Abstract
Chronic obstructive pulmonary disease is a common condition and a major cause of mortality. COPD is characterized by irreversible airflow obstruction. The physiological abnormalities observed in COPD are due to a combination of emphysema and obliteration of the small airways in association with airway inflammation. The predominant cells involved in this inflammatory response are CD8+ lymphocytes, neutrophils, and macrophages. Although eosinophilic airway inflammation is usually considered a feature of asthma, it has been demonstrated in large and small airway tissue samples and in 20%–40% of induced sputum samples from patients with stable COPD. This airway eosinophilia is increased in exacerbations. Thus, modifying eosinophilic inflammation may be a potential therapeutic target in COPD. Eosinophilic airway inflammation is resistant to inhaled corticosteroid therapy, but does respond to systemic corticosteroid therapy, and the degree of response is related to the intensity of the eosinophilic inflammation. In COPD, targeting treatment to normalize the sputum eosinophilia reduced the number of hospital admissions. Whether controlling eosinophilic inflammation in COPD patients with an airway eosinophilia will modify disease progression and possibly alter mortality is unknown, but warrants further investigation.
Keywords:
Introduction
Chronic obstructive pulmonary disease is a common condition predominantly caused by smoking. It is a major cause of mortality, and in 1999 there were approximately 30 000 deaths due to COPD in the UK. This represented 5.1% of all deaths (5.9% of all male deaths and 4.3% of all female deaths) (CitationNICE 2004). COPD is the major cause of respiratory failure and is a common cause of chronic disability. In contrast to asthma, COPD is characterized by irreversible airflow obstruction. The physiological abnormalities observed in COPD are due to a combination of emphysema and obliteration of the small airways. These two pathologies are distinct in that emphysema can occur without narrowing of the small airways, and vice versa, although the conditions usually coexist. Small airway narrowing is a consequence of inflammation, increased airway muscle mass and fibrosis in the airway wall, and the accumulation of inflammatory mucus exudates in the lumen. Subsequent increased airway wall thickness is associated with worsening disease severity as defined by the Global Initiative of Obstructive Lung Disease (GOLD) stage (CitationHogg et al 2004).
There is considerable interest in airway inflammation in COPD. This interest has been fuelled by the desire to modify airway inflammation in COPD in the anticipation that this will have an impact on lung function decline and exacerbations, which are the major determinants of the morbidity and mortality associated with this disease. Neutrophils, CD8+ T lymphocytes, and macrophages have been implicated in the disease pathogenesis of COPD, whereas, asthma is regarded as a Th2-mediated eosinophilic disease. Indeed, the presence of eosinophilic inflammation is often viewed as a distinguishing feature between asthma and COPD.
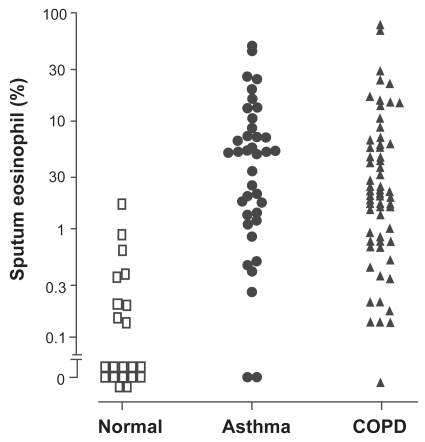
The development of sputum induction as a non-invasive test of airway inflammation has enabled clinicians to study the phenotype of airway inflammation in patients with airway disease. The sputum differential cell count has been defined in large normal populations (CitationBelda et al 2000). The normal sputum eosinophil count is <1.1%. A sputum eosinophil count >3% was associated with a good response to corticosteroids in asthma (CitationPavord et al 1999) and COPD (CitationPizzichini et al 1998). The application of sputum induction has led to the recognition that eosinophilic inflammation is present in only 50% of cases of asthma (CitationDouwes et al 2002) and in about 20%–40% of cases of COPD (CitationSaetta et al 1994; CitationConfalonieri et al 1998; CitationPizzichini et al 1998; CitationBrightling, Monteiro, et al 2000; CitationBrightling et al 2005). Hence there is considerable overlap in the presence of eosinophilic airway inflammation in COPD and asthma, as illustrated in (CitationBrightling, Monteiro, et al 2000; CitationGreen, Brightling, Woltmann, et al 2002).
Figure 1 Sputum eosinophil count in subjects with corticosteroid-naïve asthma and COPD. Data derived from CitationBrightling, Monteiro, et al (2000); CitationGreen, Brightling, Woltmann, et al (2002).
In this review we briefly summarize eosinophil biology, describe the inflammatory profile of COPD in stable disease and exacerbations and its response to treatment with particular reference to the eosinophil, and explore the potential role of a sputum eosinophil count in the management of COPD.
Eosinophil biology
Eosinophils are end-stage cells derived from the bone marrow under the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, and the late differentiation factor IL-5 (CitationDenburg 1999). In terms of their ontogeny they are closely related to the basophil rather than the neutrophil or monocyte. The selective recruitment of eosinophils into the airway is mediated by a multistep process directed by Th2 cytokine-producing T cells (CitationWardlaw et al 1999). The first step is increased production and release of eosinophils from the bone marrow under the influence of the IL-5 and specific chemoattractants such as eotaxin. Second, the target organ vasculature has increased adhesiveness for eosinophils through the specific effects of locally generated IL-4 and IL-13. These cytokines induce expression of vascular cell adhesion molecule (VCAM)-1 on lung endothelial cells, which binds through eosinophil -expressed ligands VLA-4 and P-selectin, to which eosinophils bind with greater avidity than neutrophils (CitationSymon et al 1996; CitationEdwards et al 2000; CitationWoltmann et al 2000). CC chemokines such as eotaxin released by cells in the airway wall activate the chemokine receptor CCR3 expressed by eosinophils and thus attract these cells into tissue. Here they survive for prolonged periods as a result of locally generated IL-5 and GM-CSF.
The eosinophil specific basic proteins, which are stored in the distinctive secondary granules, are major basic protein eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin. All of these proteins are toxic to bronchial epithelial cells. Eosinophils, with mast cells and basophils, are the most prominent source of cysteinyl-leukotrienes (CitationBozza et al 1997), and eosinophils also release a diverse range of cytokines. The physiological triggers in airway disease that lead to eosinophil mediator release remain uncertain but, importantly, eosinophils undergo piecemeal degranulation in most in vivo settings (CitationDvorak and Weller 2000).
Airway inflammation in COPD
Stable disease
For more than a decade, substantial effort has been made to define obstructive airways disease at an inflammatory cellular level with the aim to clarify mechanisms and improve treatment. Sputum and bronchoalveolar lavage (BAL) have been used to identify inflammatory components of the large and small airways, respectively, whereas endobronchial biopsies and lung resection tissue have been used for analysis of the bronchial wall of the large and small airway and lung parenchyma. There are several difficulties facing researchers interested in measuring lower airway inflammation in COPD. First, the functionally important inflammatory response in the small airways and surrounding lung parenchyma is in the lung periphery and is therefore difficult to access. Second, the various techniques used to assess airway inflammation differ markedly in their properties and in the profile of inflammatory cells they measure, suggesting they are accessing different lung compartments (CitationKeatings, Evans, et al 1997). Finally, when considering airway inflammation, the repeatability of such measures is not always known.
Despite these problems, a number of inflammatory cells have been shown consistently to be present in increased numbers in the airways in COPD and to relate to the severity of airflow obstruction, suggesting a causal role. The evidence is perhaps strongest for the CD8+ T lymphocyte and the neutrophil. Increased numbers of CD8+ T lymphocytes have been demonstrated at all levels of the lung (large and small airways and parenchyma) in relation to smoking and COPD (CitationO’Shaughnessy et al 1997; CitationSaetta et al 1999). The mechanism of CD8+ lymphocyte recruitment and its functional significance remain to be determined. Increased neutrophil numbers are particularly obvious in patients with established airflow obstruction. Bronchial biopsy and induced sputum studies have consistently shown a correlation between the severity of airflow obstruction and neutrophil counts, and in some studies the correlation has been close (CitationKeatings et al 1996; CitationStanescu et al 1996; CitationDi Stefano et al 1998). Furthermore, the protease-antiprotease hypothesis (CitationStockley 1995) offers a biologically plausible mechanism for the tissue destruction seen in COPD in association with neutrophilic airway inflammation.
Less attention has been paid to the presence of eosinophilic airway inflammation in stable COPD, although a sputum eosinophilia has been observed in 20%–40% of patients with COPD (CitationSaetta et al 1994; CitationConfalonieri et al 1998; CitationPizzichini et al 1998; CitationBrightling, Monteiro, et al 2000; CitationBrightling et al 2005). One bronchial biopsy study has reported an increased number of eosinophils in patients with chronic bronchitis and COPD but lower BAL concentrations of ECP than in asthmatics, suggesting that eosinophils are present but are less activated in COPD (CitationLacoste et al 1993). However, sputum ECP concentrations were increased to a greater level than seen with asthma in moderate to severe COPD (CitationGibson et al 1998; CitationBrightling, Monteiro, et al 2000; CitationBrightling, Ward, et al 2000; CitationBrightling et al 2005), suggesting that eosinophils are activated in more severe disease.
The relationship between lung function decline and eosinophilic inflammation is unclear. A negative correlation between FEV1 and the ratio of activated eosinophils to total eosinophils in endobronchial biopsies from subjects with COPD was demonstrated (CitationLams et al 2000), and a similar negative correlation between FEV1 and sputum eosinophils and ECP was found (CitationBalzano et al 1999). Conversely, in another study there was no relationship between small airway eosinophilia and severity of COPD defined by the GOLD criteria (CitationHogg et al 2004).
The origin of eosinophilic airway inflammation in COPD is unclear, although it is widely assumed that it indicates an asthmatic component to the fixed airways obstruction (CitationBarnes 1998). This is unlikely to be the case, as most studies on patients with COPD rigorously exclude subjects with variable airflow obstruction and those with clinical features suggesting asthma. It is more likely that smoking and other mechanisms that recruit neutrophils into the airway mucosa in COPD may in turn cause a minor degree of eosinophil influx. However, it is difficult to explain the very high levels of sputum eosinophilia observed in some of our subjects. An alternative and intriguing possibility is that eosinophilic COPD starts as eosinophilic bronchitis. This is a common cause of chronic cough in middle age characterized by a sputum eosinophilia but no symptoms and functional evidence of variable airflow obstruction or airway hyperresponsiveness (CitationGibson et al 1989). Although characterized by normal spirometric values at the time of diagnosis, this has been associated with an accelerated decline in FEV1 and the development of COPD (CitationBrightling et al 1999; CitationBirring et al 2002; CitationBerry et al 2005).
Exacerbations
COPD exacerbations are associated with sputum and bronchoscopic bronchial biopsy evidence of eosinophilic inflammation (CitationLacoste et al 1993; CitationSaetta et al 1994). Bronchial biopsies taken from patients during acute exacerbations and compared with stable COPD show a 30-fold increase in the total number of eosinophils and only a 3-fold increase in neutrophils (CitationSaetta et al 1994). The presence of high concentrations of tumor necrosis factor (TNF)-α (a proinflammatory cytokine that activates adhesion molecules on endothelial cells influencing eosinophil chemotaxis) and the eosinophil products ECP and EPO in induced sputum also supports a role for the eosinophil in COPD exacerbations (CitationPizzichini et al 1996; CitationGursel et al 1997; CitationKeatings and Barnes 1997; CitationKeatings, Evans, et al 1997).
Effect of treatment on airway inflammation
Intervention studies examining the effects of treatment on airway inflammation in COPD have generally used induced sputum to assess airway inflammation and inhaled or oral corticosteroids as the putative antiinflammatory agent. These studies are summarized in .
Table 1 Studies monitoring effect of corticosteroids (inhaled and oral) on airway inflammation and lung function
There is a consistent lack of effect on eosinophilic inflammation in COPD by inhaled corticosteroids (CitationKeatings, Jatakanon, et al 1997; CitationConfalonieri et al 1998; CitationCulpitt et al 1999; CitationLoppow et al 2001; CitationGizycki et al 2002; CitationBrightling et al 2005). Two studies have shown a small reduction in the sputum neutrophil count (CitationConfalonieri et al 1998; CitationYildiz et al 2000) and one a reduction in submucosal mast cell numbers (CitationGizycki et al 2002). The lack of an antiinflammatory effect of inhaled corticosteroid therapy in COPD has led to the hypothesis that COPD is a corticosteroid-resistant disease. Low levels of histone deacetylase (HDAC) demonstrated in COPD macrophages and lung tissue may be responsible for corticosteroid resistance (CitationIto et al 2005). HDAC prevents acetylation of histone, which leads to unwinding of chromatin architecture, thereby promoting transcription of proinflammatory cytokines implicated in COPD. Reduced levels of HDAC in macrophages have been seen in response to cigarette smoke. Levels are negatively correlated with increased levels of metalloproteinases, IL-8, and TNFα and a reduction in the ability of dexamethasone to reduce these mediators (CitationBarnes et al 2004). However, though studies show that COPD is relatively corticosteroid resistant compared with asthma, the response of airway inflammation in COPD to systemic corticosteroids suggests that certain aspects of the inflammatory profile in COPD are corticosteroid responsive ().
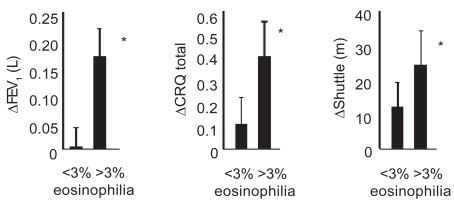
One small single-blind study has shown that following treatment with a short course of prednisolone there was no evidence of a treatment-associated change in the sputum neutrophil count or in the sputum supernatant concentration of myeloperoxidase or elastase (CitationPizzichini et al 1998). The authors observed that oral corticosteroid treatment is associated with a significant fall in the sputum eosinophil count and in the sputum supernatant concentration of ECP. Furthermore, the improvement in FEV1 and quality-of-life scores with treatment was significantly greater in those with a significant sputum eosinophilia (>3%) (CitationPizzichini et al 1998). This beneficial effect of oral corticosteroid treatment was confirmed in a randomized placebo-controlled trial of 2 weeks of prednisolone 30 mg daily. This study found that the degree of baseline eosinophilic inflammation was related to improvements in lung function and health status () (CitationBrightling, Monteiro, et al 2000). CitationFujimoto et al (1999) treated 24 emphysema subjects (defined by obstructive spirometry with demonstrable irreversibility and emphysema on CT scan) with oral prednisolone 20 mg daily for 2 weeks with analysis of sputum before and after treatment. Corticosteroids did not modulate neutrophilic inflammation, but reduction in eosinophils was observed, as were predicted improvements in FEV1. In another study, administration of 15 days of prednisolone (1.5 mg/kg daily) to 25 COPD subjects showed FEV1 improvements greater than 12% and 200 mL above baseline in 12 subjects; this subgroup exhibited raised levels of eosinophilic inflammation in BAL specimens compared with nonresponders (CitationChanez et al 1997). Hence, reversibility can be predicted from evidence of tissue eosinophilia at baseline. Recently, a large placebo-controlled study using combination inhaler therapy (fluticasone and salmeterol) has shown reduction in sputum eosinophils and neutrophils over a 12-week period when compared with placebo, with an associated improvement in lung function and reported exacerbations (CitationBarnes et al 2005; CitationQui et al 2005; CitationZhu et al 2005). Comparison between combination and single-agent inhaled therapy is required to clarify the antiinflammatory benefit of combined corticosteroid and long-acting β-agonists in COPD.
Figure 2 Improvement in post-bronchodilator FEV1, health status (Chronic Respiratory Disease Questionnaire; CRQ), and shuttle walk distance in subjects with COPD with or without a sputum eosinophilia (>3% non-squamous cells). *p < 0.05; Δ represents change after prednisolone compared with placebo. Data derived from CitationBrightling, Monteiro, et al (2000).
These observations suggest that systemic and inhaled corticosteroids have differential effects on airway inflammation in COPD. The differences between the effects of oral versus inhaled corticosteroids may reflect differences in dose or perhaps the site of action. Systemic corticosteroids are likely to exert more of an effect on small airway inflammation, which is less accessible to inhaled therapy, and systemic corticosteroids also suppress eosinophil production by the bone marrow.
Sputum inflammatory cell counts can be influenced to some extent by smoking status. A cross-sectional study showed COPD subjects who had given up smoking by 12 months had a lower percentage of eosinophils than smokers, though sputum eosinophil levels were still high in both groups (smokers 8% vs 4% ex-smokers) (CitationDomagala-Kulawik et al 2003). There was no difference in the response to oral corticosteroids between current smokers and ex-smokers (CitationBrightling, Monteiro, et al 2000). These findings are in contrast to those in smokers with asthma, who have reduced sputum eosinophil counts compared with non-smokers and were less corticosteroid responsive (CitationChalmers et al 2001; CitationTomlinson et al 2005).
There has been little exploration into the effects of other antiinflammatory treatments on COPD-related airway inflammation. Cilomilast, an oral phosphodiesterase D4 inhibitor, has been used in a randomized, double-blind 12-week trial; there was no demonstrable difference in sputum counts or FEV1 after 12 weeks, but bronchial biopsies at 10 weeks showed reductions in macrophages and CD8+ T lymphocytes compared with baseline in the cilomilast treatment arm (CitationGamble et al 2003).
Role of measuring inflammation in management of disease
One important question is whether measuring airway inflammation in COPD can influence the management of this disease. This is particularly difficult in a disease that is largely resistant to current therapy.
Several recent large, placebo-controlled studies have clarified the role of long-term treatment with inhaled corticosteroids. Regular treatment with inhaled corticosteroids in stable COPD does not alter the long-term decline in lung function (CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000; CitationThe Lung Health Study Research Group 2000), and there is conflicting evidence whether inhaled corticosteroids alter mortality (CitationSoriano et al 2002; CitationFan et al 2003). However, they do reduce the number of exacerbations and improve health status in individuals with severe COPD (CitationBurge et al 2000; CitationMahler et al 2002; CitationJones et al 2003; CitationSzafranski et al 2003). Intriguingly, subjects who had an improvement in FEV1 of >20% following short-term treatment with prednisolone had a more significant reduction in exacerbation frequency with longer-term treatment with inhaled fluticasone than those without (CitationBurge et al 2003; CitationPavord et al 2004). This suggests that the benefit from corticosteroid therapy in COPD is more marked in a subgroup of patients. Likewise in an earlier uncontrolled study, COPD subjects treated with oral corticosteroids had reduced decline in their lung function (CitationPostma et al 1988). Thus, one important question is whether these relatively minor long-term benefits are confined to a definable subgroup of patients.
Since sputum eosinophilia was also associated with an improvement in lung function after a short course of prednisolone, it is possible that the identification of eosinophilic airway inflammation might still allow corticosteroid therapy to be targeted to a population who would particularly benefit in the long term, in terms of both exacerbation rate and lung function decline. This approach has been applied to asthma, whereby in a management strategy aimed at normalizing the sputum eosinophil count there was a striking reduction in severe exacerbations (CitationGreen, Brightling, McKenna, et al 2002). We have recently applied this approach to a group of 80 subjects with COPD. Over a 12-month period, we have shown that a management approach with the additional aim of reducing the sputum eosinophil count below 3% using corticosteroids was associated with a 62% reduction in severe exacerbations of COPD requiring hospitalization when compared with traditional symptom-based management (CitationSiva et al 2005). This benefit was largely achieved by the targeted use of oral corticosteroid in the eosinophilic group. Therefore, the measurement of a sputum eosinophil count can be used to identify COPD patients with corticosteroid-responsive disease and to guide treatment.
Future treatments for eosinophilic inflammation
COPD therapies have limited efficacy. It is likely that identification of specific inflammatory phenotypes may reveal subgroups of patients who are particularly susceptible to targeted therapy. New treatments specifically aimed at modifying eosinophilic inflammation may have benefit in some patients with COPD. The best documented of these is mepolizumab, an anti-IL-5 antibody. In clinical trials, reduction of peripheral blood and bone marrow eosinophils was seen after administration but with little clinical benefit observed. This was possibly due to poor penetration of bronchial tissue, the site of activity for eosinophils in asthma (CitationFlood-Page et al 2003). In murine models, overexpression of IL-13 leads to pulmonary emphysema; potentially, treatment against IL-13 may have therapeutic benefit, though overall IL-13 levels in human emphysematous tissue are low (CitationBoutten et al 2004). A neutralizing antibody against eotaxin (which promotes eosinophil recruitment as well) has reduced lung eosinophilia in mice (CitationGonzalo et al 1996), and early clinical trials are ongoing with antibodies against receptor CCR3 on the eosinophil cell surface (CitationErin et al 2002). Whether these new therapies will be effective in COPD is unknown, and further clinical trials are required.
Summary
The mechanistic pathways behind airway inflammation in COPD are complex, and clearly there is no one inflammatory cell that is responsible for the spectrum of this disease. However, the argument is strong for a specific role of eosinophilic inflammation in COPD. Eosinophilic airway inflammation is linked to exacerbations, which contribute to both lung function and health decline. Airway eosinophilia is not evident in all COPD patients but a large subgroup can be clearly defined by simple, non-invasive sampling of sputum. This subgroup exhibits improvement in lung function and health status to systemic, but not inhaled corticosteroids. Specific targeting of eosinophilic inflammation may be effective in some patients with COPD, and validation of long-term systemic corticosteroids and new treatment regimens is warranted in COPD patients who exhibit an airway eosinophilia.
References
- BalbiBMajoriMBertaccoS2000Inhaled corticosteroids in stable COPD patients: do they have effects on cells and molecular mediators of airway inflammation?Chest1171633710858395
- BalzanoGStefanelliFIorioC1999Eosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway functionAm J Respir Crit Care Med1605Pt114869210556110
- BarnesNC1998Inhaled steroids in COPDLancet35176679519941
- BarnesNCQuiYPavordI2005Salmeterol/fluticasone proprionate: anti-inflammatory effects in COPDProc Am Thorac Soc2A543
- BarnesPJItoKAdcockIM2004Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylaseLancet363731315001333
- BeldaJLeighRParameswaranK2000Induced sputum cell counts in healthy adultsAm J Respir Crit Care Med1612Pt1475810673188
- BerryMAHargadonBMcKennaS2005Observational study of the natural history of eosinophilic bronchitisClin Exp Allergy3559860115898981
- BirringSSBrightlingCEBraddingP2002Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive studyAm J Respir Crit Care Med16610788312379551
- BouttenABonayMLaribeS2004Decreased expression of interleukin 13 in human lung emphysemaThorax59850415454650
- BozzaPTYuWPenroseJF1997Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formationJ Exp Med186909209294145
- BrightlingCEMcKennaSHargadonB2005Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax60193815741434
- BrightlingCEMonteiroWWardR2000Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trialLancet3561480511081531
- BrightlingCEWardRWoltmannG2000Induced sputum inflammatory mediator concentrations in eosinophilic bronchitis and asthmaAm J Respir Crit Care Med1623Pt18788210988099
- BrightlingCEWoltmannGWardlawAJ1999Development of irreversible airflow obstruction in a patient with eosinophilic bronchitis without asthmaEur Respir J1412283010596716
- BurgePSCalverleyPMJonesPW2000Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ320129730310807619
- BurgePSCalverleyPMAJonesPW2003Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE studyThorax58654812885977
- ChalmersGWMacleodKJThomsonL2001Smoking and airway inflammation in patients with mild asthmaChest12019172211742922
- ChanezPVignolaAMO’ShaugnessyT1997Corticosteroid reversibility in COPD is related to features of asthmaAm J Respir Crit Care Med1551529349154853
- ConfalonieriMMainardiEDella PortoR1998Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary diseaseThorax5358359797758
- CulpittSVMaziakWLoukidisS1999Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1601635910556133
- DenburgJA1999Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammationImmunol Today201111310203700
- Di StefanoACapelliALusuardiM1998Severity of airflow limitation is associated with severity of airway inflammation in smokersAm J Respir Crit Care Med1581277859769292
- Domagala-KulawikJMaskey-WarzechowsksaMKraszewskaI2003The cellular composition and macrophage phenotype in induced sputum in smokers and ex-smokers with COPDChest1231054912684293
- DouwesJGibsonPPekkanenJ2002Non-eosinophilic asthma: importance and possible mechanismsThorax57643812096210
- DvorakAMWellerPF2000Ultrastructural analysis of human eosinophilsChem Immunol7612810761302
- EdwardsBSCurryMSTsujiH2000Expression of P-selectin at low site density promotes selective attachment of eosinophils over neutrophilsJ Immunol1654041010861078
- ErinEMWilliamsTJBarnesPJ2002Eotaxin receptor (CCR3) antagonism in asthma and allergic diseaseCurr Drug Targets Inflamm Allergy12011414561201
- FanVSBrysonCLCurtisJR2003Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalization: time-dependent analysisAm J Respir Crit Care Med16814889414525798
- Flood-PagePTMenzies-GowANKayAB2003Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airwayAm J Respir Crit Care Med16719920412406833
- FujimotoKKuboKYamamotoH1999Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysemaChest11569770210084478
- GambleEGrootendorstDCBrightlingCE2003Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1689768212816740
- GibsonPGDolovichJDenburgJ1989Chronic cough: eosinophilic bronchitis without asthmaLancet1134682567371
- GibsonPGWoolleyKLCartyK1998Induced sputum eosinophil cationic protein (ECP) measurement in asthma and chronic obstructive airway disease (COAD)Clin Exp Allergy28108189761011
- GizyckiMJHattotuwaKLBarnesN2002Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissueThorax5779980312200525
- GonzaloJALloydCMKremerL1996Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptorsJ Clin Invest982332458941651
- GreenRHBrightlingCEMcKennaS2002Asthma exacerbations and sputum eosinophil counts: a randomised controlled trialLancet36017152112480423
- GreenRHBrightlingCEWoltmannG2002Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroidsThorax57875912324674
- GurselGTurktasHGokcoraN1997Comparison of sputum and serum eosinophil cationic protein (ECP) levels in nonatopic asthma and chronic obstructive pulmonary diseaseJ Asthma34313199250255
- HattotuwaKLGizyckiMJAnsariTW2002The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy studyAm J Respir Crit Care Med1651592612070058
- HoggJCChuFUtokaparchS2004The nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med35026455315215480
- ItoKItoMElliottWM2005Decreased histone deacetylase activity in chronic obstructive pulmonary diseaseN Engl J Med35219677615888697
- JonesPWWillitsLRBurgePS2003Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbationsEur Respir J21687312570111
- KeatingsVMBarnesPJ1997Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjectsAm J Respir Crit Care Med155449539032177
- KeatingsVMCollinsPDScottDM1996Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthmaAm J Respir Crit Care Med15353048564092
- KeatingsVMEvansDJO’ConnorBJ1997Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluidThorax5237249196522
- KeatingsVMJatakanonAWorsdellYM1997Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPDAm J Respir Crit Care Med15554289032192
- LacosteJYBousquetJChanezP1993Eosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol92537488409114
- LamsBESousaARReesPJ2000Subepithelial immunopathology of the large airways in smokers with and without chronic obstructive pulmonary diseaseEur Respir J155121610759445
- LoppowDSchleissMBKanniessF2001In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammationRespir Med951152111217907
- MahlerDAWirePHorstmanD2002Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm j Respir Crit Care Med16610849112379552
- [NICE] National Institute for Clinical Excellence2004Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary careThorax59Suppl I1232
- O’ShaughnessyTCAnsariTWBarnesNC1997Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1Am J Respir Crit Care Med15585279117016
- PauwelsRALofdahlCGLaitinenLA1999Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary DiseaseN Engl J Med34019485310379018
- PavordIDBrightlingCEWoltmannG1999Non-eosinophilic corticosteroid unresponsive asthmaLancet35322131410392993
- PavordIDSivaRBrightlingCE2004Prednisolone response in patients with COPDThorax5917914760166
- PizzichiniEPizzichiniMMEfthimiadisA1996Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurementsAm J Respir Crit Care Med1542Pt1308178756799
- PizzichiniEPizzichiniMMGibsonP1998Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitisAm J Respir Crit Care Med1585Pt11511179817701
- PostmaDSPetersISteenhuisE1988Moderately severe chronic airflow obstruction. Can corticosteroids slow down obstruction?Eur Respir J12263366234
- QuiYParkerDBarnesNC2005The effect of salmeterol/fluticasone proprionate on eosinophils and mast cells in COPDProc Am Thorac Soc2A132
- SaettaMBaraldoSCorbinoL1999CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1607111710430750
- SaettaMDi StefanoAMaestrelliP1994Airway eosinophilia in chronic bronchitis during exacerbationsAm j Respir Crit Care Med1506Pt11646527952628
- SivaRGreenRBrightlingCE2005Modulation of eosinophilic inflammation in COPD [abstract]European Respiratory Society 15th Annual CongressCopenhagen17–21 Sep 2005
- SorianoJBVestboJPrideNB2002Survival in COPD patients after regular use fluticasone propionate and salmeterol in general practiceEur Respir J208192512412670
- StanescuDSannaAVeriterC1996Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophilsThorax51267718779129
- StockleyRACalverleyPMAPrideN1995Biochemical and cellular mechanismsChronic obstructive pulmonary disease1st edLondonChapman and Hall93134
- SymonFALawrenceMBWilliamsonML1996Functional and structural characterization of the eosinophil P-selectin ligandJ Immunol1571711198759760
- SzafranskiWCukierARamirezA2003Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J21748112570112
- The Lung Health Study Research Group2000Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease: Lung Health Study IIN Engl J Med3431902911136260
- TomlinsonJEMcMahonADChaudriR2005Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthmaThorax60282715790982
- VestboJSorensenTLangeP1999Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trialLancet35318192310359405
- WardlawAJ1999Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigmJ Allergy Clin Immunol1049172610550733
- WoltmannGMcNultyCADewsonG2000Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flowBlood9531465210807781
- YildizFKaurACIlgazaliA2000Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary diseaseRespiration6771610705266
- ZhuJQuiYBarnesNC2005The effect of salmeterol/fluticasone on pro-inflammatory gene expression in COPDProc Am Thorac Soc2A127