Abstract
Patients with COPD may show slow, progressive deteriorations in arterial blood gases during the night, particularly during rapid eye movement (REM) sleep. This is mainly due to hypoventilation, while a deterioration of ventilation/perfusion mismatch plays a minor role. The severity of gas exchanges alterations is proportional to the degree of impairment of diurnal pulmonary function tests, particularly of partial pressure of oxygen (PaO2) and of carbon dioxide (PaCO2) in arterial blood, but correlations between diurnal and nocturnal blood gas levels are rather loose. Subjects with diurnal PaO2 of 60–70 mmHg are distinguished in “desaturators” and “nondesaturators” according to nocturnal oxyhemoglobin saturation behavior. The role of nocturnal hypoxemia as a determinant of alterations in sleep structure observed in COPD is dubious. Effects of the “desaturator” condition on pulmonary hemodynamics, evolution of diurnal blood gases, and life expectancy are also controversial. Conversely, it is generally accepted that occurrence of sleep apneas in COPD is associated with a worse evolution of the disease. Nocturnal polysomnographic monitoring in COPD is usually performed when coexistence of sleep apnea (“overlap syndrome”) is suspected, while in most other cases nocturnal oximetry may be enough. Nocturnal oxygen attenuates sleep desaturations among stable patients, without increases in PaCO2 of clinical concern. Nocturnal treatment with positive pressure ventilators may give benefit to some stable hypercapnic subjects and patients with the overlap syndrome.
Chronic obstructive pulmonary disease (COPD) is usually diagnosed in subjects with a clinical history of exposure to pulmonary risk factors who show a poorly reversible bronchial obstruction (CitationNHLBI/WHO 2005). During wakefulness, abnormalities of respiratory function are demonstrated by standard pulmonary function tests (PFTs): among them, spirometry and arterial blood gas analysis are the most generally used to define the degree of severity of COPD. A more complete evaluation of patients with this disease includes the assessment of sleep gas exchanges, which worsen to a variable extent with respect to wakefulness.
COPD is a high and increasingly prevalent disease. At present, its treatment only partially affects prognosis and quality of life. Our ability to predict prognosis of affected patients is improving (CitationCelli et al 2004), but is still imperfect. A better knowledge of the aspect of respiratory function during sleep could probably improve our approach to the disease.
Features of sleep respiratory disorders in COPD
During sleep, minute ventilation physiologically decreases with respect to wakefulness (CitationDouglas, White, Pickett, et al 1982), while pressure of oxygen in arterial blood (PaO2) falls and pressure of carbon dioxide (PaCO2) increases (CitationMidgren and Hansson 1987). In healthy young subjects, as well as in many patients with respiratory diseases who are normoxemic while awake, the PaO2 reduction does not significantly reduce oxyhemoglobin saturation (SaO2). Instead, when PaO2 is close to, or in, the steep portion of the oxyhemoglobin dissociation curve, SaO2 falls are common.
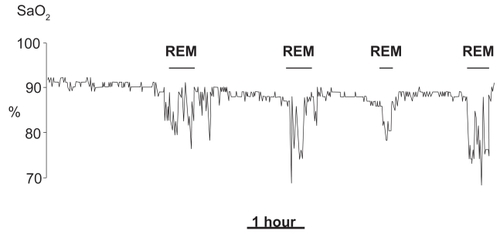
Likewise, in COPD diurnal PaO2 is correlated with nocturnal desaturations. In non-REM sleep, large SaO2 falls are uncommon, unless blood gas tensions during wakefulness are severely impaired. The most common finding is a small SaO2 reduction, while the breathing pattern remains substantially regular. Instead, prolonged oxygen desaturations are often observed throughout rapid eye movement (REM) sleep (CitationDouglas et al 1979; CitationCatterall et al 1983) (). Long duration (often several minutes) and a slow resolution distinguish such desaturations from the typical desaturations of sleep apneas that rarely exceed a minute and are rapidly reverted (CitationBonsignore et al 1990). Prolonged hypoxemic episodes in REM sleep are not specific of COPD, but can also occur in other respiratory diseases (CitationBecker et al 1999) and, to some extent, in healthy elderly subjects (CitationAber et al 1989). Polygraphic signals recorded during such episodes do not always show well-defined alterations of ventilatory activity. They often suggest the occurrence of prolonged hypoventilation (CitationDouglas et al 1979), or of hypopneas with either prevalent obstructive or central characteristics (CitationWhite et al 1995). Irregularities of the breathing pattern, resembling those of normal subjects, often occur during phasic eye movement activity; they are characterized by short changes in depth and frequency of ventilation, and are often enough to cause some oxygen desaturation (CitationGeorge et al 1987).
Figure 1 Recording of nocturnal oxyhemoglobin saturation during one night in a patient with COPD. Observe the prolonged desaturations during REM sleep stages.
Due to difficulties in monitoring, less information is available about PaCO2 changes. Invasive (CitationFletcher et al 1983; CitationCatterall et al 1985), as well as transcutaneous (CitationMidgren and Hansson 1987; CitationAubry et al 1989; CitationO’Donoghue et al 2003), measurements showed a variable increase in PaCO2 during sleep that was proportional to PaCO2 during wakefulness (CitationAubry et al 1989; CitationO’Donoghue et al 2003).
In some patients, COPD coexists with obstructive sleep apnea (OSA). This association is often referred to as “overlap syndrome” (CitationFlenley 1985). Whether prevalence of OSA among patients with COPD is higher than (CitationChaouat et al 1995; CitationLarsson et al 2001), or similar as (CitationSanders et al 2003; CitationBednarek et al 2005), in the general population is controversial. Differences in criteria of subjects’ recruitment may partly explain these contrasting results. In overlap patients, characteristics of sleep-disordered breathing (SDB) may resemble more those of OSA or of COPD, depending on which condition is preponderant.
Predictors of blood gas alterations during sleep
Among COPD patients, SaO2 values during sleep are significantly correlated with diurnal values (CitationFleetham et al 1982; CitationConnaughton et al 1988; CitationVos et al 1995). For similar wake PaO2, desaturations tend to be worse in hypercapnic than in normocapnic subjects (CitationPerez-Padilla et al 1987; CitationBradley et al 1990). This is in agreement with the early observations that desaturations during sleep are worse in “blue bloaters” than in “pink puffers” (CitationDouglas et al 1979; CitationCatterall et al 1983). As long as blood gases during wakefulness deteriorate in the natural course of the disease, nocturnal desaturations will deteriorate as well (CitationPépin et al 1989). Ventilatory responses to hypoxia (CitationTatsumi et al 1986) and hypercapnia (CitationFleetham et al 1980; CitationTatsumi et al 1986; CitationVos et al 1995) during wakefulness are inversely correlated with desaturations during sleep, but it is not clear to what extent such correlations are independent of diurnal blood gas tensions. The importance of mechanical impairment as independent predictor of nocturnal desaturations is controversial. One study found that inspiratory muscles strength was correlated to nocturnal SaO2, although its possible influence was small as compared with that of PaO2 and forced expiratory volume in one second (FEV1) (CitationHeijdra et al 1995). FEV1/forced vital capacity (FVC) was found to be a significant predictor of sleep desaturations among patients with mild COPD (CitationSanders et al 2003). By contrast, other studies showed that any predictivity of FEV1for sleep desaturations disappeared when blood gases were considered (CitationPerez-Padilla et al 1987; CitationBradley et al 1990).
Precise determination of nocturnal SaO2 has a different importance in patients with a different severity of the disease. In patients with normal or almost normal blood gas tensions during wakefulness, occurrence of significant hypoxemia during sleep is unlikely, due to the correlation between diurnal PaO2 and nocturnal SaO2. In patients with severe hypoxemia during wakefulness, SaO2 shows a further reduction during sleep; its precise evaluation could induce to administer a higher oxygen flow in the nocturnal hours. In patients with mildly reduced PaO2 during wakefulness, hypoxemia of some relevance may or may not occur during sleep. Therefore particular attention has been paid to subjects with diurnal PaO2 ranging between60 mmHg and 70 mmHg. Two criteria have been proposed to define such patients as “desaturators”: a) a SaO2 fall below 90% lasting ≥5 minutes, with a nadir level ≤85% (CitationFletcher et al 1987); b) SaO2 values <90% for ≥30% of a whole nocturnal recording (CitationLevi-Valensi et al 1990). Diurnal PaO2 and PaCO2 are worse in desaturators than in nondesaturators, but only nocturnal instrumental monitoring allows us to reliably distinguish the two groups (CitationFletcher et al 1987).
Pathogenesis of hypoxemia during sleep
Physiological modifications of respiratory control and muscular activity occurring during sleep do not result in hypoxemia in most normal subjects. In COPD patients, similar modifications interfere with the respiratory function alterations typical of the disease, and may lead to appearance or worsening of hypoxemia and hypercapnia.
Some deterioration of gas tensions may be already observed before sleep when assuming a recumbent posture, which causes a reduction in functional residual capacity (FRC) and, possibly, an increase in closing volume.
Cough reflex is lost with sleep onset (CitationSullivan et al 1979), which facilitates mucus accumulation in the airway, and enhances ventilation/perfusion mismatch throughout the whole sleep time.
In non-REM sleep the decrease in minute ventilation is not usually responsible for marked modifications in gas tensions, while more significant blood gas derangements occur during REM sleep ().
Figure 2 Mechanisms leading to appearance or worsening of hypoxemia during REM sleep in COPD.
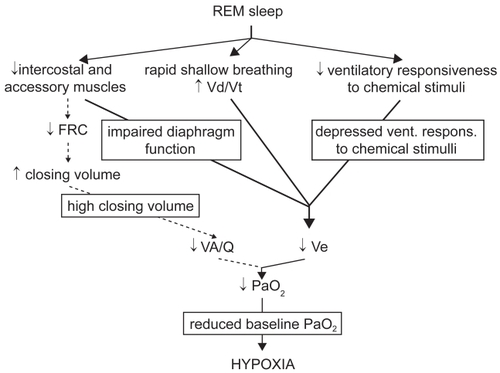
In REM sleep, several factors can contribute to reduce alveolar ventilation. In this sleep state, respiratory activity relies almost exclusively on the diaphragm, while the activity of intercostal (CitationTabachnik et al 1981) and accessory muscles (CitationJohnson and Remmers 1984) ceases. As in COPD the efficiency of diaphragmatic contraction is impaired, minute ventilation markedly decreases (CitationHudgel et al 1983; CitationBallard et al 1995). A decrease in minute ventilation may be favored by an increase in upper airway resistance and by a reduction in neuromuscular output (CitationBallard et al 1995). Ventilatory responsiveness to chemical stimuli physiologically decreases in REM sleep (CitationAnthonisen and Kryger 1982; CitationDouglas, White, Weil, et al 1982), which may be of particular concern in subjects whose responsiveness is already low during wakefulness. Eventually, bursts of rapid shallow breathing frequently occur, mainly in coincidence with eye movements (CitationGeorge et al 1987), and may cause an increase in ventilation of the dead space with a decrease in alveolar ventilation.
Invasive cardiorespiratory measurements during sleep suggested an important contribution of increased ventilation/perfusion mismatch during REM sleep to nocturnal hypoxemia in COPD (CitationFletcher et al 1983); however, the real significance of such measurements was later questioned, as they were performed in non steadystate conditions (CitationCatterall et al 1985). Theoretically, an increased ventilation/perfusion mismatch could follow loss of accessory inspiratory muscles activity (CitationJohnson and Remmers 1984), causing a decrease in FRC and an increase in closing volume. Noninvasive measurements performed by inductive plethysmography suggested that FRC could decrease during at least part of REM sleep (CitationHudgel et al 1983). Later, measurements performed in hyperinflated COPD subjects by means of body plethysmography in association with esophageal pressure ruled out this possibility (CitationBallard et al 1995); this suggests that, in COPD, hyperinflation persists during REM sleep due to loss of elastic lung recoil, despite loss of accessory muscle activity.
In summary, hypoventilation, secondary to the sleep state, is the most important determinant of REM-related hypoxemia in COPD patients. Some contribution of an increased ventilation/perfusion mismatch cannot be ruled out, but has not been clearly demonstrated. Nocturnal hypoxemia worsens along with the functional respiratory impairment, and particularly with deterioration of arterial blood gas tensions. The shape of the hemoglobin dissociation curve from oxygen accounts for the more marked drops in SaO2 among the subjects with diurnal hypoxemia.
Consequences of sleep respiratory disorders
Potential dangers of nocturnal hypoventilation episodes and of the associated hypoxemia in COPD are still uncertain ().
Table 1 Consequences of nocturnal hypoxemia and hypercapnia in COPD
In patients with severe COPD, sleep structure is usually characterized by prevalence of light non-REM sleep stages and frequent arousals (CitationBrezinova et al 1982). Such alterations were not present in patients with mild COPD with no coexistent apneas (CitationSanders et al 2003). Arousals were correlated to oxygenation during sleep in one study (CitationCormick et al 1986), and hypoxemic episodes were suspected to be responsible for sleep alterations. However, despite lower nocturnal SaO2 in “blue bloaters” than in “pink puffers”, sleep quality evaluated by electroencephalogram (EEG) was not better in the latter group (CitationCalverley et al 1982); besides, oxygen administration increased sleep duration in some experiences (CitationGoldstein et al 1984; CitationAubry et al 1989), but proved ineffective at improving sleep structure or duration in other studies (CitationFleetham et al 1982; CitationMcKeon et al 1989; CitationLin 1996). A comparison between effects of oxygen alone, which improved nocturnal SaO2, and oxygen plus ventilatory treatment, which improved both SaO2 and transcutaneous PaCO2, showed that the latter led to a significantly greater improvement in sleep duration (CitationMeecham Jones et al 1995): that supports a previous hypothesis that PaCO2 affects sleep quality more than oxygen tension (CitationFleetham et al 1982). Treatment by lung volume reduction surgery improved at the same time nocturnal oxygenation and sleep structure, but changes in sleep quality were not correlated to those in nocturnal oxygenation (CitationKrachman et al 2005). Subjective reports confirmed that sleep quality is worse in COPD than in normal subjects. According to one study symptoms like cough or wheezing, and not degree of airway obstruction, are correlated to subjective sleep disturbances (CitationKlink et al 1994), while another study pointed out the importance of comorbidities like depression or arthritis (CitationBellia et al 2003).
Hypoxemia during sleep enhances cardiac arrhythmias, at least when it is severe (CitationShepard et al 1985). Oxygen administration reduces nocturnal arrhythmias (CitationTirlapur and Mir 1982). It is unknown if such arrhythmias may influence life expectancy.
Hemodynamic monitoring during sleep in COPD showed that pulmonary arterial pressure (PAP) increases during hypoxemic episodes (CitationCoccagna et al 1978; CitationBoysen et al 1979; CitationFletcher et al 1984), and that oxygen administration prevents at the same time desaturations and PAP augmentations (CitationBoysen et al 1979; CitationFletcher et al 1984). Whether or not desaturations occurring only during sleep in COPD are followed by a stable deterioration of pulmonary hemodynamics is still debated. CitationFletcher and colleagues (1989), who adopted the first definition for “desaturator”, reported above, observed higher PAP values among desaturators than nondesaturators, and a better evolution of diurnal PAP among desaturators receiving, than among those not receiving, nocturnal oxygen (CitationFletcher et al 1992). When the second definition was used, cross-sectional studies showed either higher (CitationLevi Valensi et al 1992) or similar (CitationChaouat et al 1997) PAP values among desaturators, while later longitudinal investigations demonstrated no influence of the “desaturator” condition (CitationChaouat et al 2001) and of oxygen administration (CitationChaouat et al 1999) on PAP. CitationRasche and colleagues (2001) reported that PAP during wakefulness was significantly correlated with lowest nocturnal SaO2, but not with time spent below 90% SaO2.
It has been hypothesized that nocturnal hypoventilation may influence wake arterial blood gases. In subjects with mild diurnal hypoxemia, CitationChaouat and colleagues (2001) did not observe any trend toward a worse evolution of wake arterial blood gas tensions among desaturators than nondesaturators. By contrast, in subjects with chronic hypercapnic respiratory failure, prevention of nocturnal hypoventilation by ventilatory treatment was followed by an improvement in diurnal blood gases (CitationElliott et al 1992; CitationMeecham Jones et al 1995; CitationSivasothy et al 1998). The relatively mild nocturnal hypoxemia in the patients of the first study, as opposed to the marked desaturations recorded in the others, could account for these apparently contrasting results.
The most important consequence of nocturnal hypoventilation could be a reduced life expectancy. Early observations on COPD patients hospitalized for exacerbation pointed out that death during hospital stay occurred preferentially in the nocturnal hours, suggesting that hypoxemia during sleep could immediately influence survival (CitationMcNicholas and Fitzgerald 1984). A later multicentre study on patients on long-term oxygen therapy, evaluated retrospectively, pointed out that nocturnal death does not often occur during sleep (CitationZielinski et al 1997). Besides, in other studies nocturnal hypoxemia did not show any independent role on the evolution of the disease (CitationConnaughton et al 1988; CitationChaouat et al 2001).
Consequences of nocturnal SDB are much worse and clearer in the overlap syndrome. COPD and OSA, when associated, are of particular concern: they have a synergistic effect, often leading to impairment of diurnal arterial blood gases and to stable pulmonary hypertension that would not be warranted by the degree of severity of each of the diseases taken alone (CitationBradley et al 1986; CitationChaouat et al 1995).
Monitoring of nocturnal respiratory function
Nocturnal monitoring in COPD is performed to look for appearance of SDB, or to quantify worsening of blood gas exchanges during sleep and optimize treatment.
Polysomnography is a complex procedure consisting of simultaneous recording of sleep and cardiorespiratory parameters, and is usually performed throughout the night. It can detect disorders of ventilation and hypoxemic episodes, and allows us to temporally correlate them with sleep stages. Due to its high cost, its use must be limited. Theoretically, polysomnography is the most accurate method to explore nocturnal respiratory function, as it allows us to recognize possible underestimations of the usual degree of hypoxemia due to occasional lack of REM sleep during nocturnal monitoring. However, it has been shown that oxygen desaturations, as explored during polysomnography, are less than resulting from simple oximetry monitoring, due to poor tolerability of the polysomnographic equipment, and to a shorter sleep during polysomnographic than oximetry monitoring (CitationBrijker et al 2001). Today, in patients with COPD polysomnography is mainly performed when the coexistence of OSA is suspected, due to symptoms like heavy snoring and excessive daytime sleepiness, or to fatigue, pulmonary hypertension or polycythemia out of proportion to the diurnal PFTs alterations (CitationKushida et al 2005).
Oximetry is inexpensive, and easy to perform and to tolerate. Modern instruments can be applied for prolonged ambulatory monitoring, keeping in memory consecutive diurnal and nocturnal data. Severity of hypoxemia and need of oxygen treatment may appear quite different when SaO2 values recorded during diurnal rest and activity, as well as during the night, are taken into account at the same time, than when just instantaneous PaO2 at rest and SaO2 during exercise are considered (CitationSliwinski et al 1994; CitationPilling and Cutaia 1999; CitationFussell et al 2003). As regards SaO2 monitoring during sleep, today simple oximetry tends to be preferred over polysomnography in most COPD patients. Mean and lowest nocturnal levels, and time spent below 90% or 85% values are SaO2 measurements most commonly performed. Mean SaO2 is quite reproducible. Time spent below 90% is probably the most generally considered SaO2 parameter, as it is used to distinguish patients with mild diurnal hypoxia into desaturators and nondesaturators (CitationLevi-Valensi et al 1990); however, it is highly variable between nights, suggesting that it may be appropriate to record oximetry for multiple, rather than for a single night (CitationLewis 2003).
Treatment of sleep respiratory disorders
In COPD exacerbations, a worsening in gas exchanges during sleep must be expected; mechanical ventilation is required (CitationLightowler et al 2003), but there are no guidelines taking into account nocturnal blood gas exchanges for such treatment in this condition.
So far, most studies on respiration during sleep in COPD have regarded patients in a clinically stable state. In such patients, treatment of nocturnal hypoxia may consist of drugs, oxygen, or mechanical ventilation.
Little importance is usually attributed to drug treatment for hypoxemia during sleep in COPD. Among substances with bronchodilating effects, theophylline caused an improvement in hypoxemia, but sometimes at the expense of a worsening in sleep quality (CitationMulloy and McNicholas 1993); more recently, thiotropium bromide has been shown to determine some increase in nocturnal SaO2 without interfering with sleep quality (CitationMcNicholas et al 2004).
Oxygen and noninvasive ventilatory treatment correct more effectively nocturnal desaturations.
In stable hypoxic patients, oxygen is usually administered during part of the day and throughout the nocturnal hours (CitationNOTT 1980; CitationMRC 1981; CitationNHLBI/WHO 2005). In clinically stable hypercapnic COPD patients without sleep apnea for whom long-term oxygen therapy (LTOT) was indicated, nocturnal oxygen administration at a flow rate sufficient to ensure a diurnal SaO2 ≥90% prevented oxyhemoglobin desaturations, and caused a larger increase in transcutaneous PaCO2 than breathing room air (CitationGoldstein et al 1984; CitationAubry et al 1989), not of clinical concern (CitationGoldstein et al 1984). However, sleep desaturations are not always adequately corrected by oxygen administered at that flow rate, especially in the most hypercapnic subjects (CitationPlywaczewski et al 2000). What increase in oxygen flow is necessary in the nocturnal hours in patients on LTOT to prevent oxygen desaturation is not clear. It has been empirically proposed to increase oxygen flow at night by 1 liter/minute (CitationATS 1995), although an observation on a small number of patients suggested that a 0.5 liter/minute increase could be sufficient (CitationServera et al 1994). Nocturnal monitoring can be of help for the prescription of the most appropriate nocturnal oxygen flow.
In patients for whom LTOT is not indicated on the basis of the diurnal PaO2 level, advantages of nocturnal oxygen administration are still uncertain. The contrasting results about the effects on pulmonary hemodynamics of oxygen administered during the night for two or three years in desaturators have been mentioned above (CitationFletcher et al 1992; CitationChaouat et al 1999). One study on a small number of subjects suggested that desaturators’ survival could benefit from long-term nocturnal oxygen, although results were not statistically significant (CitationFletcher et al 1992). However, a later study on a similar group of subjects could not find any effect of nocturnal oxygen on either survival or deterioration in diurnal blood gases and delay in requirement of LTOT (CitationChaouat et al 1999).
Noninvasive nocturnal ventilatory treatment in stable COPD patients may be taken into account for some subjects with a severe form of the disease. Despite in these subjects hypoxemia is present during the whole day, in several cases mechanical ventilation may be applied only during the night, so as to correct the worst desaturations of the 24 hours, and not to interfere with daily activities. Treatment is performed by positive pressure ventilators, more often by means of pressure support or by bilevel devices. If appropriately set, during their application SaO2 and PaCO2 levels can improve at the same time (CitationElliott et al 1992; CitationMeecham-Jones et al 1995). In some subjects, nocturnal application of mechanical ventilation may be of benefit also to diurnal blood gases after a few months (CitationElliott et al 1992; CitationMeecham Jones et al 1995; CitationPerrin et al 1997; CitationJones et al 1998; CitationSivasothy et al 1998; CitationClini et al 2002). Mechanisms of such improvement are not entirely clear. It is unlikely that inspiratory muscle rest has some responsibility. Some improvement in chemosensitivity to CO2 and in pulmonary mechanics, possibly due to reduced interstitial edema, could play a role (CitationElliott et al 1991). On the average, compliance to, and benefits of, ventilatory treatment in COPD are less than in restrictive disorders (CitationLeger et al 1994; CitationSimonds and Elliott 1995). COPD patients to treat by mechanical ventilation must be carefully selected, must undergo a monitoring that demonstrates the efficacy of the ventilator setting in correcting nocturnal hypoventilation, and have to be adequately instructed and adapted to the ventilator use. Failure to fulfil some of these conditions may at least partly account for reports of unsuccessful trials with ventilatory treatment (CitationStrumpf et al 1991; CitationGay et al 1996; CitationLin 1996; CitationCasanova et al 2000). It has been proposed to prescribe long-term ventilatory treatment in association with oxygen administration to stable COPD subjects with symptoms suggestive of nocturnal hypoventilation (morning headache, apparently unwarranted fatigue or dyspnea) in one of the following conditions: a) a PaCO2 ≥55 mmHg; b) a PaCO2 ≥50 mmHg associated with recurrent hypercapnic respiratory failure; or c) a PaCO2 ≥50 mmHg associated with nocturnal SaO2 values ≤88% for at least 5 consecutive minutes despite oxygen administration at 2 liters/minute (CitationCC 1999). In addition to effects on gas exchanges, ventilatory treatment may be associated with an improvement in quality of life (CitationMeecham-Jones et al 1995; CitationPerrin 1997; CitationSivasothy et al 1998; CitationClini et al 2002) and with a more prolonged nocturnal sleep, observed at polysomnography after a few months of treatment (CitationElliott et al 1992; CitationMeecham-Jones et al 1995). An effect on survival and on number of exacerbations with an up to three-year treatment has not been demonstrated (CitationSivasothy et al 1998; CitationCasanova et al 2000).
Indications and outcomes of mechanical ventilation are different in patients with the overlap syndrome. Due to the deleterious effects of the association of OSA to COPD, previously mentioned (CitationBradley et al 1986; CitationChaouat et al 1995), treatment by nocturnal ventilation must be performed in most of these patients. Continuous positive airway pressure (CPAP) is effective to eliminate obstructive apneas, but is not always enough to correct hypoxemia during sleep. Nocturnal monitoring can help us to decide if CPAP must be replaced by other kinds of ventilations (usually bilevel positive pressure ventilation), and if it is necessary to associate oxygen administration with ventilatory treatment.
Conclusions
Important questions remain unanswered several decades after studies on respiratory function in COPD began. In particular, the real danger represented by nocturnal oxygen desaturations independently of diurnal blood gas levels, and the need to specifically address their treatment are still unclear. Most uncertainty comes from the correlation between diurnal and nocturnal blood gas levels that makes it difficult to identify the separate effects of wake and sleep hypoxemia. To separately investigate the effects of sleep hypoxemia, many studies were done on patients with hypoxemia occurring only in the nocturnal hours: however, such patients often show sleep SaO2 values only slightly below 90%, and for only part of the night. Negative results coming from such studies, rather than demonstrating lack of importance of nocturnal hypoxemia, could just indicate that moderate hypoxemia persisting only for a small portion of the 24 hours is of little concern. Severe nocturnal hypoxemia in patients with more advanced disease could be more dangerous, but its independent role is difficult to demonstrate, as it is almost always associated with a degree of hypoxemia during wakefulness whose deleterious effects are already well known. As reported above, some studies on the effects of nocturnal ventilatory treatment in hypercapnic patients suggested that correction of nocturnal blood gases derangements is of benefit, but positive results need to be confirmed. So far, all trials on treatment of nocturnal hypoxemia, either by oxygen or by mechanical ventilation, have been based on small numbers of individuals. Current indications on treatment of nocturnal hypoxemia (CitationATS 1995; CitationCC 1999) are based on a limited knowledge, and it is not surprising that the attitude of physicians towards oxygen prescription for nocturnal hypoxemia remains variable (CitationWijkstra et al 2001). Long-term studies on large cohorts could help to give an answer to the still poorly known implications of SDB in COPD.
References
- AberWRBlockAJHellardDW1989Consistency of respiratory measurements from night to night during the sleep of elderly menChest96747512791668
- AnthonisenNRKrygerMH1982Ventilatory and arousal responses to hypoxemia in sleepAm Rev Respir Dis126127091894
- [ATS] American Thoracic Society1995Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis152S77120
- AubryPRoseDArlatisS1989Bronchopneumopaties chroniques obstructives. Variations de la capnie au cours du sommeil en air ambiant et sous oxygènePresse Med1866152524037
- BallardRDCloverCWSuhBY1995Influence of sleep on respiratory function in emphysemaAm J Respir Crit Care Med151945517697271
- BeckerHFPiperAJFlynnWE1999Breathing during sleep in patients with nocturnal desaturationAm J Respir Crit Care Med15911289872827
- BednarekMPlywaczewskiRJonczakJ2005There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population studyRespiration72142915824523
- BelliaVCatalanoFScichiloneN2003Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA studySleep263182312749552
- BonsignoreGMarroneOMacalusoC1990Validation of oximetry as a screening test for obstructive sleep apnoea syndromeEur Respir J11542s4s
- BoysenPGBlockAJWynneJW1979Nocturnal pulmonary hypertension in patients with chronic obstructive pulmonary diseaseChest7653642498826
- BradleyTDMateikaJLiD1990Daytime hypercapnia in the development of nocturnal hypoxemia in COPDChest97308122298055
- BradleyTDRutherfordRLueF1986Role of diffuse airway obstruction in the hypercapnia of obstructive sleep apneaAm Rev Respir Dis13492043777688
- BrezinovaVCatterallJRDouglasNJ1982Night sleep of patients with chronic ventilatory failure and age matched controls: number and duration of the EEG episodes of intervening wakefulness and drowsinessSleep5123307100743
- BrijkerFvan den ElshoutFJHeijdraYF2001Underestimation of nocturnal hypoxemia due to monitoring conditions in patients with COPDChest1191820611399710
- BrochardLManceboJWysockiM1995Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary diseaseN Engl J Med333817227651472
- CalverleyPMBrezinovaVDouglasNJ1982The effect of oxygenation on sleep quality in chronic bronchitis and emphysemaAm Rev Respir Dis126206107103244
- CasanovaCCelliBRTostL2000Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPDChest11815829011115443
- CatterallJCalverleyPMMcNeeW1985Mechanism of transient nocturnal hypoxemia in hypoxic chronic bronchitis and emphysemaJ Appl Physiol5916987034077777
- CatterallJRDouglasNJCalverleyPM1983Transient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndromeAm Rev Respir Dis1282496870065
- [CC] Consensus Conference Report1999Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilationChest1165213410453883
- CelliBRCoteCGMarinJM2004The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med35010051214999112
- ChaouatAWeitzenblumEKesslerR1997Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemiaEur Respir J10173059272911
- ChaouatAWeitzenblumEKesslerR1999A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patientsEur Respir J141002810596681
- ChaouatAWeitzenblumEKesslerR2001Outcome of COPD patients with mild daytime hypoxaemia with or without sleep-related oxygen desaturationEur Respir J178485511488315
- ChaouatAWeitzenblumEKriegerJ1995Association of chronic obstructive pulmonary disease and sleep apnea syndromeAm J Respir Crit Care Med1518267812577
- CliniESturaniCRossiA2002The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patientsEur Respir J205293812358325
- CoccagnaGLugaresiE1978Arterial blood gases and pulmonary and systemic arterial pressure during sleep in chronic obstructive pulmonary diseaseSleep111724227027
- ConnaughtonJJCatterallJREltonRA1988Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease?Am Rev Respir Dis13834143195833
- CormickWOlsonLJHensleyMJ1986Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung diseaseThorax41846543824271
- DouglasNCalverleyPMLeggetRJ1979Transient hypoxaemia during sleep in chronic bronchitis and emphysemaLanceti1483461
- DouglasNJWhiteDPPickettCK1982Respiration during sleep in normal manThorax3784047164002
- DouglasNJWhiteDPWeilJV1982Hypercapnic ventilatory response in sleeping adultsAm Rev Respir Dis126758627149440
- DunroyHMAdamsLCorfieldDR2003CO2 retention in lung disease; could there be a pre-existing difference in respiratory physiologyRespir Physiol Neurobiol1361798612853009
- ElliottMWMulveyDAMoxhamJ1991Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensionsEur Respir J41044521756837
- ElliottMWSimondsAKCarrollMP1992Domiciliary nocturnal nasal intermittent positive pressure ventilation in hypercapnic respiratory failure due to chronic obstructive lung disease: effects on sleep and quality of lifeThorax4734281609376
- FleethamJWestPMezonB1982Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. The effect of oxygen therapyAm Rev Respir Dis126429337125332
- FleethamJAMezonBWestP1980Chemical control of ventilation and sleep arterial oxygen desaturation in patients with COPDAm Rev Respir Dis12258397436124
- FlenleyDC1985Sleep in chronic obstructive lung diseaseClin Chest Med6651612935359
- FletcherECGrayBALevinDC1983Nonapneic mechanisms of arterial oxygen desaturation during rapid-eye-movement sleepJ Appl Physiol5463296841208
- FletcherECLevinDC1984Cardiopulmonary hemodynamics during sleep in subjects with chronic obstructive pulmonary disease. The effect of short- and long-term oxygenChest856146690253
- FletcherECLuckettRAGoodnight-WhiteS1992A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mmHgAm Rev Respir Dis145107061586049
- FletcherECLuckettRAMillerT1989Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleepChest95757642924605
- FletcherECMillerJDivineGW1987Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mm HgChest9260483652746
- FussellKMAyoDSBrancaP2003Assessing need for long-term oxygen therapy: a comparison of conventional evaluation and measures of ambulatory oximetry monitoringRespir Care48115912556251
- GayPCHubmayrRDStroetzRW1996Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trialMayo Clin Proc71533428642881
- GeorgeCFWestPKrygerMH1987Oxygenation and breathing pattern during phasic and tonic REM in patients with chronic obstructive pulmonary diseaseSleep10234433629085
- GoldsteinRSRamcharanVBowesG1984Effect of supplemental nocturnal oxygen on gas exchange in patients with severe obstructive lung diseaseN Engl J Med31042596420700
- HeijdraYFDekhuijzenPNVosPJ1995Nocturnal saturation and respiratory muscle function in patients with chronic obstructive pulmonary diseaseThorax50610127638800
- HudgelDMartinRJCapehartM1983Contribution of hypoventilation to sleep oxygen desaturation in chronic obstructive pulmonary diseaseJ Appl Physiol55669776629905
- HudgelDWMartinRJCapehartM1983Contribution of hypoventilation to sleep oxygen desaturation in chronic obstructive pulmonary diseaseJ Appl Physiol55669776629905
- JohnsonMWRemmersJE1984Accessory muscle activity during sleep in chronic obstructive pulmonary diseaseJ Appl Physiol571011176501021
- JonesSEPackhamSHebdenM1998Domiciliary nocturnal intermittent positive pressure ventilation in patients with respiratory failure due to severe COPD: long-term follow up and effect on survivalThorax5349589713450
- KlinkMEDodgeRQuanSF1994The relation of sleep complaints to respiratory symptoms in a general populationChest10515148275723
- KrachmanSLChatilaWMartinUJ2005Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysemaChest1283221816304265
- KushidaCALittnerMRMorgenhalerT2005Practice parameters for the indications for polysomnography and related procedures: An Update for 2005Sleep2849952116171294
- LarssonLGLindbergAFranklinKA2001Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studiesRespiration68250511416244
- LegerPBedicamJMCornetteA1994Nasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiencyChest10510058275718
- Levi-ValensiPAubryPRidaZ1990Nocturnal hypoxemia and long-term oxygen therapy in COPD patients with daytime PaO2 60–70 mmHgLung168Suppl77052117190
- Levi-ValensiPWeitzenblumERidaZ1992Sleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patientsEur Respir J530171572442
- LewisCAEatonTEFergussonW2003Home overnight pulse oximetry in patients with COPD: more than one recording may be neededChest12311273312684303
- LightowlerJVWedzichaJAElliottMW2003Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysisBMJ32618512543832
- LinCC1996Comparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPDAm J Respir Crit Care Med15435388756806
- McKeonJLMurree-AllenKSaundersNA1989Supplemental oxygen and quality of sleep in patients with chronic obstructive lung diseaseThorax4418482650011
- McNicholasWTCalverleyPMLeeATiotropium Sleep Study in COPD Investigators2004Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturationEur Respir J238253115218993
- McNicholasWTFitzgeraldMX1984Nocturnal deaths among patients with chronic bronchitis and emphysemaBMJ2898786434121
- Meecham JonesDJPaulEAJonesPW1995Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPDAm J Respir Crit Care Med152538447633704
- MidgrenBHanssonL1987Changes in transcutaneous PCO2 with sleep in normal subjects and in patients with chronic respiratory diseasesEur J Respir Dis71388943127230
- [MRC] Medical Research Council Working Party report1981Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysemaLanceti6816
- MulloyEMcNicholasWT1993Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary diseaseAm Rev Respir Dis148103068214921
- [NHLBI/WHO] National Heart, Lung, and Blood Institute and World Health Organization2005Global initiative for chronic obstructive lung diseasePocket Guide to COPD Diagnosis, Management, and Prevention 2005 update [online]Accessed on 14 March 2006 URL: http://www.goldcopd.com
- [NOTT] Nocturnal Oxygen Therapy Trial group1980Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trialAnn Intern Med933913986776858
- O’DonoghueFJCatchesidePGEllisEE2003Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factorsEur Respir J219778412797491
- PépinJLLévyPLepaulleB1989Evolution des désaturations nocturnes de 35 patients BPCO. Relation avec les données fonctionnelles et hémodynamiquesRev Mal Respir6357642508199
- Perez-PadillaRConwayWRothT1987Hypercapnia and sleep O2 desaturation in chronic obstructive pulmonary diseaseSleep10216233629083
- PerrinCEl FarYVandenbosF1997Domiciliary nasal intermittent positive pressure ventilation in severe COPD: effects on lung function and quality of lifeEur Respir J10283599493670
- PillingJCutaiaM1999Ambulatory oximetry monitoring in patients with severe COPD: a preliminary studyChest1163142110453857
- PlywaczewskiRSliwinskiPNowinskiA2000Incidence of nocturnal desaturation while breathing oxygen in COPD patients undergoing long-term oxygen therapyChest117679310712991
- RascheKDuchnaHWOrthM2001Der Einfluss verschiedener Hypoxamiedefinitionen auf die Beziehung zwischen Pulmonalisdruck im Wachzustand und Hypoxamie im Schlaf bei COPDPneumologie552899411458436
- SandersMHNewmanABHaggertyCL2003Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway diseaseAm J Respir Crit Care Med16771412502472
- ServeraEMarin PardoJFernandezE1994Long-term oxygen therapy. Is it necessary to increase the nocturnal flow by 1 liter?Chest10613117924534
- ShepardJWJrGarrisonMWGritherDA1985Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary diseaseAm J Med7828342578248
- SimondsAKElliottMW1995Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disordersThorax5060497638799
- SivasothyPSmithIEShneersonJM1998Mask intermittent positive pressure ventilation in chronic hypercapnic respiratory failure due to chronic obstructive pulmonary diseaseEur Respir J1134409543267
- SliwinskiPLagoszMGoreckaD1994The adequacy of oxygenation in COPD patients undergoing long-term oxygen therapy assessed by pulse oximetry at homeEur Respir J727488162980
- StrumpfDAMillmanRPCarlisleCC1991Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary diseaseAm Rev Respir Dis144123491741532
- SullivanCEKozarLFMurphyE1979Arousal, ventilatory, and airway responses to bronchopulmonary stimulation in sleeping dogsJ Appl Physiol471725224019
- TabachnikEMullerNLBryanC1981Changes in ventilation and chest wall mechanics during sleep in normal adolescentsJ Appl Physiol51557647327955
- TatsumiKKimuraHKunitomoF1986Sleep arterial oxygen desaturation and chemical control of breathing during wakefulness in COPDChest9068733720388
- TirlapurVGMirMA1982Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways diseaseN Engl J Med306125307054654
- VosPJFolgeringHTvan HeerwardenCL1995Predictors for nocturnal hypoxaemia (mean SaO2 < 90%) in normoxic and mildly hypoxic patients with COPDEur Respir J87477744197
- WhiteJEDrinnanMJSmithsonAJ1995Respiratory muscle activity during rapid eye movement (REM) sleep in patients with chronic obstructive pulmonary diseaseThorax50376827785010
- WijkstraPJGuyattGHAmbrosinoN2001International approaches to the prescription of long-term oxygen therapyEur Respir J189091311829095
- ZielinskiJMacNeeWWedzichaJ1997Causes of death in patients with COPD and chronic respiratory failureMonaldi Arch Chest Dis524379151520