Abstract
Respiratory failure in patients with COPD may be caused by insufficient force production or insufficient endurance capacity of the respiratory muscles. Anabolic steroids may improve respiratory muscle function in COPD. The effect of anabolic steroids on mitochondrial function in the diaphragm in emphysema is unknown. In an emphysematous male hamster model, we investigated whether administration of the anabolic steroid nandrolone decanoate (ND) altered the activity of mitochondrial respiratory chain complexes in the diaphragm. The bodyweight of hamsters treated with ND was decreased after treatment compared with initial values, and serum testosterone levels were significantly lower in hamsters treated with ND than in control hamsters. No difference in the activity of mitochondrial respiratory chain complexes in the diaphragm between normal and emphysematous hamsters was observed. Treatment with ND did not change the activity of mitochondrial respiratory chain complexes in the diaphragm of both normal and emphysematous hamsters. In emphysematous hamsters, administration of ND decreased the activity of succinate:cytochrome c oxidoreductase compared with ND treatment in normal hamsters. We conclude that anabolic steroids have negative effects on the activity of succinate:cytochrome c oxidoreductase and anabolic status in this emphysematous hamster model.
Introduction
In patients with COPD, the diaphragm is chronically overloaded, which is more serious with increasing severity of the disorder. As a result, several adaptations occur, including a shift from fast, glycolytic, type II fibers to slow, oxidative, type I fibers (CitationLevine et al 1997; CitationMercadier et al 1998). Furthermore, metabolic changes occur, resembling those that emerge after endurance training, such as an increase in oxidative capacity (CitationNoble and Ianuzzo 1985; CitationGreen et al 1995; CitationProctor et al 1995). Mitochondrial function is enhanced in the diaphragm of patients with COPD (CitationRibera et al 2002), as indicated by increases in the maximum rate of mitochondrial respiration and efficiency of the mitochondrial electron transport chain. This is considered to be beneficial, because these changes cause an increase in endurance capacity.
Despite this shift towards a more fatigue-resistant muscle, the majority of patients with severe COPD die from respiratory failure (CitationBraghiroli et al 1997). This respiratory failure may have multiple causes, including insufficient force production or insufficient endurance capacity of the respiratory muscles, or a combination of these factors. For example, CitationLevine et al (2003) observed reduced force-generating capacity in the diaphragm of COPD patients.
Anabolic steroids may be helpful in preventing respiratory failure. Clinical studies have mainly investigated the effect of treatment with anabolic steroids on muscle force-generating capacity. For example, it has been shown that treatment with nandrolone decanoate (ND) improved respiratory muscle function and exercise capacity after a pulmonary rehabilitation program in COPD patients using oral glucocorticosteroids (CitationCreutzberg et al 2003). Treatment with testosterone resulted in increases in quadriceps muscle strength and endurance with and without a resistance training program in COPD patients (CitationCasaburi et al 2004). In this study, testosterone did not change respiratory muscle strength.
In animal models, the effects of anabolic steroid treatment on oxidative capacity in several skeletal muscles have been investigated. Controversy exists in the literature about the effect of anabolic steroids on mitochondrial function and associated endurance capacity. The effect of anabolic steroids on mitochondrial function seems to be dependent on fiber type, with fast-twitch fibers (CitationSaborido et al 1991) or aerobic muscle (CitationEgginton 1987) being most sensitive to an increase in oxidative capacity in rats.
For preventing respiratory failure, it is important to know if treatment with anabolic steroids results in increased respiratory muscle oxidative capacity and mitochondrial function, especially in emphysema. Therefore, we investigated whether administration of ND increased the activity of mitochondrial respiratory chain complexes in the diaphragm of emphysematous hamsters. For this purpose, we treated healthy and emphysematous hamsters with ND or placebo and determined the activity of several parts of the mitochondrial energy-generating system.
Methods
Animals
Male 40-week-old inbred Syrian golden hamsters (Elevage Janvier) with initial bodyweight ~100 g were used. The animals were randomly divided into normal (NH) and emphysematous (EH) groups. The study was approved by the Animal Experiments Committee of the University Medical Centre Nijmegen and performed according to the Dutch National Guidelines of Animal Care.
Induction of emphysema
Under anesthesia of a mixture of 2.5% isoflurane and N2O and (1:2), saline (NH) or porcine pancreatic elastase (18 U/100 g O2 bodyweight [CitationRaub et al 1982] [EPC, Owensville, MI, USA] in 0.50 mL normal saline) was instilled intratracheally, as described in detail previously (CitationLewis et al 1992; Citationvan der Heijden et al 1998; Citationvan Balkom et al 1999).
Determination of degree of emphysema
When the animals were killed the lungs were removed for measurement of the lung volume by fluid displacement to evaluate the extent of emphysema.
Anabolic steroid administration
Fifty-two weeks after induction of emphysema, the normal hamsters and emphysematous hamsters were divided into treated (ND) and nontreated (CON) groups. Hamsters in the treated groups were injected for 10 weeks with 2.5 mg/kg ND (Deca-Durabolin, NV Organon, Oss, The Netherlands) once a week intramuscularly. To obtain maximum effect, ND was administered in the maximum dose as recommended by the manufacturer for use in patients. Hamsters in the control groups were injected with saline for the same period. Groups were subdivided as follows: NH CON (n=9), EH CON (n=12), NH ND (n=10), and EH ND (n=14).
General procedures
Sixty-two weeks after instillation the hamsters were anesthetized with pentobarbital sodium (Nembutal, 70 mg/kg intraperitoneally). A polyethylene cannula was inserted through a tracheotomy for mechanical ventilation with 100% O2. The diaphragm was quickly excised after combined laparotomy and thoracotomy and put immediately in ice-cold SETH buffer (0.25 mol/L sucrose, 2 mmol/L EDTA, 10 mmol/L Tris, 5× 104 U heparin, pH 7.4).
Mitochondrial function
Homogenization procedure of muscle tissue
Diaphragm muscle was washed with fresh ice-cold SETH, and fat and connective tissue were disconnected. Tissue was cut in very small pieces of about 0.1 × 0.1 mm with the aid of a Sorvall TC2 tissue chopper with razor blade. Tissue was homogenized in fresh ice-cold SETH buffer (5%–10% w/v) with a Potter Elvehjem tissue homogenizer according to CitationFischer, Ruitenbeek, Stadhouders, et al (1985). Some100-μL samples of the crude homogenate were frozen immediately in liquid nitrogen and kept at −80°C for measuring protein content, cytochrome c oxidase and citrate synthase (CS). The rest of the crude homogenate was centrifuged for 10 minutes at 2°C at 600 × g. The 600 × g supernatant was frozen in 100-μL aliquots in liquid nitrogen and kept at −80°C for measuring protein content, CS activity, and activities of respiratory chain enzymes. CS activity was measured according to CitationSrere (1969) with minor modifications. Briefly, a sample of the 600 × g supernatant was diluted (1:1) with SETH buffer containing 0.5% Triton X-100. A 50-μL sample was incubated in a total volume of 1.0 mL containing 0.1 mol/L Tris, 0.1 mmol/L DTNB (5,5′-dithio bis(2-nitro)-benzoate), 0.3 mmol/L acetylcoenzyme-A, and 0.5 mmol/L oxaloacetic acid, pH 8.1. In the blank, oxaloacetic acid was omitted. Coenzyme-A production by CS was measured spectrophotometrically at 412 nm by measuring the reduction of DTNB. Protein concentration was measured according to CitationLowry et al (1951) with minor modifications.
Incubations
Incubations for determination of the activity of succinate: cytochrome c oxidoreductase (SCC) were performed according to CitationFischer, Ruitenbeek, Berden, et al (1985) with minor modifications. Incubations for determination of complex III (C-III) and complex IV (C-IV) activity were performed according to CitationBentlage et al (1996) and CitationCooperstein and Lazarow (1951), respectively. Activities of SCC, C-III, and C-IV were divided by CS activity for correction for mitochondrial content.
Serum testosterone levels
When the animals were killed, blood samples were taken from the abdominal aorta for serum testosterone concentration measurements. Serum testosterone was assessed by a 3H-radioimmunoassay (RIA) after prepurification by means of paper chromatography of ether extracts of the samples, including correction for procedural losses, as described previously (CitationSwinkels et al 1987). To summarize briefly, before extraction 3H-testosterone was added to correct for procedural losses. After chromatography, radio chromatogram scanning identified the location of the testosterone zone; the zone was cut out and soaked in buffer. The recovered radioactivity was measured by liquid scintillation counting of an aliquot from the eluate. Subsequently, testosterone tracer and antiserum were added, and after incubation, free and bound tracers were separated by means of dextrane-coated charcoal. The antibody-bound radioactivity was assessed by liquid scintillation counting of the supernatant. The calculations were performed by special software designed for correction of the mass and radioactive contribution of the recovery tracer in the RIA. The detection limit was 3.5 pmol/L.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) when appropriate. Data were analyzed with SPSS for Windows, version 12.0.1 (SPSS, Chicago, IL, USA). A one-way ANOVA (analysis of variance) test and a Tukey post hoc test were used to determine if there were differences between the groups. A paired t test was used to determine differences within the groups before and after treatment. Significance was set at the 0.05 level.
Results
Bodyweight
Bodyweight was significantly lower in both emphysematous hamster groups than in normal groups before treatment (p < 0.05). After treatment, bodyweight was significantly lower in the normal nandrolone-treated hamster group and the emphysematous nandrolone-treated group than before treatment (p < 0.05). The results are shown in .
Table 1 Bodyweights and lung volumes (mean ± SEM)
Degree of emphysema
Mean lung volume was significantly higher in the emphysematous hamster groups than in the normal groups (17.0 ± 0.5 mL vs 11.4 ± 0.5 mL; p < 0.001), indicating that in the emphysematous groups emphysema was indeed induced. Furthermore, lung volume was higher in emphysematous nandrolone-treated than emphysematous control hamsters (p = 0.004). Lung volumes are presented in .
Serum testosterone levels
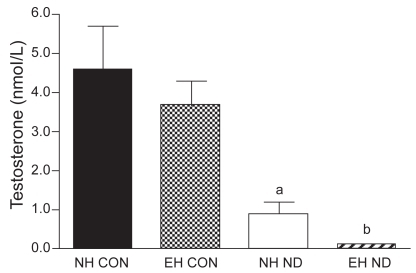
Serum testosterone levels were equal in emphysematous and normal hamsters. After treatment with ND, serum testosterone levels in normal nandrolone-treated hamsters were significantly decreased compared with those of normal control hamsters and in emphysematous nandrolone-treated hamsters compared with emphysematous controls (p = 0.001 and p < 0.001, respectively). Serum testosterone levels are presented in .
Mitochondrial function
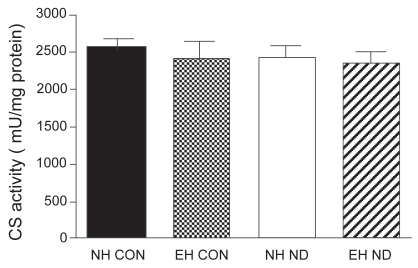
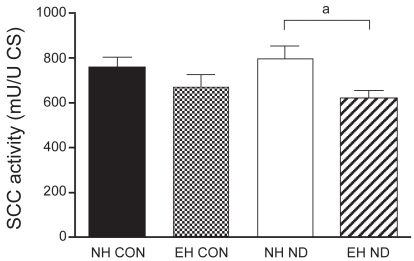
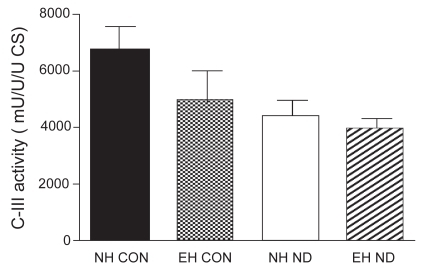
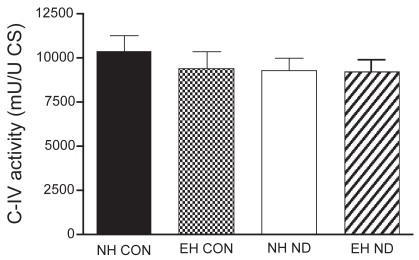
The activity of CS, C-III, and C-IV was equal between the four treatment groups. The activity of SCC was not different between normal control hamsters and emphysematous controls. However, after treatment with ND, the activity of SCC was significantly higher in the diaphragm of normal hamsters than in that of emphysematous animals. The mitochondrial function results are presented in –.
Discussion
This is the first study to investigate the effects of treatment with an anabolic steroid on the activity of mitochondrial respiratory chain complexes in the diaphragm of emphysematous hamsters. It shows that the activity of mitochondrial respiratory chain complexes in the diaphragm was not different between normal and emphysematous hamsters. Treatment with ND did not change the activity of mitochondrial respiratory chain complexes in either normal or emphysematous hamsters. However, in emphysematous animals ND treatment decreased the activity of SCC compared with ND treatment in normal hamsters. Mitochondrial content was not changed after ND treatment. Furthermore, bodyweight of hamsters treated with ND was decreased after treatment compared with initial values, and serum testosterone levels were significantly lower in animals treated with ND than in controls.
In mitochondria, energy is produced by the mitochondrial oxidative phosphorylation system, which is embedded in the mitochondrial inner membrane and comprises five enzyme complexes (I–V) and two electron carriers (coenzyme Q and cytochrome c) (CitationHatefi 1985; CitationSaraste 1999). The main function of the system is the coordinated transport of electrons and protons through the different complexes, which leads to the production of adenosine triphosphate (ATP) (CitationSmeitink et al 2001). This ATP can be used for diverse cell functions, including contractions in skeletal muscle. In our study, we found that mitochondrial energy-generating capacity of the diaphragm was not changed after treatment with anabolic steroids, either in normal or emphysematous hamsters. This is in agreement with the results of CitationSaborido et al (1991), who found that in rat extensor digitorum longus (EDL), but not in soleus muscle, mitochondrial function was enhanced after treatment with fluoxymesterone and meth-androstanolone, suggesting a higher sensitivity for anabolic steroid in fast-twitch (EDL) muscle. In the same study, the number of mitochondria was not changed after administration of anabolic steroids. In contrast, CitationEgginton (1987) suggested that aerobic muscles such as soleus and diaphragm are more susceptible to an increase of mitochondrial capacity after nandrolone phenylpropionate treatment. CitationBeck and James (1978) showed an increase in mitochondrial volume in rat diaphragm after treatment with 19-nortestosterone. This is in agreement with the findings of CitationSatoh et al (2000), who found an increase in cross-sectional areas of mitochondria after treatment with nandrolone phenylpropionate in mouse diaphragm.
Comparison with previous results is difficult, because different studies use different dose, species, duration of treatment, age, mode of administration, and activity level. These factors have been shown to influence the effects of anabolic treatment (CitationBresloff et al 1974; CitationBeck and James 1978; CitationKopera 1985; CitationPrezant et al 1993). For example, treatment of male rats with 19-nortestosterone in a low dose resulted in an increase in mitochondrial volume proportions in type I and intermediate fibers, whereas treatment with a high dose resulted in a less marked increase in mitochondrial volume proportions, particularly in intermediate fibers, suggesting that the response on anabolic steroids was lower in the high-dose group (CitationBeck and James 1978). The variation in the abovementioned factors may explain the fact that the literature is very inconsistent about the effect of treatment with anabolic steroids on mitochondrial function.
Another finding in our study is that after treatment with ND, SCC activity per mitochondrion is decreased in the diaphragm of emphysematous compared with normal hamsters. SCC activity measures complexes II and III and the electron carrier coenzyme Q, which are located in the mitochondrial inner membrane (CitationMolano et al 1999). Molano and colleagues reported decreased activity of enzymes in the mitochondrial inner membrane of rat liver after treatment with stanozolol and fluoxymesterone, but no change in CS activity, which is located in the matrix space. The authors suggested that anabolic-androgenic steroids could affect the mitochondrial membrane owing to their hydrophobic nature. In the same study, no change in C-IV activity was found after treatment with stanozolol and fluoxymesterone, suggesting that electron transport is disturbed in the electron transport chain before C-IV by these anabolic steroids (CitationMolano et al 1999). However, in our study we did not find a decrease in C-III activity after treatment with ND. This discrepancy in findings may be due to the difference in tissue studied, namely hamster diaphragm in our study versus rat liver in the study of Molano and colleagues. Furthermore, in the latter study the anabolic steroids were administered orally, which produces a first-pass effect in the liver, where the enzyme activities were measured. A possible explanation for our finding that SCC activity is decreased in the diaphragm of emphysematous hamsters treated with ND is that perhaps this specific mitochondrial part is more susceptible to the effects of ND administration in more active muscles (as is the diaphragm in emphysema).
It has recently been shown that the maximum rate of mitochondrial respiration and the efficiency of the electron transport chain for ATP production are increased in the diaphragm of COPD patients (CitationRibera et al 2002). The maximum respiratory rate of the mitochondria in the diaphragm of COPD patients was twice as high as in the diaphragm of control subjects. Endurance training elicits an increase in maximum respiratory rate of the mitochondria in human vastus lateralis muscle (CitationWalsh et al 2001). However, this increase is much less than that observed in the diaphragm of COPD patients (CitationRibera et al 2002). The respiratory rate is dependent on the muscle investigated in rats varying from 9.6 μmol O2/min/g dry weight in soleus muscle to 32μmol O2/min/g dry weight in cardiac muscle (CitationDe Sousa et al 2001). This rate also seems to be dependent on species, because the maximum mitochondrial respiration rate reported by CitationRibera et al (2002) in human diaphragm was 5.28 μmolO2/min/g dry weight, whereas the maximum respiration rate in rat diaphragm averaged 12μmol O2/min/g dry weight (CitationDe Sousa et al 2001). Data on interventions eliciting changes in human diaphragmatic mitochondrial function are not available. Therefore, it can not be predicted if the increase in mitochondrial respiration as found by Ribera and colleagues could be augmented by an intervention.
The fact that in our study we did not find an increase in the activity of mitochondrial respiratory chain complexes after ND treatment, either in normal or emphysematous hamsters, implies that treatment with ND may not increase the endurance capacity of the diaphragm. This suggests that treatment with anabolic steroids may not be able to prevent respiratory failure. However, respiratory failure may also be caused by a decrease in inspiratory muscle force-generating capacity. The role of anabolic steroids in preventing this cause of respiratory failure in patients with COPD is contradictory. For example, CitationCreutzberg et al (2003) found an increase in maximum inspiratory muscle strength after ND treatment in COPD patients (50 mg ND/2 weeks). The patients in this study performed a pulmonary rehabilitation program consisting of several endurance activities. Only in patients using oral glucocorticosteroids did ND treatment have an additional beneficial effect on respiratory muscle function above the effect of pulmonary rehabilitation. This effect of ND was not observed in patients who did not use oral glucocorticosteroids. This is in accordance with the results of CitationCasaburi et al (2004), who found no change in maximum inspiratory muscle strength after administration of testosterone in COPD patients who did not receive long-term oral corticosteroid treatment. The finding that in patients using corticosteroids ND treatment restores respiratory muscle function is in line with animal experimental studies (Citationvan Balkom et al 1998, Citation1999). In a rat model, treatment with ND prevented the loss of diaphragm force induced by long-term, low-dose methylprednisolone administration (Citationvan Balkom et al 1998). A subsequent study (Citationvan Balkom et al 1999) reported that ND was also able to prevent the loss of diaphragmatic function in emphysematous hamsters treated with long-term, low-dose methylprednisolone. These findings, combined with findings in the abovementioned studies (CitationCreutzberg et al 2003; CitationCasaburi et al 2004), suggest that treatment with anabolic steroids alone might be useful for preventing respiratory failure only in patients receiving corticosteroids.
In our study, we found that bodyweight was decreased after treatment with ND. This is in agreement with other studies, finding decreased bodyweight in male rats after treatment with ND (CitationBisschop et al 1997). Circulating endogenic androgens may play a role. Surpassing the physiological level of androgens in males has been reported to result in depression of the natural production of testosterone (CitationRyan 1981), downregulation of androgen-binding receptors (CitationRance and Max 1984), decrease in appetite (CitationKochakian and Endahl 1959), and a metabolic conversion to excess estradiol (CitationHickson and Kurowski 1986). The natural production of testosterone is indeed decreased in ND-treated hamsters in our study, which is shown by lower serum testosterone levels in the ND groups.
In conclusion, this study shows that the activity of mitochondrial respiratory chain complexes in the diaphragm in both normal and emphysematous hamsters was equal, and that treatment with ND did not change this activity. In emphysematous hamsters, administration of ND decreased the activity of SCC compared with ND treatment in normal hamsters. Furthermore, we have shown that bodyweight of hamsters treated with ND was decreased after treatment compared with initial values, and that treatment with ND resulted in significantly lower serum testosterone levels in both normal and emphysematous hamsters. Taking all results together, our data do not support the use of anabolic steroids in preventing respiratory failure caused by fatigue of the diaphragm.
Acknowledgments
We thank the technicians of the mitochondrial biochemical diagnostics lab at the Laboratory of Pediatrics and Neurology for performing the mitochondrial measurements. This study was financially supported by Boehringer-Ingelheim, the Netherlands (Grant 4922 BA12).
References
- BeckSJamesNT1978A stereological analysis of the effect of 19-nortestosterone (Durabolin) on rat skeletal muscleBr J Exp Pathol5951421718803
- BentlageHAWendelUSchaggerH1996Lethal infantile mitochondrial disease with isolated complex I deficiency in fibroblasts but with combined complex I and IV deficiencies in muscleNeurology4724388710086
- BisschopAGayan-RamirezGRollierH1997Effects of nandrolone decanoate on respiratory and peripheral muscles in male and female ratsJ Appl Physiol821112189104847
- BraghiroliAZaccariaSIoliF1997Pulmonary failure as a cause of death in COPDMonaldi Arch Chest Dis5217059203816
- BresloffPFoxPKSimAW1974The effect of nandrolone phenyl-propionate on 14 C-leucine incorporation into muscle protein in the rat and rabbit in vivoActa Endocrinol (Copenh)76403164406601
- CasaburiRBhasinSCosentinoL2004Effects of testosterone and resistance training in men with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med170870815271690
- CoopersteinSJLazarowA1951A microspectrophotometric method for the determination of cytochrome oxidaseJ Biol Chem1896657014832284
- CreutzbergECWoutersEFMostertR2003A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trialChest12417334214605042
- De SousaEVekslerVBigardX2001Dual influence of disease and increased load on diaphragm muscle in heart failureJ Mol Cell Cardiol3369971011273723
- EggintonS1987Effects of an anabolic hormone on aerobic capacity of rat striated musclePflugers Arch410356613432043
- FischerJCRuitenbeekWBerdenJA1985Differential investigation of the capacity of succinate oxidation in human skeletal muscleClin Chim Acta15323363000647
- FischerJCRuitenbeekWStadhoudersAM1985Investigation of mitochondrial metabolism in small human skeletal muscle biopsy specimens. Improvement of preparation procedureClin Chim Acta14589993978823
- GreenHJJonesSBall-BurnettM1995Adaptations in muscle metabolism to prolonged voluntary exercise and trainingJ Appl Physiol78138457713803
- HatefiY1985The mitochondrial electron transport and oxidative phosphorylation systemAnnu Rev Biochem541015692862839
- HicksonRCKurowskiTG1986Anabolic steroids and trainingClin Sports Med546193521896
- KochakianCDEndahlBR1959Changes in body weight of normal and castrated rats by different doses of testosterone propionateProc Soc Exp Biol Med100520213634193
- KoperaH1985The history of anabolic steroids and a review of clinical experience with anabolic steroidsActa Endocrinol Suppl (Copenh)27111183907240
- LevineSKaiserLLeferovichJ1997Cellular adaptations in the diaphragm in chronic obstructive pulmonary diseaseN Engl J Med33717998069400036
- LevineSNguyenTKaiserL2003Human diaphragm remodeling associated with COPD: clinical implicationsAm J Respir Crit Care Med1687061312857719
- LewisMIZhanWZSieckGC1992Adaptations of the diaphragm in emphysemaJ Appl Physiol72934431568989
- LowryOHRosebroughNJFarrAL1951Protein measurement with the Folin phenol reagentJ Biol Chem1932657514907713
- MercadierJJSchwartzKSchiaffinoS1998Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflationAm J Physiol274L527349575870
- MolanoFSaboridoADelgadoJ1999Rat liver lysosomal and mitochondrial activities are modified by anabolic-androgenic steroidsMed Sci Sports Exerc312435010063813
- NobleEGIanuzzoCD1985Influence of training on skeletal muscle enzymatic adaptations in normal and diabetic ratsAm J Physiol249E36052931994
- PrezantDJValentineDEGentryEI1993Effects of short-term and long-term androgen treatment on the diaphragm in male and female ratsJ Appl Physiol75114098226522
- ProctorDNSinningWEWalroJM1995Oxidative capacity of human muscle fiber types: effects of age and training statusJ Appl Physiol78203387665396
- RanceNEMaxSR1984Modulation of the cytosolic androgen receptor in striated muscle by sex steroidsEndocrinology11586266611254
- RaubJAMerceRRMillerFJ1982Dose response of elastase-induced emphysema in hamstersAm Rev Respir Dis12543256918202
- RiberaFN’GuessanBZollJ2002Mitochondrial electron transport chain function is enhanced in inspiratory muscles of COPD patientsAm J Respir Crit Care Med167873912493645
- RyanAJ1981Anabolic steroids are fool’s goldFed Proc40268286269903
- SaboridoAVilaJMolanoF1991Effect of anabolic steroids on mitochondria and sarcotubular system of skeletal muscleJ Appl Physiol701038431827788
- SarasteM1999Oxidative phosphorylation at the fin de siecleScience28314889310066163
- SatohKGotohTYamashitaK2000Morphological effects of an anabolic steroid on muscle fibers of the diaphragm in miceJ Electron Microsc495318
- SmeitinkJvan den HeuvelLDiMauroS2001The genetics and pathology of oxidative phosphorylationNat Rev Genet23425211331900
- SrerePALowensteinJM1969Citrate synthase, EC 4.1.3.7, citrate oxaloacetatelyase (CoA-acetylating)Methods in enzymologyXIIILondonAcademic Pr311
- SwinkelsLMRossHABenraadTJ1987A symmetric dialysis method for the determination of free testosterone in human plasmaClin Chim Acta16534193652455
- van BalkomRHDekhuijzenPNFolgeringHT1998Anabolic steroids in part reverse glucocorticoid-induced alterations in rat diaphragmJ Appl Physiol84149299572790
- van BalkomRHDekhuijzenPNvan der HeijdenHF1999Effects of anabolic steroids on diaphragm impairment induced by methylprednisolone in emphysematous hamstersEur Respir J131062910414405
- van der HeijdenHFDekhuijzenPNFolgeringH1998Long-term effects of clenbuterol on diaphragm morphology and contractile properties in emphysematous hamstersJ Appl Physiol85215229655778
- WalshBTonkonogiMSahlinK2001Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibresPflugers Arch442420511484774