those of orchiectomy. Although generally more acceptable to patients than orchiectomy, all forms of medical castration are associated with adverse events that may impact upon a patient’s quality of life. However, with appropriate follow-up and treatment, many of these adverse events can be overcome. In summary, degarelix is an effective and well-tolerated agent that provides a useful addition to the hormonal armamentarium for prostate cancer, offering patients with hormone-sensitive advanced disease a valuable alternative treatment option.
Acknowledgments and disclosures
Reconstitution data for degarelix were provided by Pernille Rosted, Keith Fox and Jette Toft from Ferring Pharmaceuticals. Medical writing support was provided by Dawn Batty PhD of Bioscript Stirling Ltd and funded by Ferring Pharmaceuticals.
References
- HugginsCStevensREJHodgesCVStudies in prostatic cancer II. The effects of castration on advanced carcinoma of the prostate glandArch Surg194143209223
- FDA, US Food and Drug AdministrationCancer drug approval endpoints for prostate cancer 2009 Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/CancerDrugs/ucm094586.htm; Accessed Nov 16 2009.
- FlemingMTMorrisMJHellerGScherHIPost-therapy changes in PSA as an outcome measure in prostate cancer clinical trialsNat Clin Pract Oncol200631265866717139317
- LiljaHUlmertDVickersAJProstate-specific antigen and prostate cancer: prediction, detection and monitoringNat Rev Cancer20088426827818337732
- HuhtaniemiIWhiteRMcArdleCAPerssonBEWill GnRH antagonists improve prostate cancer treatment?Trends Endocrinol Metab2009201435019008119
- RohlHFBeukeHPEffect of orchidectomy on serum concentrations of testosterone and dihydrotestosterone in patients with prostatic cancerScand J Urol Nephrol199226111141631501
- HeidenreichABollaMJoniauSGuidelines on Prostate CancerEuropean Association of Urology2009 Available from: http://www.uroweb.org/professional-resources/guidelines/online; Accessed Oct 26 2009.
- ConnPMCrowleyWFJrGonadotropin-releasing hormone and its analoguesN Engl J Med19913242931031984190
- SasagawaIKubotaYNakadaTInfluence of luteinizing hormone-releasing hormone analogues on serum levels of prostatic acid phosphatase and prostatic specific antigen in patients with metastatic carcinoma of the prostateInt Urol Nephrol199830674575310195870
- TomeraKGleasonDGittelmanMThe gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: initial results of endocrinological and biochemical efficacies in patients with prostate cancerJ Urol200116551585158911342922
- ThompsonIMZeidmanEJRodriguezFRSudden death due to disease flare with luteinizing hormone-releasing hormone agonist therapy for carcinoma of the prostateJ Urol19901446147914802122011
- Van PoppelHNilssonSTestosterone surge: rationale for gonadotropin-releasing hormone blockers?Urology20087161001100618407326
- PerlmutterMALeporHAndrogen deprivation therapy in the treatment of advanced prostate cancerRev Urol20079Suppl 1S3S817387371
- KaisaryAVIversenPTyrrellCJCarrollKMorrisTIs there a role for antiandrogen monotherapy in patients with metastatic prostate cancer?Prostate Cancer Prostatic Dis20014419620312497018
- HeidenreichHAusGAbbouCCGuidelines on prostate cancerEuropean Association of Urology2007 Available from: http://www.uroweb.org/fileadmin/user_upload/Guidelines/07_Prostate_Cancer_2007.pdf; Accessed Aug 10 2009.
- SharifiRBrownellerRSerum testosterone suppression and potential for agonistic stimulation during chronic treatment with monthly and 3-month depot formulations of leuprolide acetate for advanced prostate cancerJ Urol200216831001100412187208
- ZinnerNRBidairMCentenoATomeraKSimilar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trialUrology20046461177118115596193
- MoroteJOrsolaAPlanasJRedefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapyJ Urol20071784 Pt 11290129517698136
- PerachinoMCavalliVBraviFTestosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance?BJU Int2009828 [Epub ahead of print].
- JockenhovelFBhasinSSteinerBSRivierJEValeWWSwerdloffRSHormonal effects of single gonadotropin-releasing hormone antagonist doses in menJ Clin Endocrinol Metab1988665106510703129447
- SalamehWBhasinSSteinerBMcAdamsLAPetersonMSwerdloffRMarked suppression of gonadotropins and testosterone by an antagonist analog of gonadotropin-releasing hormone in menFertil Steril19915511561641898888
- BagatellCJConnPMBremnerWJSingle-dose administration of the gonadotropin-releasing hormone antagonist, Nal-Lys (antide) to healthy menFertil Steril19936046806858405525
- BagatellCJRivierJEBremnerWJDose effects of the gonadotropin-releasing hormone antagonist, Nal-Glu, combined with testosterone enanthate on gonadotropin levels in normal menFertil Steril19956411391457789550
- KlingmullerDSchepkeMEnzweilerCBidlingmaierFHormonal responses to the new potent GnRH antagonist CetrorelixActa Endocrinol (Copenh)1993128115188447189
- BroquaPRivierePJConnPMRivierJEAubertMLJunienJLPharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelixJ Pharmacol Exp Ther200230119510211907162
- DebruyneFBhatGGarnickMBAbarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancerFuture Oncol20062667769617155895
- PR NewswireFDA approves Ferring Pharmaceuticals’ Degarelix (generic name) for the treatment of advanced prostate cancerPR Newswire, Europe Ltd2008 Available from: http://www.prnewswire.co.uk/cgi/news/release?id=245656; Accessed Mar 2 2009.
- JiangGStalewskiJGalyeanRGnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6J Med Chem200144345346711462984
- European Medicines AgencyAssessment report for Firmagon (Doc. Ref: EMEA/CHMP/635761/2008). Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/firmagon/H-986-en6.pdf; Accessed Apr 28 2009.
- KoechlingWEffect of various GnRH antagonists on histamine release from human skinPoster presented at the 8th International Symposium on GnRH Analogues in Cancer and Human Reproduction2005 Nov 2Salzburg, Austria
- AgersoHKoechlingWKnutssonMHjortkjaerRKarlssonMOThe dosing solution influence on the pharmacokinetics of degarelix, a new GnRH antagonist, after s.c. administration to beagle dogsEur J Pharm Sci200320333534014592699
- DoehnCSommerauerMJochamDDrug evaluation: Degarelix – a potential new therapy for prostate cancerIDrugs20069856557216871466
- Degarelix US prescribing information2008 Available from: http://www.fda.gov/cder/foi/label/2008/022201lbl.pdf; Accessed Apr 28 2009.
- BalchenTAgersoHOlesenTKJensenJKSenderovitzTPharmacokinetics, pharmacodynamics, and safety of a novel fast-acting gonadotropin-releasing hormone receptor blocker, degarelix, in healthy menPoster presented at the 8th International Symposium on GnRH Analogues in Cancer and Human Reproduction2005 Nov 2Salzburg, Austria
- IversenPVan PoppelHTammelaTDetermining the optimal initiation and maintenance dose of degarelix for hormone therapy of prostate cancer patientsPoster presented at the 28th Congress of the Société Internationale d’Urologie2006 Nov 12–15Cape Town, South Africa
- Van PoppelHTombalBde la RosetteJJPerssonBEJensenJKKoldOTDegarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker – results from a 1-yr, multicentre, randomised, phase II dosage-finding study in the treatment of prostate cancerEur Urol200854480581318538469
- GittelmanMPommervillePJPerssonBEJensenJKOlesenTKA 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North AmericaJ Urol200818051986199218801505
- KlotzLBoccon-GibodLShoreNDThe efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancerBJU Int2008102111531153819035858
- TombalBMillerKBoccon-GibodLAdditional analysis of the secondary endpoint of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in patients with prostate cancer segmented by baseline characteristicsEur Urol20091120 [Epub ahead of print].
- JungKLeinMStephanCComparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implicationsInt J Cancer2004111578379115252851
- DamberJETammelaTAbrahamssonPAComparing testosterone and PSA for different baseline testosterone concentrations during initiation of degarelix and leuprolide treatment [abstract]Eur Urol2009Suppl 8130
- SchröderFHTombalBMillerKChanges in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12-month, comparative, phase III studyBJU Int20091113 [Epub ahead of print].
- IversenPJensenJKOlesenTKPerssonBEPhase III trial of degarelix vs leuprolide: comparing safety and tolerabilityPresented at the World Congress on Controversies in Urology2009 Feb 5Lisbon, Portugal Abstract 618148.
- CassilethBRSolowayMSVogelzangNJQuality of life and psychosocial status in stage D prostate cancer. Zoladex Prostate Cancer Study GroupQual Life Res1992153233291299464
- GomellaLGContemporary use of hormonal therapy in prostate cancer: managing complications and addressing quality-of-life issuesBJU Int200799Suppl 1252917229166
- MichaelsonMDCotterSEGargolloPCZietmanALDahlDMSmithMRManagement of complications of prostate cancer treatmentCA Cancer J Clin200858419621318502900
- GleaveMKlotzLTanejaSSThe continued debate: intermittent vs continuous hormonal ablation for metastatic prostate cancerUrol Oncol2009271818619111804
- GerberGSZagajaGPRayPSRukstalisDBTransdermal estrogen in the treatment of hot flushes in men with prostate cancerUrology20005519710110654902
- LoprinziCLMichalakJCQuellaSKMegestrol acetate for the prevention of hot flashesN Engl J Med199433163473528028614
- LoprinziCLPisanskyTMFonsecaRPilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivorsJ Clin Oncol1998167237723819667254
- LoprinziCLBartonDLCarpenterLAPilot evaluation of paroxetine for treating hot flashes in menMayo Clin Proc200479101247125115473404
- WidmarkAFossaSDLundmoPDoes prophylactic breast irradiation prevent antiandrogen-induced gynecomastia? Evaluation of 253 patients in the randomized Scandinavian trial SPCG-7/SFUO-3Urology200361114515112559286
- SaltzsteinDSieberPMorrisTGalloJPrevention and management of bicalutamide-induced gynecomastia and breast pain: randomized endocrinologic and clinical studies with tamoxifen and anastrozoleProstate Cancer Prostatic Dis200581758315685254
- LeinMWirthMMillerKSerial markers of bone turnover in men with metastatic prostate cancer treated with zoledronic acid for detection of bone metastases progressionEur Urol20075251381138717321667
- SmithMRMcGovernFJZietmanALPamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancerN Engl J Med20013451394895511575286
- RyanCWHuoDBylowKSuppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acidBJU Int20071001707517552955
- LebretTBouregbaARoles of the urologist and nurse from the perspective of patients with prostate cancer receiving luteinizing hormone-releasing hormone analogue therapyBJU Int2008102101419142418549431
- AndersonJDegarelix: a novel gonadotropin-releasing hormone blocker for the treatment of prostate cancerFuture Oncol20095443344319450172