Abstract
Oxidative stress is an important feature in the pathogenesis of COPD. Targeting oxidative stress with antioxidants or boosting the endogenous levels of antioxidants is likely to be beneficial in the treatment of COPD. Antioxidant agents such as thiol molecules (glutathione and mucolytic drugs, such as N-acetyl-L-cysteine and N-acystelyn), dietary polyphenols (curcumin, resveratrol, green tea, catechins/quercetin), erdosteine, and carbocysteine lysine salt, all have been reported to control nuclear factor-kappaB (NF-κ B) activation, regulation of glutathione biosynthesis genes, chromatin remodeling, and hence inflammatory gene expression. Specific spin traps such as α-phenyl-N-tert-butyl nitrone, a catalytic antioxidant (ECSOD mimetic), porphyrins (AEOL 10150 and AEOL 10113), and a superoxide dismutase mimetic M40419 have also been reported to inhibit cigarette smoke-induced inflammatory responses in vivo. Since a variety of oxidants, free radicals, and aldehydes are implicated in the pathogenesis of COPD, it is possible that therapeutic administration of multiple antioxidants will be effective in the treatment of COPD. Various approaches to enhance lung antioxidant capacity and clinical trials of antioxidant compounds in COPD are discussed.
Introduction
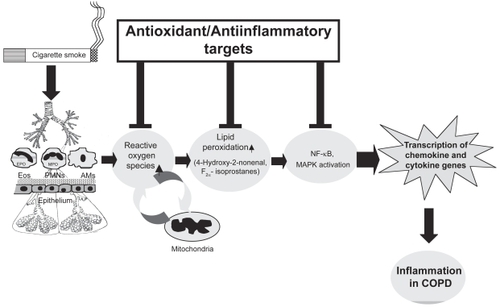
Reactive oxygen species (ROS), such as superoxide anion (O2•−) and the hydroxyl radical (•OH), are highly unstable species with unpaired electrons capable of initiating oxidation. Lungs are continuously exposed to oxidants, generated either endogenously by metabolic reactions (eg, from mitochondrial electron transport during respiration or during activation of phagocytes) or exogenously, such as from air pollutants or cigarette smoke. Production of ROS has been directly linked to oxidation of proteins, DNA, and lipids, which may cause direct lung injury or induce a variety of cellular responses through the generation of secondary metabolic reactive species. ROS may alter remodeling of extracellular matrix and blood vessels, stimulate mucus secretion, inactivate antiproteases, cause apoptosis, and regulate cell proliferation (CitationRahman and MacNee 1996a, Citation1999). Furthermore, increased levels of ROS have been implicated in initiating inflammatory responses in the lungs through the activation of transcription factors such as nuclear factor-kappaB (NF-κ B) and activator protein-1 (AP-1), signal transduction, chromatin remodeling, and gene expression of proinflammatory mediators (CitationRahman and MacNee 1998; CitationRahman 2003) (). Since a variety of oxidants, free radicals, and aldehydes are implicated in the pathogenesis of COPD, it is possible that therapeutic administration of a variety of antioxidants will be effective in the treatment of COPD.
Figure 1 Mechanism of reactive oxygen species (ROS)-mediated lung inflammation. Inflammatory response is mediated by oxidants inhaled and/or released by the activated neutrophils, alveolar macrophages, eosinophils, and epithelial cells, leading to production of ROS and membrane lipid peroxidation. Activation of transcription of the proinflammatory cytokine and chemokine genes, up-regulation of adhesion molecules, and increased release of proinflammatory mediators are involved in the inflammatory responses in patients with COPD.
This paper discusses the rationale for antioxidant therapeutic intervention in the light of oxidative stress in the management of COPD.
Inhaled oxidants and cigarette smoke
COPD is a slow, progressive condition characterized by airflow limitation, which is largely irreversible (CitationATS 1995; CitationPauwels et al 2001). Cigarette smoking is the major etiological factor in this condition. More than 90% of patients with COPD are smokers, but only 15%–20% of cigarette smokers show a rapid decline FEV1 and develop the disease (CitationSnider 1989). An increased oxidant burden in smokers derives from the fact that cigarette smoke contains more than 1017 oxidant/free radical molecules per puff and more than 4700 chemicals (CitationChurch and Pryor 1985).
Cell-derived ROS
The common feature of COPD is the development of an inflammatory response, characterized by activation of epithelial cells and resident macrophages and the recruitment and activation of neutrophils, eosinophils, monocytes, and lymphocytes (CitationRahman and MacNee 1996a). The activation of macrophages, neutrophils, and eosinophils generates O2•−, which is rapidly converted to H2O2 under the influence of superoxide dismutase (SOD), and •OH is formed non-enzymatically in the presence of Fe2+ as a secondary reaction. ROS can also be generated intracellularly from several sources. The primary ROS-generating enzyme is NADPH oxidase, a complex enzyme system that is present in phagocytes and epithelial cells. In addition to NADPH oxidase, phagocytes employ other enzymes to produce ROS, which involves the activity of heme peroxidases (myeloperoxidase, MPO) or eosinophil peroxidase (EPO). Superoxide anion and H2O2 can also be generated by mitochondria and the xanthine/xanthine oxidase (XO) reaction. XO activity has been shown to be increased in cell-free bronchoalveolar lavage (BAL) fluid and plasma from COPD patients, compared with normal individuals. This has been associated with increased O2•− and lipid peroxide levels (CitationPinamonti et al 1998).
Oxidative stress biomarkers in exhaled breath condensate
Identification of non-invasive biomarkers of oxidative stress is important because antioxidant clinical trials or supplementation studies could potentially be monitored by measuring markers of oxidative stress. Recent studies have focused on applying non-invasive techniques, such as biomarkers in exhaled breath condensate to evaluate oxidative stress in COPD (CitationRahman and Kelly 2003; CitationRahman and Biswas 2004). Smokers and patients with COPD have higher levels of exhaled H2O2 than non-smokers, and levels are even higher during exacerbations of COPD (CitationKharitonov and Barnes 2001; CitationMontuschi and Barnes 2002). The levels of 8-iso-prostaglandin F2α (8-isoprostane) in exhaled breath condensate are elevated in healthy smokers and more markedly in patients with COPD, reflecting the degree of oxidative stress (CitationRahman and MacNee 1998; CitationMontuschi et al 2000). Non-specific lipid peroxidation products, such as thiobarbituric acid reactive substances (TBARS), have also been shown to be elevated in breath condensate and in lungs of patients with stable COPD (CitationPratico et al 1998; CitationNowak et al 1999). Other specific products of lipid peroxidation such as malondialdehyde and 4-HNE have also been shown to be increased in exhaled breath condensate of COPD patients (CitationRahman et al 2000; CitationRahman, van Schadewijk, et al 2002; CitationAoshiba et al 2003).
Antioxidant therapeutic interventions
Systemic antioxidant capacity and antioxidant vitamins
Smoking and exacerbations of COPD result in decreased antioxidant capacity in plasma in association with depleted protein sulfhydryls in the plasma (CitationRahman et al 1996, Citation1997; CitationCorradi et al 2003). The decrease in antioxidant capacity in smokers occurs transiently during smoking and resolves rapidly after smoking cessation. In exacerbations of COPD, however, antioxidant capacity remains low for several days after the onset of the exacerbation, tending to return towards normal values at the time of recovery from the exacerbation (CitationRahman et al 1997). The depletion of antioxidant capacity could in part be explained by the increased release of ROS from peripheral blood neutrophils, as shown by a significant negative correlation between neutrophil superoxide anion release and plasma antioxidant capacity (CitationRahman et al 1996). Thus, there is clear evidence that oxidants in cigarette smoke markedly decrease plasma antioxidants, which may play an important role in the pathogenesis of COPD. Furthermore, it is possible that interindividual differences in antioxidant capacity may contribute to the differences in susceptibility to cigarette smoke-induced COPD. Therefore, it is imperative to propose the rationale for antioxidant therapy ameliorating the increased oxidative stress and consequently the inflammatory response in COPD. There are various options to enhance the lung antioxidant screen ().
Table 1 Antioxidant therapeutic interventions in COPD
Depletion of total antioxidant capacity in smokers is associated with decreased levels of major plasma antioxidants in smokers (CitationPelletier 1970; CitationPetruzzelli et al 1990; CitationDuthie et al 1991; CitationAntwerpen et al 1993; CitationBridges et al 1993; CitationMezzetti et al 1995; CitationRahman and MacNee 1996a). These studies show depletion of vitamin C, vitamin E, β-carotene, and selenium in the serum of chronic smokers and in patients with COPD (CitationPelletier 1970; CitationChow et al 1986; CitationDuthie et al 1991; CitationAntwerpen et al 1993; CitationBridges et al 1993; CitationMezzetti et al 1995; CitationTug et al 2004). Moreover, decreased vitamin E and vitamin C levels were reported in leukocytes and BAL fluids from smokers (CitationBarton and Roath 1976; CitationBridges et al 1990; CitationTheron et al 1990). Ascorbate appears to be a particularly important antioxidant in the plasma (CitationPacht et al 1988). Cigarette smoke-induced lipid peroxidation of plasma in vitro is decreased by ascorbate (CitationCross et al 1994). Reduced levels of vitamin E and a marginal increase in vitamin C have been shown in the BAL fluid of smokers, compared with non-smokers (CitationRahman and MacNee 1996b). Similarly, alveolar macrophages from smokers have both increased levels of vitamin C and augmented uptake of ascorbate, suggesting that these cells are trying to redress their antioxidant balance (CitationRahman and MacNee 1996b).
Dietary antioxidant supplementation is one of the simplest approaches to boost antioxidant defense systems. Supplementation of vitamin C, vitamin E, and β-carotene has been attempted in cigarette smokers and patients with COPD (CitationCross et al 1993; CitationAllard et al 1994; CitationRautalahti et al 1997; CitationSteinberg and Chait 1998; CitationAghdassi et al 1999; CitationHabib et al 1999; CitationLykkesfeldt et al 2000) (). In the general population there is a positive association between dietary intake of antioxidant vitamins and lung function. Epidemiological studies have demonstrated negative associations of dietary antioxidant intake with pulmonary function and with obstructive airway disease (CitationGrievink et al 1998). CitationBritton and co-workers (1995) showed a positive association between dietary intake of the antioxidant vitamin E and lung function in a population of 2633 subjects, supporting the hypothesis that this antioxidant may have a role in protecting against the development of COPD. Another study has suggested that antioxidant levels in the diet could be a possible explanation for differences in COPD mortality in different populations (CitationSargeant et al 2000). Dietary polyunsaturated fatty acids may also protect cigarette smokers against the development of COPD (CitationShahar et al 1999). These studies support the concept that dietary antioxidant supplementation including polyphenols may be a possible therapy to prevent or inhibit oxidative stress and inflammatory responses, which are key features in the development of COPD.
Table 2 Clinical trials conducted for the efficacy of antioxidants in smokers and in COPD
Antioxidant vitamin supplementation reduces oxidant stress in smokers, measured as a decrease in pentane levels in breath as an indication of lipid peroxides in the airways (CitationEuler et al 1996). CitationDietrich and colleagues (2002) have recently shown that vitamin C or an antioxidant mixture containing vitamin C, α-lipoic acid, and vitamin E decreases plasma F2-isoprostane levels in smokers with high body mass index, suggesting modulation of lung oxidative stress with these dietary supplements. This study suggested that cigarette smoking depletes a variety of multiple antioxidants needed to quench an array of free radicals present in cigarette smoke and inhibit inflammatory response induced by cigarette smoking. However, robust clinical trials using dietary antioxidant vitamins and polyphenols are urgently needed to address the beneficial effects of these antioxidants in COPD.
Directly increasing lung antioxidant capacity
The development and progress of COPD are associated with increased oxidative stress or decreased antioxidant resources (CitationBoots et al 2003). The most direct way to redress the oxidant imbalance in COPD would be to increase the lung’s capacity to produce antioxidants. A variety of means by which to do this have been attempted with varying success.
Lung thiols: glutathione and its biosynthesis
The thiol antioxidant glutathione (GSH) is concentrated in epithelial lining fluid compared with plasma (CitationCantin et al 1987; CitationPacht et al 1988) and has an important protective role in the airspaces and intracellularly in epithelial cells. Several studies have suggested that GSH homeostasis may play a central role in the maintenance of the integrity of the lung airspace epithelial barrier. Decreasing the levels of GSH in epithelial cells leads to loss of barrier function and increased permeability (CitationLi et al 1996; CitationMorrison et al 1999). Human studies have shown elevated levels of GSH in epithelial lining fluid in chronic cigarette smokers compared with non-smokers (CitationCantin et al 1987; CitationMorrison et al 1999). However, this increase is not present immediately after acute cigarette smoking (CitationMorrison et al 1999). The twofold increase in BAL fluid GSH in chronic smokers may not be sufficient to deal with the excessive oxidant burden during smoking, when acute depletion of GSH may occur (CitationHarju et al 2002). CitationHarju and colleagues (2002) found that the immunoreactivity of glutamate cysteine ligase (GCL), the rate-limiting enzyme in GSH synthesis, was decreased in the airways of smokers compared with non-smokers, suggesting that cigarette smoke predisposes lung cells to ongoing oxidant stress. CitationNeurohr and colleagues (2003) recently showed that decreased GSH levels in BAL fluid cells of chronic smokers were associated with a decreased expression of GCL light subunit without a change in GCL heavy subunit expression. Increasing the activity of GCL would be expected to increase cellular GSH levels. The induction of GCL by molecular means to increase cellular GSH levels or GCL gene therapy also holds great promise in protection against chronic inflammation and oxidant-mediated injury in COPD.
Direct increase of lung cellular levels of GSH would be a logical approach to enhance the antioxidant potential in the treatment of COPD. In fact, extracellular augmentation of GSH has been tried through intravenous administration of GSH, oral ingestion of GSH, and aerosol inhalation of nebulized GSH in an attempt to reduce inflammation in various lung diseases (CitationRahman and MacNee 1999, Citation2000). However, all these routes of administration lead to undesirable effects, which suggests that direct GSH therapy may not be an appropriate way of increasing GSH levels in lung epithelial lining fluid and cells in COPD. The bioavailability of GSH, pH, and osmolality in the inflammatory microenvironment, and resultant formation of toxic products (GSSG and GSH-adducts) are further challenges for direct GSH administration. Alternative formulations may address bioavailability, such as liposomal delivery, but at present it seems that direct administration of GSH will not be successful in treating COPD.
N-acetyl-L-cysteine (NAC)
NAC, a cysteine-donating reducing compound, acts as a cellular precursor of GSH and becomes deacetylated in the gut to cysteine following oral administration (CitationCotgreave 1997). NAC may also reduce cystine to cysteine, which is an important mechanism for intracellular GSH elevation in vivo in lungs. It reduces disulfide bonds (a property of a good reducing agent), but also has the potential to interact directly with oxidants. NAC is also used as a mucolytic agent, to reduce mucus viscosity and to improve mucociliary clearance. A Cochrane systematic review evaluated the effects of treatment with mucolytic agents in patients with COPD. Mucolytic treatment was associated with a significant reduction of 0.79 exacerbations per patient per year compared with placebo, a 29% decrease. How mucolytic agents work is unknown, although they may reduce exacerbations by altering mucus production, by breakdown of sulfhydryl groups, or through antibacterial or immunostimulatory effects (CitationPoole and Black 2001, Citation2003). Although small-scale trials failed to demonstrate any clear clinical benefits, a few meta-analyses have shown a small but significant clinical benefit in COPD (CitationGrandjean et al 2000; CitationDekhuijzen 2004).
Pharmacological administration of NAC has been used, with varying success, in an attempt to enhance lung GSH in patients with COPD (CitationRasmusse and Glennow 1988; CitationBridgemen et al 1994). CitationVan Schooten et al (2002) have reported that in a randomized, double-blind, placebo-controlled phase II trial, a 6-month oral dose of 600 mg twice daily reduced various plasma and BAL fluid oxidative biomarkers in smokers. Similarly, it has been shown that treatment with NAC 600 mg once daily for 12 months also reduced the concentration of H2O2 in exhaled breath condensate compared with placebo in stable COPD patients (CitationKasielski and Nowak 2001). A more recent clinical trial also proves that oral administration of NAC 600 mg twice daily for 2 months rapidly reduces the oxidant burden in airways of stable COPD patients (CitationDe Benedetto et al 2005). This reduction in oxidative biomarkers results in clinical benefit such as reduction in bronchial hypersecretion (CitationAylward et al 1980) in addition to a decline in FEV1 and in exacerbations (CitationStey et al 2000). Orally administered NAC has been shown to increase phagocytic activity of BAL macrophages from healthy smokers (CitationLinden et al 1993); similar results were not seen in COPD patients, possibly owing to active concentrations of NAC not reaching the lung, as in vitro analysis of cells supports an induction of phagocytosis by NAC (CitationVecchiarelli et al 1994). It has also been reported recently that orally administered NAC increased the quadriceps endurance time of severe COPD patients (CitationKoechlin et al 2004), thus suggesting that NAC administration may have beneficial effects on the systemic oxidative stress associated with COPD. However, a multicenter study using NAC delivered by metered-dose inhalers in patients with chronic cough failed to show a positive effect on wellbeing, sensation of dyspnea, cough, or lung function (CitationDueholm et al 1992).
Although there is a body of evidence that the administration of NAC provides benefit for COPD patients, it is not clear whether this represents a maintenance therapy (CitationDecramer et al 2001). A phase III multicenter trial, Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS), has recently been completed, with the aim of addressing this question and determining whether the effectiveness of NAC as an “antioxidant” results in an alteration in the rate of decline in FEV1, exacerbation rate, and quality of life in patients with moderate to severe COPD and hence supporting NAC administration as a maintenance therapy for COPD (CitationGerrits et al 2003) (). The results of the phase III BRONCUS trial showed no effect on decline in FEV1 but a reduction in overinflation in patients with severe COPD and exacerbation rate in patients not treated with inhaled glucocorticoids (CitationDecramer et al 2005).
N-acystelyn (NAL)
NAL, a lysine salt of NAC, is a mucolytic and antioxidant (reducing) thiol compound. The advantage of NAL over NAC is that it has a neutral pH in solution, whereas NAC is acidic. NAL can be aerosolized into the lung without causing significant side effects (CitationGillissen et al 1997). Gillissen and co-workers compared the effect of NAL and NAC and found that both drugs enhanced intracellular GSH in alveolar epithelial cells and inhibited hydrogen peroxide and O2• − released from human blood-derived polymorphonuclear leukocytes from smokers with COPD. NAL also inhibited ROS generation induced by serumopsonized zymosan by human polymorphonuclear neutrophils. This inhibitory response was comparable to the effects of NAC (CitationGillissen et al 1997). Recently, CitationAntonicelli and colleagues (2002) have shown that NAL inhibited oxidant-mediated interleukin (IL)-8 release in alveolar epithelial A549 cells, suggesting an antiinflammatory effect of NAL. Therefore, NAL may represent an interesting alternative approach to augment the antioxidant screen, thereby inhibiting inflammatory responses in the lungs, and it has the advantage over other antioxidant agents in that it may be administered by inhalation. A clinical trial using NAL in the treatment of COPD is in progress.
N-isobutyrylcysteine (NIC)
Because NAC becomes hydrolyzed in biological systems, the measured bioavailability of the drug is low. Thus, it was speculated that a drug might be synthesized that had greater bioavailability than NAC and could be used as a more effective treatment for chronic bronchitis. NIC is a NAC-like thiol compound that does not undergo effective first-pass hydrolysis and hence has higher oral bio-availability than NAC (CitationEkberg-Jansson et al 1999). The oral bioavailability can be as high as 80%, depending on food intake. However, when evaluated as a therapy for exacerbations of chronic bronchitis, NIC performed no better than placebo and not as well as NAC (CitationGillissen et al 1997). Furthermore, a study of NIC also failed to reduce exacerbation rates in patients with COPD (CitationEkberg-Jansson et al 1999).
Erdosteine
Erdosteine is a new thiol compound that acts as an antioxidant, but in addition has mucoactive properties and reduces bacterial adhesiveness. In the “Equalife” randomized, placebo-controlled clinical study, erdosteine was administered orally 300 mg twice daily for 8 months (CitationMoretti et al 2004). Patients receiving erdosteine had significantly fewer exacerbations and spent fewer days in hospital than the placebo group. Moreover, patients receiving erdosteine showed no reduction in lung function over this period and showed a significant improvement in health-related quality of life. It is not clear whether this clinical benefit is due to antioxidant effects of erdosteine. The mucolytic effect of erdosteine is perhaps due to the presence of a sulfhydryl group. It may be possible that erdosteine reduces bacterial colonization through a direct effect on adhesion.
Procysteine
Procysteine (L-2-oxothiazolidine-4-carboxylate) is a cysteine-donating compound that increases the cysteine levels of the cells and has greater bioavailability than NAC. This thiol compound is well tolerated and has been shown to increase mitochondrial levels of GSH in alveolar type II cells (CitationGuidot and Brown 2000). Glutathione esters, particularly GSH monoethyl esters, can increase the GSH levels of the cells by cleavage of ester bond (an ethyl group esterified to glycine). GSH esters have been shown to increase GSH levels in the lungs of rats; however, this compound can be cytotoxic, and variation in the uptake levels of GSH has been shown in various cellular models (CitationButterworth et al 1993).
Antioxidant enzyme mimetics and spin traps
Increased activity of antioxidant enzymes (SOD and superoxide catalase) in alveolar macrophages from young smokers has been reported (CitationMcCusker and Hoidal 1990). However, CitationKondo and co-workers (1994) found that the increased superoxide generation by alveolar macrophages in elderly smokers was associated with decreased antioxidant enzyme activity when compared with that of non-smokers. The activities of CuZn superoxide dismutase (CuZnSOD), glutathione-S-transferase, and glutathione peroxidase are all decreased in alveolar macrophages from elderly smokers (CitationGilks et al 1998).
The activities of SOD and glutathione peroxidase have been shown to be higher in the lungs of rats exposed to cigarette smoke (CitationYork et al 1976). CitationMcCusker and Hoidal (1990) have also demonstrated enhanced antioxidant enzyme activity in alveolar macrophages from hamsters after cigarette smoke exposure, which resulted in reduced mortality when the animals were subsequently exposed to >95% oxygen. They speculated that alveolar macrophages undergo an adaptive response to chronic oxidant exposure that ameliorates potential damage to lung cells from further oxidant stress. The mechanisms for the induction of antioxidant enzymes in erythrocytes (CitationToth et al 1986), alveolar macrophages (CitationMcCusker and Hoidal 1990), and lungs (CitationYork et al 1976) by cigarette smoke exposure are currently unknown.
Spin traps such as α-phenyl-N-tert-butyl nitrone react directly with reactive oxygen and reactive nitrogen species at the site of inflammation (CitationChabrier et al 1999). CitationSmith and colleagues (2002) have shown that intratracheal instillation of a catalytic antioxidant, manganese(III) mesotetrakis (N,N’-diethyl-1,3-imidazolium-2-yl) porphyrin (AEOL 10150 and AEOL 10113), inhibited the cigarette smoke-induced inflammatory response (decreased number of neutrophils and macrophages) in rats after 2 days or 8 weeks (6 hours/day, 3 days/week) exposure. These compounds also mimic extracellular SOD and superoxide catalase, scavenging both lipid peroxides and peroxynitrite, and have been shown to be effective in a number of animal models of lung disease.
SOD mimetic M40419 has been shown to block the development of emphysema and significantly reduce lung markers of oxidative stress in an animal model (CitationTuder et al 2003). Animal studies have shown that recombinant SOD treatment can prevent the neutrophil influx to the airspaces and IL-8 release induced by cigarette smoking through a mechanism involving down-regulation of NF-κ B (CitationNishikawa et al 1999). This further substantiates the idea that compounds with antioxidant enzyme properties may be able to act as novel antiinflammatory drugs by regulating the molecular events in COPD.
Glutathione peroxidase mimic
Small molecules with enzymatic activity similar to glutathione peroxidase, such as the selenoorganic compound ebselen, have been developed. Ebselen increases the efficiency of GSH as an antioxidant and can thus be used as therapy against oxidative stress and inflammation. Recent studies have shown that ebselen inhibits airway inflammation (neutrophil recruitment and chemokine expression) in response to lipopolysaccharide in various animal models (CitationHaddad et al 2002; CitationZhang et al 2002). It would be interesting to see whether similar results can be obtained with ebselen in response to smoking in vivo.
Redox sensor molecules
There is a range of small redox molecules such as β-strand mimetic templates MOL-294 and PNRI-299 that have been shown to inhibit NF-κ B and AP-1-mediated transcription and block allergic airway inflammation in a mouse asthma model (CitationHenderson et al 2002). The mechanism of inhibition is based on the reversible inhibition of redox sensor proteins (similar to redox effector factor-1). These redox compounds are novel and have been shown to reduce airway eosinophil infiltration, mucus hypersecretion, edema, and cytokine release in a mouse asthma model. However, the activity of these compounds in cigarette smoke-induced oxidative stress and proinflammatory mediator release assays in vitro or in vivo has not been reported.
Inhibition of superoxide production from inflammatory neutrophils: phosphodiesterase 4 inhibitor
Various mechanisms inhibit superoxide production in vitro through increasing intracellular levels of cyclic adenosine monophosphate (cAMP); the most advanced of these being the use of phosphodiesterase 4 (PDE4) inhibitors. These inhibitors act by increasing intracellular concentrations of cAMP, which has a broad range of antiinflammatory effects on various key effector cells involved in asthma and COPD (CitationLipworth 2005). Raising cAMP in neutrophils blocks the assembly of NADPH oxidase and hence inhibits superoxide production. These compounds also potently inhibit expression of a variety of cytokines, such as tumor necrosis factor (TNF)-α and monocyte inflammatory protein (MIP)-1β, and therefore may have a broad antiinflammatory profile. Clinical benefit of PDE4 inhibitors has been demonstrated in COPD (CitationCompton et al 1999; CitationSoto and Hanania 2005), although these compounds are dose limited by emetic and cardiac effects (CitationSturton and Fitzgerald 2002). This is perhaps due to inhibition of a wrong isoform of PDE4 isoenzyme (PDE4D), instead of PDE4B (antiinflammatory). Currently the PDE4 inhibitor daxos and roflumilast (Altana) is in phase III trials and may represent the most likely new mechanism to enter the market for the treatment of COPD in the short term.
Modulation of redox-sensitive transcription factors and inflammatory pathways
A number of transcription factors involved in the regulation of a variety of inflammatory genes, such as NF-κ B and AP-1, are activated by oxidative stress (CitationRahman 2002). NF-κ B exists as a heterodimeric complex usually of p50 and p65/RelA subunits. CitationDi Stefano and colleagues (2002) demonstrated increased expression of the p65 protein of NF-κ B in bronchial epithelium of smokers and patients with COPD. The increased expression of p65 in epithelial cells was correlated with the degree of airflow limitation in patients with COPD. Similarly, CitationCaramori and co-workers (2003) have shown that the p65 subunit of NF-κ B was increased in sputum macrophages but not in sputum neutrophils during exacerbations of COPD, suggesting cell-specific activation of this factor. The activation of NF-κ B in monocytes/macrophages can then trigger the release of proinflammatory mediators in lung epithelial fluid, which would then amplify the inflammatory cascade by activation of epithelial cells as well as recruitment of neutrophils to the airways. Activation of NF-κ B by oxidative stress is inhibited by co-incubation with NAC in vitro, providing evidence for activation of this transcription factor in these patients being due to oxidative stress (CitationSchreck et al 1992). Small molecule inhibition of this pathway is currently of great interest as a potential means to down-regulate the inflammation characteristic of COPD. I-κ B kinase-2 (IKK)-mediated phosphorylation of I-κ B is required for its subsequent ubiquitination and degradation; therefore small molecule inhibitors of this enzyme would be expected to block the nuclear translocation of NF-κ B. A variety of compounds are in preclinical development for this target from GlaxoSmithKline, AstraZeneca, Altana, and Tularik, but as yet no compound suitable for clinical studies has been reported in the literature. Alternative means to inhibit NF-κ B activation, such as inhibitors of ubiquitin ligase (CitationWertz et al 2004), peptide inhibitors of NEMO (a protein that forms a complex with IKK 2) (CitationChoi et al 2003), PPARα agonists, and direct inhibition of NF-κ B transport through nuclear pores, are being investigated in preclinical studies.
A recent study reported on the potential therapeutic effect of the small molecule antagonist siRNA against NF-κ B subunit p65 in airway epithelial cell lines (CitationMcIntyre et al 2003). In this in vitro study, cells treated with TNFα showed reduced NF-κ B p65 expression and concomitant IL-6 and CXCL8 expression. An antisense antagonist for NF-κ B p65 may be beneficial in inhibiting the NF-κ B-mediated inflammatory genes in patients with COPD.
NF-κ B-targeted therapies, inhibitors of IKK, may prove to be useful in antiinflammatory therapies (). The potential disadvantage of using inhibitors of cell signaling molecules is that mitogen-activating protein kinase (MAPK), NF-κ B, and IKK are general signal transduction proteins involved in multiple processes and therefore inhibitors may have unacceptable side effects. In addition, inhibition of these molecules may also impair defense mechanisms against infections.
Table 3 Direct and indirect antioxidant drugs under development for the treatment of COPD
Oxidative stress and steroid efficacy in COPD
It has been suggested that oxidative stress may have a role in the poor efficacy of corticosteroids in COPD. CitationIto and coworkers (2001) showed a role for histone acetylation and deacetylation in IL-1β-induced TNFα release in alveolar macrophages derived from cigarette smokers. They also suggested that oxidants may play an important role in the modulation of histone deacetylase (HDAC) and inflammatory cytokine gene transcription. Furthermore, we have shown that both cigarette smoke/H2O2 and TNFα caused an increase in histone acetylation (HAT activity) leading to IL-8 expression in monocytes and alveolar epithelial cells both in vitro and in vivo in rat lungs (CitationRahman, Gilmour, et al 2002; CitationMarwick et al 2004; CitationMoodie et al 2004).
Glucocorticoid suppression of inflammatory genes requires recruitment of HDAC2 to the transcription activation complex by the glucocorticoid receptor (CitationIto et al 2001; CitationRahman et al 2004). This results in deacetylation of histones and a decrease in inflammatory gene transcription. A reduced level of HDAC2 was associated with increased proinflammatory response and reduced responsiveness to glucocorticoids in alveolar macrophages obtained from smokers (CitationIto et al 2001; CitationRahman, Gilmour, et al 2002; CitationMarwick et al 2004; CitationMoodie et al 2004; CitationRahman et al 2004). Culpitt and co-workers have shown that cigarette smoke solution stimulated release of IL-8 and granulocyte-macrophage colony-stimulating factor, which was not inhibited by dexamethasone, in alveolar macrophages obtained from patients with COPD compared with those of smokers (CitationCulpitt, Rogers, Shah, et al 2003). They suggested that the lack of efficacy of corticosteroids in COPD might be due to steroid insensitivity of macrophages in the respiratory tract. Thus, the cigarette smoke/oxidant-mediated reduction in HDAC2 levels in alveolar epithelial cells and macrophages will not only increase inflammatory gene expression but also cause a decrease in glucocorticoid function in patients with COPD (). HDAC activity has also been measured in bronchial biopsies and alveolar macrophages from COPD patients and smoking controls, demonstrating a significant decrease in HDAC activity, the magnitude of which increased with severity of disease (CitationIto et al 2005). Moreover, protein expression of HDAC2 was decreased in a similar manner in COPD patients.
Figure 2 Model showing the possible mechanism of histone acetylation by oxidative stress and its repression by corticosteroids (GCs), leading to inhibition of gene transcription. Mitogen-activating protein kinase (MAPK) signaling pathways may be activated by oxidative stress, leading to histone acetylation. Direct interaction between co-activators (HAT), histone deacetylase (HDAC), and the glucocorticoid receptor (GR) may result in repression of the expression of proinflammatory genes. HDAC forms a bridge with HAT to inhibit gene transcription. However, when the HDAC is inhibited by oxidants or the NF-κ B subunit p65 is acetylated, steroids may not be able to recruit HDACs into the transcriptional complex to inhibit proinflammatory gene expression.
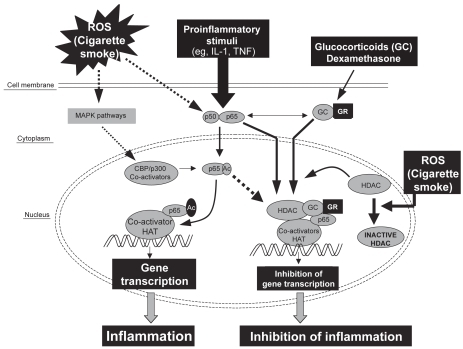
Consequently, a potential means by which to treat COPD would be to increase HDAC2 expression and activity such that steroids regain their antiinflammatory activity. We have shown that co-incubation of cells with NAC and H2O2 protects HDAC2 from down-regulation and reduction of specific activity (CitationMoodie et al 2004). In addition, it has been reported that theophylline has a similar effect in lung macrophage cells, increasing HDAC2 expression and re-sensitizing the cells to steroids (CitationBorja et al 2004). Alternative means of up-regulating HDAC2 activity would be of great interest for potential combination therapies of the future.
Polyphenols
Dietary polyphenols have antioxidant and antiinflammatory properties that may explain their beneficial effects (CitationArts and Hollman 2005). Curcumin is an active principle of the perennial herb Curcuma longa (commonly known as turmeric). Turmeric has a long traditional use in the Orient for many ailments, particularly as an antiinflammatory agent. Recent studies have reported that curcumin inhibits NF-κ B expression/activation, IL-8 release, cyclo-oxygenase (COX)-2, heme oxygenase-1, cytokines, and neutrophil recruitment in the lungs (CitationShishodia et al 2003; CitationBiswas et al 2005). Curcumin has multiple properties to protect against cigarette smoke-mediated oxidative stress (CitationShishodia et al 2003). It acts as oxygen radical and hydroxyl radical scavenger, increases antioxidant glutathione levels by induction of GCL, and acts as an antiinflammatory agent through inhibition of NF-κ B and IL-8 release in lung cells. Resveratrol, a flavonoid found in red wine, is an effective inhibitor of inflammatory cytokine release from macrophages in COPD patients (CitationCulpitt, Rogers, Fenwick, et al 2003). This antiinflammatory property of resveratrol may be due to its ability to induce sirtuins and HDAC activity (CitationHowitz et al 2003). A recent in vivo study has shown that resveratrol inhibits inflammatory cytokine expression in response to lipopolysaccharide in rat lungs (CitationBirrell et al 2005). The molecular mechanisms of antiinflammatory properties of dietary polyphenols against cigarette smoke/ oxidative stress have not yet been studied. This compound may induce phase II detoxifying genes by Nrf-2-dependent mechanisms.
Recent studies from our laboratory show that these dietary polyphenols restore glucocorticoid functions in response to oxidative stress imposed by cigarette smoke by up-regulation of HDAC activity in the monocyte/ macrophage (U937) and MonoMac6 cell lines (CitationRahman et al 2005). This was associated with restoration of HDAC1, HDAC2, and HDAC3 levels, suggesting that dietary polyphenol-mediated inhibition of proinflammatory cytokines increases formation of HDAC-p65 complex with glucocorticoid receptor, hence rendering NF-κ B ineffective. The other possible mechanism of polyphenol-mediated inhibition of inflammatory response is by quenching oxidants and aldehydes and inhibiting histone deacetyl-transferase activity. Catechins present in green tea (epigal-locatechin-3-gallate) in addition to theophylline may be effective in cigarette smoke-mediated oxidative stress and inflammatory response (CitationSchwartz et al 2005). However, this compound has never been tested in in vitro or in vivo smoking models. Overall, these dietary polyphenols and flavonols may not only act as antioxidant/antiinflammatory agents, but also possibly increase the efficacy of glucocorticoids in COPD.
Bioflavonoids possess both antioxidant and antiinflammatory properties and hence may influence chronic inflammatory diseases such as COPD. CitationTabak and colleagues (2001) studied the intake of catechins, flavonols, and flavones in relation to pulmonary function and COPD symptoms in 13 651 adults from three Dutch cities. Dietary intake of catechin (eg, green tea polyphenols, epigallocatechin gallate), flavonol (eg, quercetin and kaempferol), and flavone (eg, apigenin and luteolin) was positively associated with FEV1 and inversely associated with chronic cough and breathlessness, but not chronic phlegm. More importantly, single-component (such as catechin) intake was independently associated with FEV1 and all three COPD symptoms, whereas flavonol and flavone intake was independently associated with chronic cough only. The importance of this study was further substantiated by a study by CitationWalda and colleagues (2002), who showed the beneficial protective effect of fruit containing polyphenols and vitamin E against COPD symptoms in 20-year COPD mortality from three European countries, in Finnish, Italian, and Dutch cohorts. These important studies certainly encourage further multinational studies to demonstrate the beneficial effects of a high intake of nutraceuticals (polyphenols/bioflavonoids) against COPD symptoms.
Conclusions
There is now considerable evidence for the increased generation of ROS in COPD, which may be important in the pathogenesis of this condition. There are several small molecule compounds in clinical trials that target oxidant signaling or quench oxidants derived from cigarette smoke. Antioxidant and/or antiinflammatory agents such as thiol molecules, spin traps, dietary polyphenols, antioxidant mimetics, and inhibitors of oxidative stress-induced signaling pathways present potential means by which to treat this element of COPD. Antioxidant compounds may also enhance the efficacy of glucocorticoids by quenching oxidants and aldehydes, increasing histone deacetylase activity in COPD patients. Dietary polyphenols, such as resveratrol and curcumin, inhibit cigarette smoke/oxidant-induced NF-κ B activation, histone acetylation, and proinflammatory cytokine release and restore glucocorticoid functions via a mechanism involving up-regulation of HDAC activity. Thus, dietary polyphenols regulate inflammatory response at the molecular level, and possibly this is a way forward to restore glucocorticoid efficacy in the treatment of smoking-induced chronic inflammatory diseases. An effective wide-spectrum antioxidant therapy that has good bioavailability and potency is urgently needed to control the localized oxidative and inflammatory processes that occur in the pathogenesis of COPD. Although thiol antioxidant treatments have shown promising effects in targeting ROS and oxidant-mediated cellular alterations, in vivo cigarette smoke studies and human clinical trials of other small molecule antioxidants with dual activities (antioxidant and antiinflammatory) are urgently needed to validate these compounds as clinical therapies.
Acknowledgments
This study was supported by the Environmental Health Sciences Center (EHSC) Support ES01247 and an EHSC pilot project grant.
References
- AghdassiERoyallDAllardJP1999Oxidative stress in smokers supplemented with vitamin CInt J Vitam Nutr Res69455110052021
- AllardJPRoyallDKurianR1994Critical assessment of body-composition measurements in malnourished subjects with Crohn’s disease: the role of bioelectric impedance analysisAm J Clin Nutr59884908147334
- AntonicelliFParmentierMDrostEM2002Nacystelyn inhibits oxidant-mediated interleukin-8 expression and NF-kappaB nuclear binding in alveolar epithelial cellsFree Radic Biol Med3249250211958950
- AntwerpenLVTheronAJMyerMS1993Cigarette smoke-mediated oxidant stress, phagocytes, vitamin C, vitamin E, and tissue injuryAnn N Y Acad Sci68653658512261
- AoshibaKKoinumaMYokohoriN2003Immunohistochemical evaluation of oxidative stress in murine lungs after cigarette smoke exposureInhal Toxicol1510293812928978
- ArtsICHollmanPC2005Polyphenols and disease risk in epidemiologic studiesAm J Clin Nutr811 Suppl317S325S15640497
- [ATS] American Thoracic Society1995Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med152S771207582322
- AylwardMMaddockJDewlandP1980Clinical evaluation of acetyl-cysteine in the treatment of patients with chronic obstructive bronchitis: a balanced double-blind trial with placebo controlEur J Respir Dis61Suppl III819
- BartonGMRoathOS1976Leucocyte ascorbic acid in abnormal leucocyte statesInt J Vitam Nutr Res462714977212
- BirrellMAMcCluskieKWongS2005Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanismFASEB J19840115734790
- BiswasSKMcClureDJimenezLA2005Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activityAntioxid Redox Signal7324115650394
- BootsAWHaenenGRBastA2003Oxidant metabolism in chronic obstructive pulmonary diseaseEur Respir J Suppl4614S27S14621103
- BorjaGCLoukiaTKazuhiroI2004Theophylline restores histone deacetylase activity and steroid responses in COPD macrophagesJ Exp Med2006899515337792
- BridgemenMMEMarsdenMSelbyC1994Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissueThorax4967058066561
- BridgesABScottNAParryGJ1993Age, sex, cigarette smoking and indices of free radical activity in healthy humansEur J Med220588261071
- BridgesRBChowCKRehmSR1990Micronutrient status and immune function in smokersAnn N Y Acad Sci587218312113786
- BrittonJRPavordIDRichardsKA1995Dietary antioxidant vitamin intake and lung function in the general populationAm J Respir Crit Care Med151138377735589
- ButterworthMUpshallDGHobbsM1993Elevation of cysteine and replenishment of glutathione in rat lung slices by cysteine isopropylester and other cysteine precursorsBiochem Pharmacol451769748494535
- CantinAMNorthSLHubbardRC1987Normal alveolar epithelial lung fluid contains high levels of glutathioneJ Appl Physiol6315273040659
- CaramoriGRomagnoliMCasolariP2003Nuclear localization of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbationsThorax583485112668802
- ChabrierPEAuguetMSpinnewynB1999BN 80933, a dual inhibitor of neuronal nitric oxide synthase and lipid peroxidation: a promising neuroprotective strategyProc Natl Acad Sci U S A9610824910485910
- ChoiMRolleSWellnerM2003Inhibition of NF-kappaB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosisBlood10222596712763940
- ChowCKThackeRBridgesRB1986Lower levels of vitamin C and carotenes in plasma of cigarette smokersJ Am Coll Nutr5305123734276
- ChurchDFPryorWA1985Free radical chemistry of cigarette smoke and its toxicological implicationsEnviron Health Perspect64111263007083
- ComptonCHGubbJCedarE1999An oral, selective PDE4 inhibitor improves health status in patients with COPDEur Respir J14281s
- CorradiMRubinsteinIAndreoliRl2003Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1671380612522029
- CotgreaveIA1997N-acetylcysteine: pharmacological considerations and experimental and clinical applicationsAdv Pharmacol38205278895810
- CrossCEO’NeillCAReznickAZ1993Cigarette smoke oxidation of human plasma constituentsAnn N Y Acad Sci68672908512263
- CrossCEVan der VlietAO’NeillCA1994Oxidants, antioxidants, and respiratory tract lining fluidsEnviron Health Perspect102185917705296
- CulpittSVRogersDFFenwickPS2003Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPDThorax58942614586044
- CulpittSVRogersDFShahP2003Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from COPD patientsAm J Respir Crit Care Med16243112406856
- De BenedettoFAcetoADraganiB2005Long-term oral N-ace-tylcysteine reduces exhaled hydrogen peroxide in stable COPDPulm Pharmacol Ther1841715607126
- DecramerMDekhuijzenPNTroostersT2001The Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS): hypothesis and design. BRONCUS-trial CommitteeEur Respir J173293611405507
- DecramerMRutten-van MolkenMDekhuijzenPN2005Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS); a randomised placebo-controlled trialLancet36515526015866309
- DekhuijzenPN2004Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary diseaseEur Respir J236293615083766
- Di StefanoACaramoriGOatesT2002Increased expression of nuclear factor-κ B in bronchial biopsies from smokers and patients with COPDEur Respir J205566312358328
- DietrichMBlockGHudesM2002Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokersCancer Epidemiol Biomarkers Prev1171311815395
- DueholmMNielsonCThorshaugeH1992N-acetylcysteine by metered dose inhaler in the treatment of chronic bronchitis: a multi-centre studyRespir Med8689921615189
- DuthieGGArthurJJamesWPT1991Effects of smoking and vitamin E on blood antioxidant statusAm J Clin Nutr531061S1063S2012019
- Ekberg-JanssonALarsonMMacNeeW1999N-isobutyrylcysteine, a donor of systemic thiols, does not reduce the exacerbation rate in chronic bronchitisEur Respir J138293410362048
- EulerDEDaveSJGuoH1996Effect of cigarette smoking on pentane excretion in alveolar breathClin Chem4230388595728
- GerritsCMGMHeringsRMCLeufkensHGM2003N-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary diseaseEur Respir J21795812765423
- GilksCBPriceKWrightJL1998Antioxidant gene expression in rat lung after exposure to cigarette smokeAm J Pathol152269789422544
- GillissenAJaworskaMOrthM1997Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitroRespir Med91159689135855
- GrandjeanEMBerthetPRuffmannR2000Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trialsClin Ther222092110743980
- GrievinkLSmitHAOckeMC1998Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function; the MORGEN studyThorax53166719659349
- GuidotDMBrownLA2000Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed ratsAlcohol Clin Exp Res241070610924012
- HabibMPTankLJLaneLC1999Effect of vitamin E on exhaled ethane in cigarette smokersChest1156849010084476
- Haddad elBMcCluskieKBirrellMA2002Differential effects of ebselen on neutrophil recruitment, chemokine, and inflammatory mediator expression in a rat model of lipopolysaccharide-induced pulmonary inflammationJ Immunol1699748212097404
- HarjuTKaarteenaho-WiikRSoiniY2002Diminished immunoreactivity of gamma-glutamylcysteine synthetase in the airways of smokers’ lungAm J Respir Crit Care Med166754912204877
- HendersonWRJrChiEYTeoJL2002A small molecule inhibitor of redox-regulated NF-kappa B and activator protein-1 transcription blocks allergic airway inflammation in a mouse asthma modelImmunology16952949
- HowitzKTBittermanKJCohenHY2003Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespanNature425191612939617
- ItoKItoMElliottWM2005Decreased histone deacetylase activity in chronic obstructive pulmonary diseaseN Engl J Med35219677615888697
- ItoKLimGCaramoriG2001Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophagesFASEB J1511101211292684
- KasielskiMNowakD2001Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary diseaseRespir Med954485611421501
- KharitonovSABarnesPJ2001Exhaled markers of pulmonary diseaseAm J Respir Crit Care Med163169372211401895
- KoechlinCCouillardACristolJP2004Does systemic inflammation trigger local exercise-induced oxidative stress in COPD?Eur Respir J235384415083751
- KondoTTagamiSYoshiokaA1994Current smoking of elderly men reduces antioxidants in alveolar macrophagesAm J Respir Crit Care Med149178828111579
- LiXYRahmanIDonaldsonK1996Mechanisms of cigarette smoke induced increased airspace permeabilityThorax51465718711672
- LindenMRasmussenJBPiitulainenE1993Airway inflammation in smokers with nonobstructive and obstructive chronic bronchitisAm Rev Respir Dis1481226328239158
- LipworthBJ2005Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary diseaseLancet3651677515639300
- LykkesfeldtJChristenSWallockLM2000Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakesAm J Clin Nutr71530610648268
- MarwickJAKirkhamPAStevensonCS2004Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungsAm J Respir Cell Mol Biol316334215333327
- McCuskerKHoidalJ1990Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamstersAm Rev Respir Dis141678822310098
- McIntyreKWShusterDJGilloolyKM2003A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in miceArthritis Rheum482652913130486
- MezzettiALapennaDPierdomenicoSD1995Vitamins E, C and lipid peroxidation in plasma and arterial tissue of smokers and non-smokersAtherosclerosis1129197772072
- MontuschiPBarnesPJ2002Analysis of exhaled breath condensate for monitoring airway inflammationTrends Pharmacol Sci23232712008001
- MontuschiPCollinsJVCiabattoniG2000Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokersAm J Respir Crit Care Med1621175710988150
- MoodieFMMarwickJAAndersonCS2004Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cellsFASEB J181897915456740
- MorettiMBottrighiPDallariR2004The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE StudyExp Clin Res3014352
- MorrisonDRahmanILannanS1999Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokersAm J Respir Crit Care Med15947399927360
- NeurohrCLenzA-GDingI2003Glutamate-cysteine ligase modulatory subunit in BAL alveolar macrophages of healthy smokersEur Respir J2282712882455
- NishikawaMKakemizuNItoT1999Superoxide mediates cigarette smoke-induced infiltration of neutrophils into the airways through nuclear factor-kappaB activation and IL-8 mRNA expression in guinea pigs in vivoAm J Respir Cell Mol Biol20189989922209
- NowakDKasielskiMAntczakA1999Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: no significant effect of cigarette smokingResp Med9338996
- PachtERKasekiHMohammedJR1988Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicityJ Clin Invest77789963949977
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med16312567611316667
- PelletierO1970Vitamin C status of cigarette smokers and nonsmokersAm J Clin Nutr2352085444988
- PetruzzelliSHietanenEBartschH1990Pulmonary lipid peroxidation in cigarette smokers and lung cancer patientsChest9893052209151
- PinamontiSLeisMBarbieriA1998Detection of xanthine oxidase activity products by EPR and HPLC in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary diseaseFree Radic Biol Med2577199823542
- PoolePJBlackPN2001Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic reviewBMJ3221271411375228
- PoolePJBlackPN2003Preventing exacerbations of chronic bronchitis and COPD: therapeutic potential of mucolytic agentsAm J Respir Med23677014719989
- PraticoDBasiliSVieriM1998Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2alpha-III, an index of oxidant stressAm J Respir Crit Care Med1581709149847257
- RahmanI2002Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targetsCurr Drug Targets Inflamm Allergy129131514561194
- RahmanI2003Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseasesJ Biochem Mol Biol369510912542980
- RahmanIBauterMRMejaK2005Curcumin-restores glucocorticoid function and inhibits cigarette-mediated IL-8 release in oxidant stress monocytic U-937 cellsAm J Respir Crit Care Med2A395
- RahmanIBiswasSK2004Non-invasive biomarkers of oxidative stress: reproducibility and methodological issuesRedox Rep91254315327743
- RahmanIGilmourPJimenezLA2002Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammationMol Cell Biochem2342394812162440
- RahmanIKellyF2003Biomarkers in breath condensate: a promising new non-invasive technique in free radical researchFree Radic Res3712536614753750
- RahmanIMacNeeW1996aRole of oxidants/antioxidants in smoking-induced lung diseasesFree Radic Biol Med21669818891669
- RahmanIMacNeeW1996bOxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary diseaseThorax51348508733482
- RahmanIMacNeeW1998Role of transcription factors in inflammatory lung diseasesThorax53601129797762
- RahmanIMacNeeW1999Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway diseaseAm J Physiol277L10678810600876
- RahmanIMacNeeW2000Oxidative stress and regulation of glutathione in lung inflammationEur Respir J165345411028671
- RahmanIMarwickJKirkhamP2004Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expressionBiochem Pharmacol6812556715313424
- RahmanIMorrisonDDonaldsonK1996Systemic oxidative stress in asthma, COPD, and smokersAm J Respir Crit Care Med1541055608887607
- RahmanISkwarskaEMacNeeW1997Attenuation of oxidant/antioxidant imbalance during treatment of exacerbations of chronic obstructive pulmonary diseaseThorax5256589227727
- RahmanISwarskaEHenryM2000Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease?Thorax551899310679536
- RahmanIvan SchadewijkAACrowtherA20024-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med166490512186826
- RasmusseJBGlennowC1988Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitisEur J Respir Dis13515
- RautalahtiMVirtamoJHaukkaJ1997The effect of alphatocopherol and beta-carotene supplementation on COPD symptomsAm J Respir Crit Care Med1561447529372659
- SargeantLAJaeckelAWarehamNJ2000Interaction of vitamin C with the relation between smoking and obstructive airways disease in EPIC Norfolk. European Prospective Investigation into Cancer and NutritionEur Respir J1639740311028650
- SchreckRAlbermannKBaeuerlePA1992Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cellsFree Radic Res Commun17221371473734
- SchwartzJLBakerVLariousE2005Molecular and cellular effects of green tea on oral cells of smokers: a pilot studyMol Nutr Food Res49435115538715
- ShaharEBolandLLFolsomAR1999Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The Atherosclerosis Risk in Communities Study InvestigatorsAm J Respir Crit Care Med1591780510351918
- ShishodiaSPotdarPGairolaCG2003Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1Carcinogenesis2412697912807725
- SmithKRUyeminamiDLKodavantiUP2002Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidantFree Radic Biol Med3311061412374622
- SniderGL1989Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesisAnn Rev Med40411292658758
- SotoFJHananiaNA2005Selective phosphodiesterase-4 inhibitors in chronic obstructive lung diseaseCurr Opin Pulm Med111293415699784
- SteinbergFMChaitA1998Antioxidant vitamin supplementation and lipid peroxidation in smokersAm J Clin Nutr68319279701189
- SteyCSteurerJBachmannS2000The effect of oral N-acetyl-cysteine in chronic bronchitis: a quantitative systematic reviewEur Respir J162536210968500
- SturtonGFitzgeraldM2002Phosphodiesterase 4 inhibitors for the treatment of COPDChest121192S196S12010850
- TabakCIljaCArtsCW2001Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN StudyAm J Respir Crit Care Med16461411435239
- TheronAJRichardsGARensburgAJ1990Investigation of the role of phagocytes and anti-oxidant nutrients in oxidant stress mediated by cigarette smokeInt J Vitam Nutr Res6026162276884
- TothKMBergerEMBeehlerCJ1986Erythrocytes from cigarette smokers contain more glutathione and catalase and protect endothelial cells from hydrogen peroxide better than do erythrocytes from nonsmokersAm Rev Respir Dis13428143740654
- TuderRMZhenLChoCY2003Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockadeAm J Respir Cell Mol Biol29889712600822
- TugTKaratasFTerziSM2004Antioxidant vitamins (A, C and E) and malondialdehyde levels in acute exacerbation and stable periods of patients with chronic obstructive pulmonary diseaseClin Invest Med27123815305803
- Van SchootenFJNiaABDe FloraS2002Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokersCancer Epidemiol Biomarkers Prev111677511867504
- VecchiarelliADottoriniMPietrellaD1994Macrophage activation by N-acetyl-cysteine in COPD patientsChest105806118131544
- WaldaICTabakCSmitHA2002Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countriesEur J Clin Nutr566384312080403
- WertzIEO’RourkeKMZhouH2004Deubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signallingNature5694915258597
- YorkGKPierceTHSchwartzLS1976Stimulation by cigarette smoke of glutathione peroxidase system enzyme activities in rat lungArch Environ Health3128690999340
- ZhangMNomuraAUchidaY2002Ebselen suppresses late airway responses and airway inflammation in guinea pigsFree Radic Biol Med324546411864785