Abstract
The budesonide–formoterol dry powder inhaler (Symbicort® Turbuhaler® 160/4.5–640/18 μg/day) contains the long-acting β2-adrenoreceptor agonist formoterol and the inhaled corticosteroid budesonide. Two large, 12-month trials examined the effect of budesonide–formoterol 160/4.5 μg twice daily in COPD patients who met these criteria. The studies were identical, except one in which the patients had received oral prednisolone 30 mg/day and had inhaled formoterol 4.5 μg twice daily for 2 weeks before randomization. In terms of the FEV1, budesonide–formoterol produced an effect greater than that of both budesonide alone and formoterol alone reported in previous studies. The combination was generally more effective than either of the components in terms of peak expiratory flow, symptoms, and exacerbations. These advantages of the combination over those of either budesonide alone or formoterol alone were quite consistent. Improving lung function and decreasing symptoms significantly, budesonide–formoterol combination therapy provides significant clinical improvements in COPD, despite the limited reversibility of impaired lung function in the disease.
Introduction
COPD is an important cause of morbidity, mortality, and hospital admissions. This disorder is characterized by airflow limitation that is not fully reversible despite bronchodilator therapy. This limitation in airflow, which is associated with an exaggerated inflammatory response of the lung to the inhalation of noxious particles or gases, usually worsens progressively (CitationPauwels et al 2001; CitationFabbri and Hurd 2003; CitationNHLBI/WHO workshop report 2004).
COPD is a multicomponent disease that includes airway inflammation, airflow limitation, mucociliary dysfunction, and airway structural changes (CitationPauwels et al 2001). Oxidative stress and an imbalance between proteases and antiproteases in the lung have also been implicated in the pathophysiology of the condition (CitationBarnes 2000a). All COPD components contribute to a complex of lung function changes, symptoms, and exacerbations, which affect the patient’s health and, ultimately, survival (CitationPauwels et al 2001). Therefore, the correct therapeutic approach must be applied to interfere with these components. According to Global Initiative for Chronic Obstructive Lung Disease Guidelines (GOLD), the main goals of COPD treatment are maintenance of improvement in lung function, relief of symptoms, prevention of exacerbations and hospitalizations, improvement in quality of life, decrease in accelerated decline in lung function, increase in life expectancy, accomplishment of all of these in a cost-effective manner, and provision of drugs with minimal side-effects (CitationNHLBI/WHO workshop report 2004).
The fixed combination of budesonide–formoterol in a single dry powder inhaler (Symbicort® Turbuhaler® [hereafter referred to as budesonide–formoterol]; AstraZeneca UK Limited) has been instructed to patients with COPD as an alternative to treatment of concurrent inhaled corticosteroid and a long-acting β2-agonist, administered via separate inhalers, or as an alternative to inhaled corticosteroids alone (CitationDoherty 2002; CitationLalloo 2002; CitationCazzola and Dahl 2004; CitationReynolds et al 2004). This article reviews the pharmacology and clinical profile of budesonide–formoterol in treating patients with COPD.
Pharmacodynamics
The pharmacodynamics of both budesonide and formoterol have been documented and reviewed (CitationBartow and Brogden 1998; CitationBoobis 1998; CitationSzefler 1999; CitationHvizdos and Jarvis 2000; CitationMcGavin et al 2001; CitationCheer and Scott 2002). Budesonide uptake is rapid because of its high affinity for the glucocorticoid receptor, leading to high initial saturation of the glucocorticoid receptors (CitationHvizdos and Jarvis 2000). Reversible esterification of budesonide on inhalation prolongs the duration of action in airways and lung tissue, while the moderate lipophilicity of unesterified budesonide limits peripheral tissue retention (CitationSzefler 1999; CitationHvizdos and Jarvis 2000; CitationMcGavin et al 2001). Budesonide inhibits the activities of a wide range of inflammatory cells including eosinophils, T lymphocytes, mast cells, macrophages, dendritic cells, and neutrophils (CitationAstraZeneca 2005). Budesonide is widely distributed into tissues; the mean volume of distribution at steady state is approximately 3 L/kg in both children and adults (CitationAstraZeneca 2005). At concentrations achieved with recommended dosages, 85%–90% of the medication binds to plasma proteins (CitationAstraZeneca 2005).
Formoterol is a long-acting, selective, β2-agonist bronchodilator with a fast onset of action (within 1–3 minutes) and a long duration (12 hours) of broncholytic effect. It bronchodilates as rapidly as salbutamol (CitationCazzola et al 2001), and its efficacy and duration of action are similar to those of salmeterol (CitationCazzola et al 1995). A significant effect occurs with formoterol within minutes of inhalation of a therapeutic dose (CitationFaulds et al 1991; CitationCazzola et al 2004).
Formoterol has also been shown to induce mean peak bronchodilation (increase in FEV1 over baseline values) more rapidly than salmeterol (CitationCazzola et al 1995; CitationAalbers et al 2002). In addition, Celik et al showed that after 10 minutes, formoterol induced a clinically and statistically significant improvement in FEV1 compared with placebo, whereas salmeterol required 20 minutes (CitationCelik et al 1999; CitationJohnson and Rennard 2001).
Few data have been published on the pharmacodynamics of the combined product in patients with COPD (CitationFenton and Keating 2004; CitationGoldsmith and Keating 2004; CitationAstraZeneca 2005; CitationGlaxoSmithKline 2005). Formoterol and budesonide have demonstrated additive and synergistic effects when used in combination. Therefore, budesonide–formoterol combination provides an effective treatment option in COPD patients (CitationCalverley et al 2003; CitationSzafranski et al 2003). The concomitant use of a long-acting β2-agonist and an inhaled corticosteroid can influence both airway obstruction (smooth muscle contraction, increased cholinergic tone, and loss of elastic recoil), and airway inflammation (increased numbers of neutrophils, macrophages and CD8+lymphocytes, elevated IL-8 and TNF-a, and protease–antiprotease imbalance) (CitationCazzola and Dahl 2004; CitationCazzola et al 2004).
A small increase in cortisol suppression was observed following administration of budesonide–formoterol compared with the individual components. Nevertheless, this increase was not considered clinically relevant (CitationReynolds et al 2004; CitationAstraZeneca 2005).
Pulmonary effects
As mentioned above, budesonide and formoterol have demonstrated additive and synergistic effects when used in combination. Both lung function and health status of COPD patients improved significantly when patients were treated with budesonide–formoterol delivered in a single inhaler instead of monotherapy by either budesonide or formoterol (CitationCalverley et al 2003; CitationSzafranski et al 2003; CitationReynolds et al 2004).
In a double-blind, randomized, crossover study in moderate-to-severe COPD patients, inhalations of budesonide–formoterol increased FEV1. Budesonide–formoterol was administered via a Turbuhaler dry powder inhaler (CitationCazzola et al 2003). Budesonide–formoterol recipients showed a mean maximum improvement from baseline in FEV1 earlier than fluticasone–salmeterol recipients (120 vs 300 minutes; mean maximum improvements of 0.29 vs 0.32 L). Between-group differences in FEV1 occurred at 120 and 360 minutes (CitationCazzola et al 2003). However, at 12 hours the mean increase from baseline in FEV1 was the same for both combinations (0.10 L) (CitationCazzola et al 2003). Similar results were reported in other 12-month studies (CitationCalverley et al 2003; CitationSzafranski et al 2003).
The effects of budesonide–formoterol combination treatment in COPD patients have been investigated. In 2 large, 12-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter trials, moderate-to-severe COPD patients received inhalations of budesonide–formoterol (160/4.5 μg), budesonide (200 μg) alone, formoterol (4.5 μg) alone, or placebo twice daily (CitationCalverley et al 2003; CitationSzafranski et al 2003). These studies showed that budesonide–formoterol significantly reduces both the risk of exacerbations and the need for medical intervention, and improves lung function and decreases symptoms (CitationCalverley et al 2003; CitationSzafranski et al 2003).
Anti-inflammatory effects
Increasingly, COPD is being recognized as an inflammatory disorder of both the large and small airways, characterized by airway remodeling and emphysematous changes in the lung parenchyma (CitationBarnes 2000b). Importantly, the severity of airway inflammation is directly related to the severity of the underlying COPD. In established COPD, the peripheral airways show wall inflammation, fibrosis, smooth muscle hypertrophy, and goblet cell metaplasia in the epithelium, and the airway lumen is often plugged by mucus (CitationCasio et al 1978; CitationXie et al 2003; CitationScola et al 2004; CitationSin et al 2004).
Formoterol acts by stimulating the adenylate cyclase pathways, which, in turn, increases intracellular concentrations of cyclic adenosine monophosphate (CitationScola et al 2004). Although formoterol, itself has weak anti-inflammatory effect, in vitro studies suggest that it can materially amplify the effects of budesonide when administered in combination therapy, making it possible to achieve large anti-inflammatory effects even with relatively low doses of budesonide. Formoterol also appears to increase the effectiveness of budesonide in suppressing expression of adhesion molecules such as intercellular adhesion molecule-1. The bronchodilatory effect of formoterol may also facilitate budesonide deposition into areas of the lung, wherein active inflammation is present and prominent. Furthermore long-acting β2-agonists enhance nuclear localization of glucocorticoid receptors and suppression of inflammatory mediators by corticosteroids (CitationSin et al 2004).
Corticosteroids attenuate neutrophil recruitment activation, and chemotaxis (CitationLomas et al 1991; CitationLlewellyn-Jones et al 1994). Moreover, they reduce the numbers of mucosal and subepithelial mast cells, and reduce the epithelial CD8/CD4 ratio, although they have no effect on the major inflammatory cell types in COPD (CitationGizycki et al 2002; CitationHattotuwa et al 2002). Inhaled corticosteroids (ICS) also significantly reduce bronchoalveolar lavage fluid levels of IL-8 and myeloperoxidase, and also cell numbers, and reduce the proportion of neutrophils, symptom score, and bronchitis index (CitationBalbi et al 2000). These findings seem to support the use of ICS in treating COPD, although long-term treatment with ICS must be associated with a high risk of adverse systemic effects and involves unnecessary expense (CitationBarnes 2000a).
In vivo studies have demonstrated that combined usage of formoterol and budesonide produces a greater effect on airway inflammation than either drug alone with a complementary interaction (CitationSin et al 2004).
Pharmacokinetics
The pharmacokinetics of inhaled forms of both budesonide and formoterol have previously been reviewed in detail (CitationBartow and Brogden 1998; CitationBoobis 1998; CitationEdsbäcker 1999; CitationDonnelly and Seal 2001; CitationCheer and Scott 2002).
Data on combination budesonide–formoterol other than the one present in the manufacturer’s summary are difficult to find (CitationAstraZeneca 2005). In this study, the mean lung deposition of budesonide after inhalation via a Turbuhaler device was reported as 32%–44% of the delivered dose and the amount of formoterol that reaches the lungs from a Turbuhaler was reported as 28%–49% of the delivered dose (CitationAstraZeneca 2005). The pharmacokinetics of the individual components are briefly described below.
Absorption and distribution
Following inhalation, budesonide fatty acid esterification in the airways (CitationEdsbäcker and Brattsand 2002) rapidly dissolves in bronchial secretions (CitationEdsbäcker 1999). It was concluded that budesonide reaches maximum plasma concentration (Cmax) at 17–30 minutes (CitationThorsson et al 1994; CitationEdsbäcker 1999; CitationDonnelly and Seal 2001; CitationAstraZeneca 2005). The budesonide–formoterol Turbuhaler delivers half of the labeled dose as fine particles. The systemic bioavailability of budesonide is nearly half of the delivered dose (CitationAstraZeneca 2005).
Following inhalation, absorption of inhaled formoterol is rapid and reaches maximum Cmax at 10 minutes. Its systemic bioavailability is approximately 61% of the delivered dose (CitationAstraZeneca 2005).
Therapeutic efficacy
The effects of budesonide–formoterol combination treatment via Turbuhaler in COPD patients also have been investigated. This section reviews studies assessing the efficacy of budesonide–formoterol combination as maintenance therapy with COPD and the effect of this combination on health-related quality of life (HRQL) (CitationCalverley et al 2003; CitationSzafranski et al 2003; CitationCazzola et al 2004).
The efficacy of budesonide–formoterol in COPD patients has been studied in 2 large, 12-month, randomized, double-blind, placebo-controlled, parallel-groups, multicenter clinical trials. These studies showed that budesonide–formoterol significantly reduces both the risk of exacerbations and the need for medical intervention, and provides rapid, sustained improvement in lung function and symptom relief (CitationCalverley et al 2003; CitationSzafranski et al 2003).
In the study of Calverley et al, 1022 patients (mean age, 64 years; FEV1, 0.98 L and 36% predicted) with severe COPD (FEV1 < 50% predicted normal) and a history of exacerbations were included. Initially, treatment was intensified with the oral steroid prednisolone (30 mg once daily) and inhaled formoterol Turbuhaler (4.5 μg twice daily) for 2 weeks, in an attempt to optimize patients’ health status. Following the intensification period, patients were randomized to receive budesonide–formoterol Turbuhaler (160/4.5 μg, twice daily), budesonide Turbuhaler alone (200 μg, twice daily), formoterol Turbuhaler alone (4.5 μg, twice daily), or placebo for 1 year (CitationCalverley et al 2003).
The study of Szafranski et al involved 812 patients (mean age, 64 years; FEV1, 0.96 L and 36% predicted) with severe COPD (FEV1 < 50% predicted normal) and a history of exacerbations. Patients were randomized to receive budesonide–formoterol Turbuhaler (160/4.5 μg, twice daily), budesonide Turbuhaler alone (200 μg, twice daily), formoterol Turbuhaler alone (4.5 μg, twice daily), or placebo for 1 year, after a 2-week run-in period when maintenance medication was withdrawn and only terbutaline was allowed as rescue (CitationSzafranski et al 2003).
Effect on lung function
Budesonide–formoterol combination therapy provides a rapid and sustained improvement in lung function (CitationCalverley et al 2003; CitationSzafranski et al 2003). CitationCalverley et al (2003) showed that budesonide–formoterol therapy was significantly better than that with long-acting β2-agonists alone, corticosteroids alone, or placebo in maintaining the lung function improvement achieved after optimization. Mean FEV1 values in the budesonide–formoterol group remained 14%, 11%, and 5% above the values in the placebo group, budesonide group, and formoterol group, respectively (). Morning peak expiratory flow (PEF) in the budesonide–formoterol group was 18 L/minute, 15 L/minute, and 7 L/minute above the value in the placebo group, budesonide group, and formoterol group, respectively. The value for evening PEF in the budesonide–formoterol group was 14 L/minute, 12 L/minute, and 5 L/minute above the value in the placebo group, budesonide group, and formoterol group, respectively. Forced vital capacity value in the budesonide–formoterol group was 9%, 8%, and 2% above that in the placebo group, budesonide group, and formoterol group, respectively (CitationCalverley et al 2003).
Figure 1 Changes in mean FEV1 in the 4 treatment groups from randomisation to the average of all available measurements during the 12-month treatment period. Budesonide–formoterol (▪) vs budesonide (●), p < 0.001; budesonide–formoterol vs formoterol (□), p = 0.002; budesonide vs placebo (○), p = 0.145; formoterol vs placebo, p < 0.001; budesonide–formoterol vs placebo, p < 0.001. Reprinted from CitationCalverley PM, Boonsawat Z, Zhong N, et al. 2003. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J, 22:912–9. Copyright © 2003, with permission from European Respiratory Society Journals Ltd.
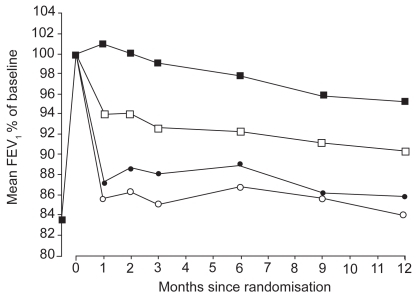
CitationSzafranski et al (2003) reported that at trial endpoint, patients receiving budesonide–formoterol had FEV1 values 15%, 9%, and 1% greater than those receiving placebo, budesonide recipients, and formoterol alone, respectively. Mean FEV1 values at baseline across treatment groups ranged from 0.96 to 1.01L. These lung function improvements were maintained throughout the 12-month study. Significant improvements in morning and evening PEF values were observed during the 12 months of therapy with budesonide–formoterol compared with all other treatment groups (adjusted mean change from run-in: morning PEF, 24, 16, and 12 L/min vs placebo, budesonide alone, and formoterol alone, respectively; evening PEF, 20, 15, and 11 L/min vs placebo, budesonide alone, and formoterol alone, respectively) (CitationSzafranski et al 2003).
In a double-blind, randomized, crossover study in moderate-to-severe COPD patients, inhalations of budesonide–formoterol increased FEV1. Budesonide–formoterol was administered via a Turbuhaler dry powder inhaler (CitationCazzola et al 2003). Budesonide–formoterol recipients showed a mean maximum improvement from baseline in FEV1 earlier than fluticasone–salmeterol recipients (120 vs 300 minutes; mean maximum improvements of 0.29 vs 0.32 L). Between-group differences in FEV1 occurred at 120 and 360 minutes, but at 12 hours the mean increase from baseline in FEV1 was the same for both combinations (0.10 L) (CitationCazzola et al 2003). Similar results were reported in other 12-month studies (CitationCalverley et al 2003; CitationSzafranski et al 2003).
Effect on COPD symptoms
Budesonide–formoterol combination therapy provides significantly greater reduction in the symptoms of COPD. The improvement in lung function observed after budesonide–formoterol treatment must be considered to be important, but the greater capacity of budesonide–formoterol to decrease the mean total symptom score (night-time awakenings, shortness of breath, cough, and chest tightness), to increase the number of days free from shortness of breath by 12% vs placebo, and to increase the number of awakening-free nights by 14% vs placebo, each of which is equivalent to approximately 1 extra day/night per week, is likely to have a substantial impact on health (CitationSzafranski et al 2003). This effect was sustained for 12 months for budesonide–formoterol vs placebo and budesonide alone (CitationCalverley et al 2003; CitationSzafranski et al 2003).
The reduction in shortness of breath score was significantly greater in patients receiving budesonide–formoterol than in those receiving budesonide, an improvement that was sustained throughout the 12-month study period without any signs of tachyphylaxis (CitationCalverley et al 2003).
Exacerbations of COPD
In addition to physical deterioration, COPD exacerbations have a major impact on patients’ feelings of well-being and HRQL, and are extremely distressing for patients (CitationDonaldson et al 2002). As the disease worsens, patients find it increasingly difficult to perform even the activities of everyday living. Patients who have exacerbations more frequently have a worse HRQL (CitationSeemungal et al 1998).
Budesonide–formoterol combination therapy was shown to significantly reduce exacerbations vs formoterol alone and placebo. Budesonide–formoterol has been shown to extend the time interval to first severe exacerbation by 158, 100, and 76 days more than placebo, formoterol alone, and budesonide alone, respectively (CitationCalverley et al 2003). CitationSzafranski et al (2003) reported that budesonide–formoterol extended the time interval to first severe exacerbation by 156, 100, and 76 days more than placebo, formoterol alone, and budesonide alone, respectively. These reductions in exacerbations provide a significant improvement in COPD patients’ quality of life, and give patients the confidence to return to everyday activities. Budesonide–formoterol therapy also reduced the risk of exacerbation by 29%, 30%, and 23% vs placebo, formoterol alone, and budesonide alone, respectively (CitationCalverley et al 2003). Budesonide–formoterol was seen to reduce the risk of exacerbations by 23%, 24%, and 11% vs placebo, formoterol alone, and budesonide alone, respectively (CitationSzafranski et al 2003).
Effect on HRQL
The HRQL of patients with COPD can be measured using the St. George’s Respiratory Questionnaire (SGRQ), which measures the impact of the disease on well-being and daily life, and has been validated for use in patients with COPD (CitationJones 1992). A reduction in score of 4 is considered a clinically relevant improvement, noticeable by the patient. A worse quality of life predicts a worse clinical outcome (CitationOsman et al 1997).
Adding inhaled corticosteroids to the treatment has been shown to significantly improve HRQL in patients with COPD (CitationYildiz et al 2004). Budesonide–formoterol has been shown to provide a meaningful improvement in COPD patients’ HRQL (CitationCalverley et al 2003; CitationSzafranski et al 2003). CitationSzafranski et al (2003) reported that the reduction from baseline in mean SGRQ total score was significantly greater in patients treated with budesonide–formoterol than in placebo recipients (–3.9 vs –0.03 units). In patients receiving budesonide–formoterol, the SGRQ symptom and impact scores were reduced by 5.9 and 4.7 units more than the reduction in the SGRQ symptom and impact scores in placebo recipients. Mean reductions from baseline in the SGRQ total score were –1.9 and –3.6 units in budesonide and formoterol recipients, respectively. SGRQ total scores at baseline, before which patients received rescue-medication only, ranged from 51 to 54 in the 4 groups.
CitationCalverley et al (2003) reported that budesonide–formoterol combination therapy provided a sustained reduction of –7.5 in SGRQ score, compared with the reduction in SGRQ score in placebo, a superior improvement to that seen with formoterol alone (reduction of –4.1 vs placebo) and budesonide alone (reduction of –3.0 vs placebo). Budesonide–formoterol improved SGRQ scores significantly when compared with budesonide and formoterol alone, by 4.5 and 3.4 units, respectively.
Tolerability
Budesonide–formoterol combination therapy is well tolerated in COPD patients and shows a similar frequency of adverse events when compared with budesonide alone and formoterol alone. The adverse effects of inhaled budesonide (CitationPauwels et al 1999; CitationJohnell et al 2002) and formoterol (CitationCheer and Scott 2002; Dahl et al 2002) in patients with COPD have been described in detail elsewhere. Tolerability data have also been previously reported (CitationCalverley et al 2003; CitationSzafranski et al 2003; CitationAstraZeneca 2005).
Budesonide–formoterol combination therapy was generally well tolerated in patients with COPD. Statistical analysis of tolerability data was reported for withdrawal rates only (CitationSzafranski et al 2003) ().
Figure 2 Kaplan-Meier plot of discontinuations, by treatment group. –– Symbicort; ..... budesonide; ----- formoterol; –..– placebo. Reprinted from CitationSzafranski W, Cukier A, Ramirez A, et al. 2003. Efficacy and safety of budesonide–formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J, 21:74–81. Copyright © 2003, with permission from European Respiratory Society Journals Ltd.
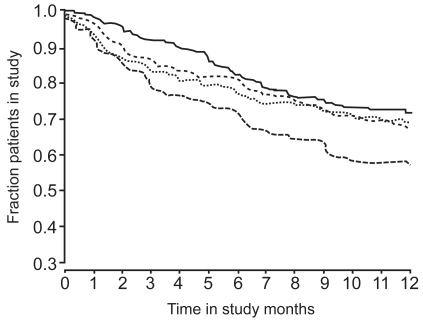
CitationCalverley et al (2003) reported the withdrawal of 393 of 1022 randomized patients (38%), 193 because of deterioration of COPD. CitationSzafranski et al (2003) reported the withdrawal of 275 of 812 randomized patients (34%), 115 because of deterioration of COPD. In both trials, a significantly higher number of withdrawals due to deterioration of COPD occurred in the placebo groups (23% and 21%) compared with the budesonide–formoterol groups (11% and 10%). A significantly smaller proportion of patients withdrew from the budesonide–formoterol group due to deterioration of COPD than from the budesonide (18%; p = 0.038) or formoterol (23%; p < 0.001) groups in the larger study (CitationCalverley et al 2003).
Adverse effects of the combination of budesonide–formoterol in a double-blind, randomized trial with COPD were respiratory infection (14%), pneumonia (3%), pharyngitis (3%), fever (2%), dyspnea worsening (2%), chest pain (3%), back pain (3%), diysphonia (2%), rhinitis (4%), hypertension (2%), and moniliazis (2%) (CitationCalverley et al 2003).
Fewer patients withdrew from the study in the budesonide–formoterol group than in the budesonide alone, formoterol alone, or placebo groups (CitationCalverley et al 2003).
Dosage and administration
One inhalation twice daily of budesonide–formoterol 200/6 μg and 400/12 μg via the Turbuhaler device is approved in many countries for the symptomatic treatment of patients with severe COPD (FEV1 < 50% predicted normal) and a history of repeated exacerbations who have significant symptoms despite regular therapy with long-acting bronchodilators (CitationAstraZeneca 2005). The budesonide–formoterol 200/6 μg inhaler delivers 160 μg of budesonide and 4.5 μg of formoterol per dose; the 400/12 μg inhaler delivers 320 μg of budesonide and 9 μg of formoterol per dose. The recommended dosage for the treatment of COPD in adults is 320/9 μg twice daily (CitationAstraZeneca 2005). Formoterol is a long-acting bronchodilator and should not be used for rescue medication. In elderly patients dosage adjustment is not required (CitationAstraZeneca 2005).
Budesonide–formoterol: current status
Budesonide–formoterol combination therapy administered via the Turbuhaler dry powder inhaler is currently approved in many countries for the treatment of patients with severe COPD. The recommended dosage of the drug is 320/9 μg twice a day. In clinical trials with COPD, combined budesonide–formoterol administered via the Turbuhaler dry powder inhaler has proven more effective in improving lung function and reducing exacerbations when compared with the same dose of budesonide or formoterol given alone. Budesonide–formoterol combination therapy is also safer and tolerated better than the same dose of budesonide or formoterol given alone.
References
- AalbersRAyresJBackerV2002Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trialEur Respir J199364312030736
- AstraZeneca UK Limited2005Symbicort® Turbuhaler® summary of product characteristics [online]Accessed 20 August 2005 URL: http://emc.medicines.org.uk
- BalbiBMajoriMBertaccoS2000Inhaled corticosteroids in stable COPD patients: do they have effects on cells and molecular mediators of airway inflammation?Chest1171633710858395
- BarnesPJ2000aInhaled corticosteroids are not beneficial in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med161342410673166
- BarnesPJ2000bChronic obstructive pulmonary disease. ReviewN Engl J Med3432698010911010
- BartowRABrogdenRN1998Formoterol: an update of its pharmacological properties and therapeutic efficacy in the management of asthmaDrugs2303229506248
- BoobisA1998Comparative physiochemical and pharmacokinetic profiles of inhaled beclomethasone dipropionate and budesonideRespir Med92Suppl B2610193529
- CalverleyPMBoonsawatZZhongN2003Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J22912914680078
- CasioMGhezzoHHoggJC1978The relations between structural changes in small airways and pulmonary function testsN Eng J Med298127781
- CazzolaMCentanniSRegordaC2001Onset of action of single doses of formoterol administered via Turbuhaler in patients with stable COPDPulm Pharmacol Ther1441511162418
- CazzolaMDahlR2004Inhaled combination therapy with long-acting β2-agonists and corticosteroids in stable COPDChest1262203715249466
- CazzolaMMateraMGSantangeloG1995Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response studyRespir Med89357627638371
- CazzolaMSantusPDi MarcoF2003Bronchodilator effect of an inhaled combination therapy with salmeterol + fluticasone and formoterol + budesonide in patients with COPDRespir Med97453712735659
- CazzolaMSantusPDi MarcoF2004Onset of action of formoterol/budesonide in single inhaler vs. formoterol in patients with COPDPulm Pharmacol Ther17121515123220
- CelikGKayacanOBederS1999Formoterol and salmeterol in partially reversible chronic obstructive pulmonary disease: a crossover, placebo-controlled comparison of onset and duration of actionRespiration66434910516540
- CheerSMScottLJ2002Formoterol: a review of its use in chronic obstructive pulmonary diseaseAm J Respir Med128530014720051
- DahlRKristufekPGreefhorstAPM2000The cardiac safety profile of formoterol dry powder is similar to placebo in patients with COPD [Abstract]Eur Respir J16Suppl 3151sP497
- DohertyDE2002Early detection and management of COPD. What you can do to reduce the impact of this disabling diseasePostgrad Med11141412082920
- DonaldsonGCSeemungalTARBhowmikA2002Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax578475212324669
- DonnellyRSealJP2001Clinical pharmacokinetics of inhaled budesonideClin Pharmacokinet404274011475468
- EdsbäckerS1999Pharmacological factors that influence the choice of inhaled corticosteroidsDrugs58Suppl 4716
- EdsbäckerSBrattsandR2002Budesonide fatty-acid esterification: a novel mechanism prolonging binding to airway tissue. Review of available dataAnn Allergy Asthma Immunol886091612086369
- FabbriLMHurdSSFor the GOLD Scientific Committee2003Global strategy for the diagnosis, management and prevention of COPDEur Respir J221212882441
- FauldsDHollingsheadLMGoaKL1991Formoterol. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways diseaseDrugs42115371718682
- FentonCKeatingGM2004Inhaled salmeterol/fluticasone propionate: a review of its use in chronic obstructive pulmonary diseaseDrugs6419759615329047
- GlaxoSmithKline2005Advair Diskus 100/50 250/50 500/50 Prescribing Information [online]Accessed 25 August 2005 URL: http://www.gsk.com/products/advair_us.htm
- GizyckiMJHattotuwaKLBarnesN2002Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissueThorax5779980312200525
- GoldsmithDRKeatingGM2004Budesonide–formoterol: a review of its use in asthmaDrugs64159761815233594
- HattotuwaKLGizyckiMJAnsariTW2002The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebocontrolled biopsy studyAm J Respir Crit Care Med1651592612070058
- HvizdosKMJarvisB2000Budesonide inhalation suspension: a review of its use in infants, children and adults with inflammatory respiratory disordersDrugs60138
- JohnellOPauwelsRLöfdahlC-G2002Bone mineral density in patient with chronic obstructive pulmonary disease treated with budesonide Turbuhaler®Eur Respir J1910586312108857
- JohnsonMRennardS2001Alternative mechanisms for long acting β2-adrenergic agonists in COPDChest1202587011451847
- JonesPWQuirkFHBaveystockCM1992A self-complete measure of health status for chronic airflow limitationAm Rev Respir Dis145132171595997
- LallooU2002Symbicort®: controlling asthma in adultsRespir Med96Suppl A1622
- Llewellyn-JonesCGHillSLStockleyRA1994Effect of fluticasone propionate on neutrophil chemotaxis, superoxide generation, and extracellular proteolytic activity in vitroThorax49207128202875
- LomasDAIpMChambaA1991The effect of in vitro and in vivo dexamethasone on human neutrophil functionAgents Actions33279851659155
- McGavinJKGoaKLJarvisB2001Inhaled budesonide–formoterol combinationDrugs6171811217872
- NHLBI/WHO2004Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung DiseaseNational Heart, Lung and Blood Institute Update of the management sections [online]Accessed July 2004 URL: http://www.goldcopd.com
- OsmanLMGoddenDJFriendJARDouglasJG1997Quality of life and hospital re-admission in patients with chronic obstructive pulmonary diseaseThorax5267719039248
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summaryAm J Respir Crit Care Med16312567611316667
- PauwelsRALöfdahlCGLaitinenLA1999Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking: European Respiratory Society Study on Chronic Obstructive Pulmonary DiseaseN Engl J Med34019485310379018
- ReynoldsNAPerryCMKeatingGM2004Budesonide–formoterol in chronic obstructive pulmonary diseaseDrugs644314114969576
- ScolaAMChongLKSuvamaSK2004Desensitization of mast cell beta2-adrenoceptor-mediated responses by salmeterol and formoterolBr J Pharmacol1411637114662724
- SeemungalTARDonaldsonGCPaulEA1998Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1571418229603117
- SinDDJohnsonMQi GanWPaul ManSF2004Combination therapy of inhaled corticosteroids and long-acting β2-adrenergic in management of patients with chronic obstructive lung diseaseCurr Pharm Des1035476015579052
- SzafranskiWCukierARamirezA2003Efficacy and safety of budesonide–formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J21748112570112
- SzeflerSJ1999Pharmacodynamics and pharmacokinetics of budesonide: a new nebulized corticosteroidJ Allergy Clin Immunol104S17583
- ThorssonLEdsbäckerSConradsonT-B1994Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDIEur Respir J71839447828694
- XieQMChenJQShenWH2003Comparison of bronchodilating and antiinflammatory activities of oral formoterol and its (R,R)-enantiomerslActa Pharmacol Sin242778212617779
- YildizFBasyigitIYildirimE2004Does addition of inhaled steroid to combined bronchodilator therapy affect health status in patients with COPD?Respirology9352515363007