 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by poorly reversible airflow limitation. The pathological hallmarks of COPD are inflammation of the peripheral airways and destruction of lung parenchyma or emphysema. The functional consequences of these abnormalities are expiratory airflow limitation and dynamic hyperinflation, which then increase the elastic load of the respiratory system and decrease the performance of the respiratory muscles. These pathophysiologic features contribute significantly to the development of dyspnea, exercise intolerance and ventilatory failure. Several treatments may palliate flow limitation, including interventions that modify the respiratory pattern (deeper, slower) such as pursed lip breathing, exercise training, oxygen, and some drugs. Other therapies are aimed at its amelioration, such as bronchodilators, lung volume reduction surgery or breathing mixtures of helium and oxygen. Finally some interventions, such as inspiratory pressure support, alleviate the threshold load associated to flow limitation. The degree of flow limitation can be assessed by certain spirometry indexes, such as vital capacity and inspiratory capacity, or by other more complexes indexes such as residual volume/total lung capacity or functional residual capacity/total lung capacity. Two of the best methods to measure flow limitation are to superimpose a flow–volume loop of a tidal breath within a maximum flow–volume curve, or to use negative expiratory pressure technique. Likely this method is more accurate and can be used during spontaneous breathing. A definitive definition of dynamic hyperinflation is lacking in the literature, but serial measurements of inspiratory capacity during exercise will document the trend of end-expiratory lung volume and allow establishing relationships with other measurements such as dyspnea, respiratory pattern, exercise tolerance, and gas exchange.
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a disease state characterized by poorly reversible airflow limitation and loss of pulmonary capillary bed. It is usually progressive and associated with abnormal inflammatory responses in the lung (CitationPauwels et al 2001). Chronic inflammation is a predominant feature of COPD and involves the airways (CitationSaetta et al 2001), lung parenchyma (CitationSaetta et al 1999), and pulmonary vasculature (CitationPeinado et al 1999). It is caused by exposure to inhaled noxious particles and gases present in tobacco smoke (CitationVon Essen et al 1995; CitationSalvi et al 1999) and likely in other air pollutants that may be inhaled during breathing. Macrophages, T lymphocytes (predominately CD8+), and neutrophils are increased numerically as well as activated (CitationPesci et al 1998), this results in the release of a variety of mediators (CitationKeatings et al 1996; CitationMueller et al 1996; CitationYamamoto et al 1997; CitationBeeh et al 2003) that are believed to be capable of unbalance the protease-antiprotease equilibrium (CitationGottlieb et al 1996; CitationStockley et al 1999) and damage lung structures (CitationLiu et al 1999; CitationShapiro and Senior 1999).
The pathological hallmarks of COPD are inflammation of the peripheral airways and destruction of lung parenchyma or emphysema (CitationThurlbeck 1991). The functional consequence of these abnormalities is expiratory airflow limitation. Since the determinants of expiratory flow through the airways are both the driving alveolar pressure that promotes flow (elastic recoil of the lung) and the opposing resistance of the airways, the reduction in flow occurring in COPD is defined more accurately as airflow limitation rather than airflow obstruction, since both loss of elastic recoil and increase in airway resistance play an important role (CitationPride and Green 1997). Emphysema will contribute to the airflow limitation by reducing the elastic recoil of the lung through parenchymal destruction, as well as by reducing the elastic load applied to the airways through destruction of alveolar attachments. On the other hand, inflammation of the peripheral airways will contribute to the airflow limitation by increasing the thickness of the airway wall which, together with fibrosis and smooth muscle hypertrophy, may cause airway narrowing (CitationThurlbeck 1985). The role of mucus hypersecretion in the development of chronic airflow limitation is still controversial (CitationPeto et al 1983; CitationVestbo et al 1996).
Expiratory flow limitation and dynamic hyperinflation (DH) are clinical and pathologic concepts that have been present for well over 100 years. Recent developments have revitalized the interest on this crucial psychopathological consequence of obstructive disease. In accordance with this renewed interest, a provocative hypothesis has been put forward recently that proposes that the transition from peripheral airways disease to COPD follows three pathophysiological stages defined by the severity of expiratory flow limitation: In Stage I, closing volume eventually exceeds the functional residual capacity (FRC); in Stage II tidal volume expiratory flow limitation (EFL) develops; and in Stage III, DH increases to a point that produces dyspnea and exercise limitation. The presence of airway closure (Stage I) and EFL (Stage II) may promote peripheral airway injury and accelerate the abnormalities of lung function (CitationMilic-Emili 2004).
Pathophysiology of dynamic hyperinflation
Nonmuscular factors
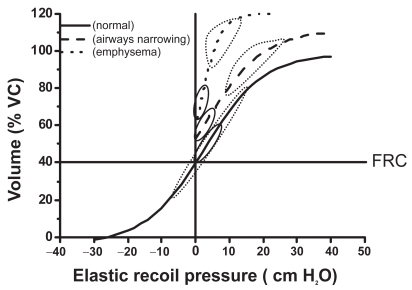
The respiratory system (ie, the combined elastic recoil of the lung and chest wall) is an elastic structure able to change its shape and volume in order to breathe the necessary air in, and the alveolar gas out, to sustain the amount of gas exchange needed to match metabolic needs. Under normal physiological conditions the respiratory muscles provide the power to produce such changes in volume. For a given change in pleural pressure generated by the respiratory muscles, the attainable end-inspiratory and end-expiratory volumes are determined by the passive pressure–volume (P–V) relationship of the respiratory system () (CitationMead et al 1967; CitationAgostoni and Hyatt 1986). The P–V behavior of the respiratory system is distinctly sigmoid () since the respiratory system is most compliant between 20% to 80% of the vital capacity (VC) (CitationMead et al 1967; CitationAgostoni and Hyatt 1986). To state this in a clearer way, the elastic work of breathing is minimized by maintaining tidal volume within the 20%–80% of the VC range. In COPD there are different forms and degrees of damage to the alveolar wall resulting in pathophysiological changes in the lung elastic recoil. These changes might reduce the amount of pressure required to achieve a specific change in volume (CitationCherniack et al 1963; CitationSaksena and Burrows 1966), or simply shift the normal relationship upwards near passive FRC () (CitationSharp et al 1968). There is limited information about passive elastic recoil characteristics of the lung and chest wall during exercise, but at least in healthy subjects, they remain essentially unchanged, or decrease slightly (CitationStubbing et al 1980; CitationYounes and Kivinen 1984). Therefore total lung capacity (TLC) does not change significantly during physical activity either in normal subjects or in COPD patients (CitationStubbing et al 1980).
Figure 1 Pressure volume relationship of the passive respiratory system. Lower and upper boundaries of the elastic recoil pressure–volume relationship of the respiratory system in healthy subjects (---), in patients with narrowed airways (—), and in patients with loss of lung elastic recoil (·····). The loops represent tidal breathing at rest (—) and during exercise (·····).
While the P–V relationship determines the attainable volume extremes for a given muscular pressure, the actual volume change in a given amount of inspiratory or expiratory time depends on the pressure–flow (P–F) relationship, which is highly dependent on the airways resistance (in a linear system, the volume at the time tx [Vtx] is given by the expression
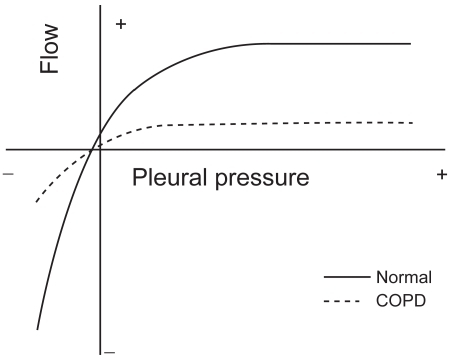
where P is the pressure applied, C the compliance of the system, tx the time elapsed, and R the resistance. The product RC is the time constant, which determines the speed of the change), as can be seen in . During inspiration, even when inspiratory resistances are high, flow down the airways can be increased by increasing the force of inspiratory muscle contraction up to its maximum capacity (CitationYounes and Riddle 1981). The same is true during expiration at relative low pressures, but during forced expiration in both normal and COPD, increments in alveolar pressure (generated by increasing muscle effort) produce progressively smaller increments in expiratory flow, until flow ultimately reaches a plateau () where it is independent of any increase of driving pressure (CitationHyatt and Wilcox 1963). This phenomenon is called flow limitation and it seems to be related to the dynamic compression of the intra-thoracic airways at a segment progressively distal as lung volume decreases (CitationGreen and Pride 1997). The resulting increase in airways resistance offsets the pressure generated by the additional increase in muscular effort, and therefore expiratory flow is actually largely independent of muscle effort and is determined by the respiratory system elastic recoil. Elastic recoil of the system is proportional to lung volume and the expiratory airways conductance (ie, the inverse of resistance) (CitationHyatt 1983). Respiratory mechanics in COPD are characterized by both elevated inspiratory and expiratory resistance to airflow (CitationMcGregor and Becklake 1961; CitationCitterio 1981; CitationAldrich et al 1989). An increased inspiratory airflow resistance can be compensated by augmenting the activation of the inspiratory muscles (CitationIm Hof et al 1986), but the increased expiratory resistance, together with the reduced lung elastic recoil present in these patients, further limits expiratory flow (CitationHyatt 1961). This is physiologically much more deleterious because expiration is primarily effort independent (), and cannot be compensated by increasing expiratory muscle effort (CitationPoon et al 1987). The consequence in COPD is that the time needed for lung units to empty their volume and achieve their passive equilibrium point is significantly increased and many of them do not reach their relaxation volume before a new inspiration is initiated. As a result, part of the gas that would have been expired in a normal lung remains “trapped” (ie, more gas remains inside that what would be if those units were not altered) and the alveolar pressure at the end of the expiration is higher than the atmospheric pressure (intrinsic PEEP) (CitationPride and Macklem 1986; CitationYounes 1991). In young healthy subjects RV is about 25% of the TLC, and FRC is about 50% of the TLC (CitationStocks and Quanjer 1995). In older healthy subjects, RV and FRC are about 30% and 55% of TLC respectively (CitationStocks and Quanjer 1995). In COPD, values of RV and FRC are increased to values as high as 70% and 85% of the TLC, respectively (CitationFishman et al 2003). Since tidal breathing is performed at FRC, COPD must breathe at volumes that are very close to TLC where the system is much less distensible ().
Figure 2 Effects of time and resistance on the change in expiratory volume. Rates of changes of volume after a similar given change of alveolar pressure in a subject with normal respiratory system resistance (—) and a resistance 3 times greater (---) such as in COPD, assuming a constant compliance. Note the marked effect of resistance on the volume change, particularly when the available time is shortened.
Abbreviations: COPD, chronic obstructive pulmonary disease.
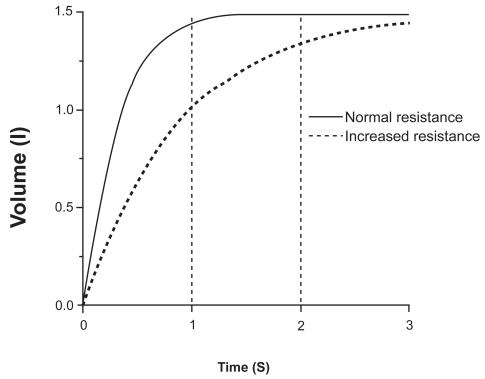
Figure 3 Iso-volume pressure-flow relationship. Schematic representation of the pressure-flow relationship in a healthy (normal) subject and a patient with COPD, showing the effect of the increased expiratory resistance upon the maximum expiratory flow and in both cases the independence of the maximum flow from the pleural pressure. Copyright © 1986. Modified with permission from CitationPride NB, Macklem PT. 1986. Lung mechanics in disease. In: Fishman AP (ed). Handbook of physiology, Section 3, Volume III, Part 2: The respiratory system. Bethesda MD: American Physiological Society, pp 659–92.
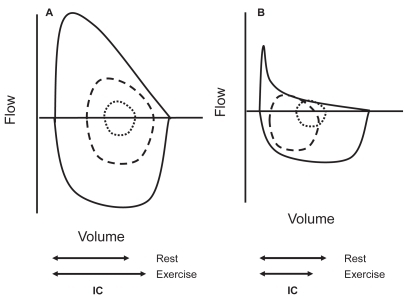
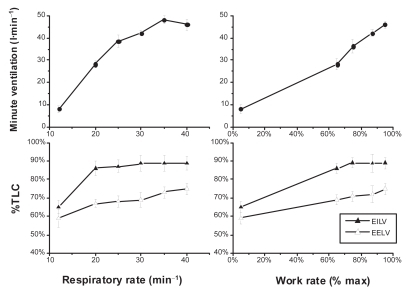
During exercise minute ventilation must increase to meet the increased metabolic demands. This is achieved by increasing both the tidal volume (VT) and the respiratory frequency (fR), what means that the expiratory time necessary to reach passive FRC or below is reduced. In normal young subjects expiratory flow is fast enough to decrease end-expiratory lung volume (EELV) up to 0.5–1L below the resting FRC with increasing work. This is due to the activation of expiratory muscles (CitationPride and Macklem 1986; CitationHenke et al 1988). The decreased EELV not only allows increasing VT within the most compliant part of the P–V relationship, but also has a beneficial inspiratory effect because, as the expiratory muscles relax, the passive tendency of the respiratory system below FRC is towards inspiration. In older subjects, EELV also decreases during moderate exercise, but thereafter, tends to increase back to near resting levels (CitationJohnson et al 1991). In COPD, due to the increased flow limitation, more and more units are unable to empty their gas as expiratory time decreases when the respiratory rate increases, and EELV typically rises ( and ) (CitationHyatt 1961; CitationGrimby et al 1968; CitationPotter et al 1971; CitationDodd et al 1984; CitationPride and Macklem 1986; CitationBabb et al 1991; CitationYounes 1991; CitationYan et al 1997; CitationDiaz et al 2000; O’Donell et al 2001; CitationOga et al 2002, Citation2003; CitationO’Donnell and Webb 2003; CitationPuente-Maestu et al 2005) in spite of expiratory muscles activity (CitationDodd et al 1984; CitationYounes 1991). This temporary increase in EELV in COPD above its baseline (admittedly already elevated) value is termed DH. The extent of DH depends on the degree of expiratory flow limitation, the prevailing ventilation, the breathing pattern for a given ventilation, and is inversely related to the level of resting lung hyperinflation (CitationO’Donnell et al 2001; CitationPuente-Maestu et al 2005).
Figure 4 Maximum and tidal flow-volume curves in subjects with and without flow limitation. In this figure it can be seen an schematic representation of the spontaneous flow-volume curves generated at tidal volume at rest (inner dotted line ····) and peak exercise (dashed line ----) compared with the maximum flow volume curve in a subject without flow limitation able to reduce its end-expiratory lung volume (Panel A) and a flow-limited COPD patient with dynamic hyperinflation (Panel B).
Muscle function
To move the air into the alveoli, sufficient force must be exerted by the respiratory muscles to expand the lungs and the chest wall. In addition, respiratory muscles must overcome the resistance and inertia in the system so that air will flow into the airways. This force is provided by the respiratory muscles and, thus, breathing is the results of their cyclic activation. Whether the net force is inspiratory or expiratory will depend on the balance between the pressure generated by the muscles and the elastic recoil of the respiratory system. The maximum inspiratory and expiratory pressures that the respiratory muscles are able to generate are related to several factors such as age (CitationBlack and Hyatt 1969), muscle training (CitationLeith and Bradley 1976), the integrity of the inervation (CitationGross et al 1980) and, more important for the present discussion, of the length and shape (usually termed as “configuration”) of the respiratory muscles, and particularly of the diaphragm (Marshall 1962). According to the Laplace’s relationship (the Laplace relationship establishes the relationship between the pressure generated across a curved surface “P”, the tension of the surface “T” and its radius “r”: P=T/r), the less curved a surface is, the lower the pressure difference created across it. Thus at high lung volumes, a given neural output would be likely to cause less change in trans-diaphragmatic pressure than at low lung volumes. Furthermore, inspiratory muscles are able to generate less pressure for a given neural input because of their length–tension relationship (CitationKim et al 1976; CitationSimilowski et al 1991; CitationPolkey et al 1998). The ability of the respiratory muscles to sustain a given level of pressure output (endurance) is defined by its pressure time index (PTI) (CitationMcGregor and Becklake 1961; CitationBellee and Grassino 1982, Citation1983)
Where “Pimus” is the inspiratory muscular pressure needed to achieve a certain displacement of the respiratory system, “MIP” is the maximum inspiratory pressure, “Ti” the inspiratory time and “Ttot” the total respiratory time. Thus the PTI is determined to a considerable degree by how high MIP is, and the fraction of MIP required to sustaining a given VT. MIP decreases as lung volume increases, while maximum expiratory pressure changes in the opposite direction (CitationAgostoni and Hyatt 1986), therefore at high volumes a greater fraction of maximum effort is required to generate the same pressure. Paradoxically, thus, while flow limitation is primarily an expiratory phenomenon, its consequences are mainly suffered by the inspiratory muscles because expiratory muscles cannot appreciably increase flow () (CitationHyatt 1961, Citation1983; CitationHyatt and Wilcox 1963; CitationPride and Macklem 1986; CitationGreen and Pride 1997) to force the emptying of the lungs. In consequence the volume at which the respiratory system has to operate is increased (CitationHyatt 1961; CitationGrimby et al 1968; CitationPotter et al 1971; CitationDodd et al 1984; CitationPride and Macklem 1986; CitationBabb et al 1991; CitationYounes 1991; CitationYan et al 1997; CitationDiaz et al 2000; CitationO’Donnell et al 2001; CitationOga et al 2002, Citation2003; CitationO’Donnell and Webb 2003; CitationPuente-Maestu et al 2005) and hence the elastic work needed for a given displacement of the thorax ( and ) (CitationCherniack et al 1963; CitationSaksena and Burrows 1966; CitationMead et al 1967; CitationSharp et al 1968; CitationYounes and Kivinen 1984; CitationAgostoni and Hyatt 1986). Second inspiratory muscles have to generate a substantial inspiratory pressure (intrinsic PEEP) before inspiratory airflow can occur. PEEP behaves actually as an inspiratory threshold load (CitationPepe and Marini 1982; CitationPride and Macklem 1986; CitationHaluszka et al 1990; CitationYounes 1991), and finally, as we have seen above, at high lung volume efficacy of respiratory muscles as pressure generators is greatly reduced (Marshall 1962; CitationKim et al 1976; CitationSimilowski et al 1991; CitationPolkey et al 1998).
Clinical consequences of dynamic hyperinflation
Exercise limitation
Patients with COPD characteristically show a poor exercise performance which is manifested as marked reduction in peak oxygen uptake and decreased endurance to submaximal levels of exercise () (CitationGallagher 1994; CitationCasaburi et al 1999; CitationO’Donnell and Webb 2003). The origin of this poor exercise tolerance is multifactorial and includes abnormal lung mechanics, impaired pulmonary gas exchange, destruction of the pulmonary vascular bed, impaired cardiac function and peripheral muscle dysfunction (CitationGallagher 1994; CitationCasaburi et al 1999; CitationO’Donnell and Webb 2003). Dynamic hyperinflation has a detrimental impact on exercise tolerance via three important physiopathological mechanisms. The relatively rapid shallow breathing pattern in COPD compared with healthy subjects, reflects the mechanical constraints on tidal volume expansion, which has an exaggerated frequency-dependency in COPD, fR increases with exercise in COPD up to a maximum of about 25–35min−1 (CitationDiaz et al 2000; CitationNield et al 2003; CitationPuente-Maestu et al 2005). At increased respiratory rates, the inversely proportional decrease in expiratory time, even though there is a slight reduction of Ti/Ttot to 0.40–0.45 (CitationDiaz et al 2000; CitationPuente-Maestu et al 2005), and as result of the decreased expiratory time further DH ensues. When EELV reaches approximately 0.5L tidal volume, ventilation reaches a plateau (or even a slight decreases), exercise soon stops () (CitationO’Donnell et al 2001; CitationPuente-Maestu et al 2005). This encroachment of tidal volume not only hampers the ventilatory response to the metabolic load of exercise, it contributes to reducing the efficiency of ventilation (ie, increases the dead space) as well (CitationGallagher 1994). This effect, together with the augmented mechanical impedance of the respiratory system results in an increased work and O2 cost of breathing at any given metabolic load compared with age-matched healthy controls, (CitationRoussos et al 1982; CitationDonahoe et al 1989; CitationShindoh et al 1994; CitationMannix et al 1999; CitationTakayama et al 2003). In one study this additional oxygen cost has been found to be as much as 40% of the total oxygen uptake (CitationLevison and Cherniack 1968).
Table 1 Main clinical consequences of dynamic hyperinflation
Figure 5 Tidal volume encroachment by dynamic hyperinflation in COPD. In this figure the effects of respiratory rate and work rate on the end-inspiratory (EILV), end-expiratory lung volume (EELV), tidal volume (ie, EILV–EELV) and minute ventilation in subjects with severe COPD is displayed. While EILV increases with increasing respiratory rate from 20 to 30min−1 (lower left panel), so does EELV resulting in an almost constant (encroached) tidal volume. At respiratory rates higher than 30, though, EILV does not increase any more, however, EELV further increases resulting in a reduced tidal volume and even a drop in ventilation (left upper panel) at respiratory rates higher than 35min−1. In the right lower panel we can see how during progressive exercise tidal volume also decreases at high intensity due again to increase in EELV without parallel increase in EILV (Constructed with data from CitationPuente-Maestu et al 2005).
Dyspnea
Dyspnea is believed to be the unpleasant awareness of the respiratory muscle effort. As it was discussed above DH both increases the pressure changes needed to achieve a given tidal volume and decreases the ability of the respiratory muscles to generate this pressure (Marshall 1962; CitationKim et al 1976; CitationSimilowski et al 1991; CitationPolkey et al 1998). This then increases the amount of pressure needed to achieve a certain tidal volume with respect to the maximum pressure (CitationKillian et al 1992). The change in lung mechanics during exacerbation of COPD has been show to be directly related to dyspnea during spontaneously breathing. The improvement in operating lung volumes (ie, increase in inspiratory capacity [IC]) as the exacerbation resolves is correlated to symptomatic improvement and reductions in dyspnea (CitationParker et al 2005; CitationStevenson et al 2005). While COPD patients do not usually stop exercise at higher rates of perceived dyspnea than normal subjects, the ratio of symptoms to the metabolic load and minute ventilation are usually increased (CitationKillian and Jones 1994; CitationHamilton et al 1996). In these patients there exists a close correlation between breathlessness during exercise and DH (CitationO’Donnell et al 1993; CitationEltayara et al 1996; CitationPuente-Maestu et al 2005; CitationVogiatzis et al 2005). In a recent study, arm exercise in COPD has been found to produce DH as well (CitationGigliotti et al 2005).
Hypoventilation/hypercapnia
The increase in inspiratory and expiratory airway resistance characteristic of COPD would cause only minor problems in ventilatory function (ie, for the respiratory muscles) if the disease, through expiratory flow limitation did not cause dynamic hyperventilation, and its corollaries that include increased elastic recoil, encroached tidal volume, the threshold load imposed by intrinsic PEEP, and the changes in the configuration of the thorax that greatly reduce their ability to generate pressure. When COPD patients with expiratory flow-limitation need to increase their respiratory rate to increase minute ventilation, there is potential for inspiratory muscle failure due to DH (). During exacerbations, the inflammatory process, the ventilation/perfusion (V′A/Q′) mismatching, increased airflow resistance, and DH, expose the respiratory muscles to the risk of fatigue, eventually leading to ventilatory pump failure and hypercapnia (CitationRossi et al 1997). DH is one of the major mechanisms involved in the development of hypercapnia and (secondarily hypoxemia) during exercise in COPD patients (CitationDiaz et al 2000; CitationO’Donnell et al 2002).
Cardiac dysfunction
Lung hyperinflation and excessive expiratory muscle recruitment can impair venous return and reduce right ventricular preload in COPD (CitationMahler et al 1984; CitationMiller, Pegelow, et al 2005). Moreover, large intrathoracic pressure swings generated during exercise to overcome the increased elastic and resistive loads may result in left ventricular dysfunction (increased left ventricular afterload) (CitationOswald-Mammosser et al 1991; CitationMontes de Oca et al 1996). Right ventricular afterload during exercise is also increased because of the increased pulmonary vascular resistance associated with breathing at lung volumes close to TLC (CitationRanieri et al 1996; CitationOswald-Mammosser et al 1998).
Weaning failure
The incidence of EFL was very high in a small sample of patients with COPD receiving invasive mechanical ventilation for acute respiratory failure. In this series IC was lower in patients who failed weaning than those who were successfully weaned (CitationAlvisi et al 2003).
Hypotension and barotrauma in the mechanical ventilated patient
Dynamic hyperinflation is a potential cause of hypotension and barotrauma in mechanically ventilated patients with EFLs (). DH should be minimized by the use of bronchodilators and appropriately setting minute ventilation (higher tidal volumes, slower respiratory rate, higher I:E ratio) to maximize expiratory time (CitationSchumaker et al 2004).
Exercise training
Most rehabilitation programs are based on constant-load exercise (CLE) training, consisting of sustained exercise for 30–40min (CitationACCP/AACVPR 1997). Generally, high-intensity training is argued to be needed for the improvement of exercise capacity (CitationCasaburi et al 2001). Although patients with moderately severe COPD (mean forced expiratory volume in one second [FEV1] >45% predicted) can tolerate high levels (80%) of their peak tolerance for several minutes (CitationNeder et al 2000), more severe disease (FEV1 <45% predicted) are unable to tolerate such high exercise intensities for long periods of time (CitationMaltais et al 1997). Together with peripheral muscle dysfunction, the major factor that limit exercise tolerance in these patients is the development of DH, and the concurrent mechanical constraints on ventilation that contribute importantly to perceived respiratory discomfort (CitationDiaz et al 2000; CitationPuente-Maestu et al 2005). Dynamic hyperventilation is evident even with short exercise bouts in interval training (CitationVogiatzis et al 2004). In one study, a correlation was found between resting hyperinflation (measured as RV/TLC%) and the increase in endurance time after 8 weeks of leg muscle rehabilitation (CitationPuente-Maestu et al 2003). In another study, IC was found as a significant predictor of the long term effects after a rehabilitation program, but in a multiple logistic regression model, only pressure of carbon dioxide (PaCO2) was identified as predictor for the maintenance of improvement in health-related quality of life over one year (CitationNishiyama et al 2005).
Survival
Certain variables closely linked to DH (CitationO’Donnell et al 1993, Citation2001, Citation2002; CitationDiaz et al 2000; CitationMarin et al 2001) such as exercise capacity (maximal oxygen uptake) (CitationGerardi et al 1996; CitationBowen et al 2000; CitationMyers et al 2002; CitationHiraga et al 2003; CitationOga et al 2003) or 6 minute walking distance (CitationPinto-Plata et al 2004), dyspnea (CitationCelli et al 2004), and oxygen desaturation (CitationNishimura et al 2002; CitationHiraga et al 2003; CitationTojo et al 2005) during exercise have been show to be powerful predictors of survival in COPD patients (). A recent study of 689 COPD patients, with a mean follow up of 34 month, showed that the IC/TLC was an independent risk factor for mortality in subjects with COPD (CitationCasanova et al 2005). Using the criterion of IC/TLC <25% with a mean follow up period of 34 months, the adjusted hazard ratio for death of any cause was 1.97 and 2.04 for death caused by acute respiratory failure (CitationCasanova et al 2005). In another study RV/TLC was also a prognostic factor for survival at 5 years but not independent of age, FEV1, and arterial oxygen pressure at maximal exercise (CitationTojo et al 2005).
Therapeutic interventions directed to ameliorate dynamic hyperinflation
The treatment of flow limitation and DH has a long history that goes back as far as the 19th century, but the recent recognition of the importance of DH has modified the target physiological variables that we use to evaluate the therapeutic interventions, such as bronchodilators or rehabilitation, and has led to a renewed interest in specific treatments such as the lung volume reduction surgery (LVRS). In this section we will discuss those therapies grouped according their target physiological variable.
Treatments that primarily decrease respiratory rate and increase VT
Pursed-lip breathing
Pursed-lip breathing (PLB) (CitationBarach et al 1995) is aimed to reduce breathing frequency and to diminish the impact of intrinsic PEEP on respiratory muscles. PLB produces a substantial increase in VT along with a reduced ventilatory rate and minute ventilation (CitationThoman et al 1966; CitationMueller et al 1970; CitationBianchi et al 2004). There is a direct relationship between the efficacy of PLB in reducing dyspnea and the effect on respiratory rate (CitationMueller et al 1970). This technique is able to increase expiratory airways pressure thus inhibiting expiratory airways collapse (CitationIngram et al 1967). It appears that patients that do not adopt PLB instinctively did not assume it naturally for long periods of time even when properly taught (CitationTiep et al 1986). In a recent study imposed PLB did not improved 6 minute-walking distance, but improved the rate of dyspnea recovery to basal levels (CitationGarrod et al 2005). In another study, PLB reduced respiratory rate in patients with COPD during exercise on a cycle-ergometer, but the effect of imposed PLB on dyspnea were variable and related to the change that it promoted in the tidal volume and EELV (CitationSpahija et al 2005).
Peripheral muscle training
A number of studies have shown that leg training decreases respiratory rate during exercise (Cassaburi et al 1997; CitationPuente-Maestu et al 2000; CitationVogiatzis et al 2002; CitationGigliotti et al 2003; CitationRuiz de Oña et al 2004), and three previous studies have directly analyzed the impact of leg training on DH at high intensity exercise. In two uncontrolled studies, a reduction of respiratory rate and an increase in IC was seen (CitationGigliotti et al 2003; CitationPorszasz et al 2005). In another controlled study, the same effect was seen after 8 weeks of leg training on a bike compared with no training in moderate to severe COPD (CitationPuente-Maestu et al 2006). In another study the opposite was observed (CitationPellegrino 1999). However, this latter study included eight quite unusual COPD subjects (ie, 38 [11] years, FEV1 3.5 (0.5) L and V O2 2.86 (0.6) L min−1) that can not be regarded as a typical population of COPD patients. Arm training also reduced DH during arm exercise in a small uncontrolled study of COPD subjects (CitationGigliotti et al 2005).
Oxygen
Several observations in nonhypoxemic COPD patients demonstrate the favorable effect of oxygen supplementation during exercise in COPD patients without clinically significant hypoxemia (CitationWoodcock, Gross, Geddes, et al 1981; CitationDean et al 1992; CitationO’Donnell, Bain, et al 1997). Endurance time increased with supplemental oxygen at several different inhaled concentrations of oxygen both in a small group (10) of severe mildly hypoxemic COPD patients and 7 healthy controls. In both groups oxygen reduced fR, dyspnea, and increased VT, and endurance time in a dose-dependent fashion, but the effects were relatively larger in the COPD patients. In the healthy subjects, differences were only appreciable with FIO2 > 0.5 or more. The improvement in endurance time was correlated with a decrease in EELV (CitationSomfay et al 2001). In a group of 18 severe COPD patients, oxygen reduced the degree of DH during recovery from exercise but did not reduce breathlessness compared with air, which suggests that lung mechanics may play a different role in the genesis of dyspnea during recovery from exercise (CitationStevenson et al 2004).
Opioids
Opioids have been show to decrease dyspnea during exercise (CitationWoodcock, Gross, Gellert, et al 1981; CitationLight et al 1989), but to our knowledge no study has addressed their effect on DH. One study reported a decrease in fR for a given work-rate, and increases in VT and ventilation were found (CitationLight et al 1996). The reported side-effects of opioids and their tendency to lead to tolerance and addiction preclude their use except in highly specific cases and end-of-life situations. Benzodiazepines do not seem to improve exercise capacity or dyspnea (CitationHaas et al 1993) and have a deleterious effect on inspiratory muscle function, reducing both VT and minute ventilation (CitationJolly et al 1996).
Treatments that primarily reduce flow limitation
One of the most exciting aspects of the renewed interest in DH is the realization that bronchodilator drugs may significantly improve DH, and thus exercise tolerance (CitationBelman et al 1996; CitationO’Donnell et al 1998), and that these changes may not be detectable by isolated resting pulmonary function test (PFT) measurements like the FEV1 (CitationHadcroft and Calverley 2001).
Bronchodilators
Several studies have addressed the effect of bronchodilators on lung hyperinflation (). In 13 COPD patients randomly assigned to receive either inhaled placebo or salbutamol, the bronchodilator caused significant increase in both FEV1 and forced vital capacity (FVC). There was also a significant reduction in the peak exercise EELV/TLC and esophageal inspiratory pressure/peak inspiratory esophageal pressure. Moreover a significant reduction in breathlessness that correlated with changes in end-inspiratory lung volume/TLC was also seen (CitationBelman et al 1996). In a retrospective review of 281 patients with TLC >133% predicted and 676 with TLC between 115% to 133% predicted, 200μg of salbutamol produced a significant reduction of FRC, RV, and increased the IC and FVC. The FEV1 improved in only a minority (around 30%). If lung volume measurements are also considered, the overall bronchodilator response may improve up to 76% of the severely hyperinflated group and up to 62% of the moderately hyperinflated ones. Changes in volumes correlated poorly with changes in maximal airflows. Surprisingly TLC was also slightly reduced (about 2.5%) by bronchodilators suggesting that the changes in airways resistance or the familiarity with the method may have affected the constant volume plethysmographic technique used to measure volumes (CitationNewton et al 2002). Unless salbutamol had produced changes in the ability to generate force by the respiratory muscles, or the elastic properties of the lung, both of which it are unlikely. CitationRamirez-Venegas and colleagues (1997) found that the use of salmeterol reduced not only dyspnea but improved lung function in patients with COPD. Patients showed an increase in FVC, a reduction in RV and FRC, and no changes in TLC following inhalation of salmeterol. CitationBoni and colleagues (2002) studied 20 COPD, 11 with flow limitation using the negative expiratory pressure before and after the inhalation of 400μg salbutamol. Following salbutamol, IC did not change in non-flow limited patients but increased significantly in the flow-limited ones. Dyspnea decreased in relation to IC at rest even in the absence of a significant improvement in FEV1. Di Marco and colleagues (1993) found that in patients with decreased baseline inspiratory capacities; there was a much greater increase of IC after administration of bronchodilators such as salbutamol, formoterol, salmeterol, and oxitropium. This increase correlated closely with improvement in a sensation of dyspnea. CitationCelli and colleagues (2003) evaluated the long-term effects (4 weeks) of 18 μg/day of tiotropium. Significant similar improvements in the area under the curve of FEV1 and IC and over the curve in FRC were observed, reflecting sustained improvements in hyperinflation. CitationO’Donnell, Fluge, and colleagues (2004) and CitationMaltais and colleagues (2005) conducted a multicentric study on the long-term effects (6 weeks) of 18μg/day of tiotropium. Tiotropium reduced lung hyperinflation indices at rest and during exercise, and improved exercise tolerance 8 h after the inhalation of the drug. Finally CitationO’Donnell, Voduc, and colleagues (2004) studied the effects of 50μg of inhaled salmeterol. After salmeterol versus placebo at rest, IC increased and FRC decreased at a standardized time during exercise. In addition, IC and VT increased and dyspnea decreased. Significant increments in peak ventilation, oxygen uptake, and the duration of the incremental test were also seen. Man and colleagues (2004) studied 16 patients with “irreversible” severe COPD after two weeks of treatment with 50μg of salmeterol twice a day. Salmeterol significantly reduced the transdiaphragmatic pressure-time product, DH, and Borg scores during endurance treadmill walk, however there was no significant change in exercise endurance time.
Table 2 Summary of trials on BD measuring resting or dynamic hyperinflations an outcome
In contrast with the results of , bronchodilators have shown wide variations in regards to changes in exercise capacity (CitationLiesker et al 2002). Earlier negative studies may have included subjects whose exercise capacity was not ventilatory-limited. In fact, several studies in demonstrate that the effects of bronchodilators are larger in those with flow limitation than in those without flow limitation. Flow limitations are a major determinant of exercise capacity (CitationDiaz et al 2000). Alternatively, they may have included a great proportion of patients that were not responsive to bronchodilators (about 1/3 of COPD patients do not improve either FEV1 or IC with bronchodilators) (CitationNewton et al 2002). Third, some of those studies may have used insufficient doses and only a few included long-acting bronchodilators, and in particular, none included tiotropium (CitationLiesker et al 2002). Fourth, most studies included 6 minute walking tests to measure exercise capacity; however, 6 minute walking distance is sensitive to a learning effect. Two training sessions are necessary to eliminate a learning effect in walking tests (CitationKervio et al 2003; Knox et al 1988). Constant work load tests appears to be more appropriate than other tests, as they allow for placebo-controlled comparisons of symptoms and physiological parameters, including exercise endurance time at a standardized work rate or power output (CitationO’Donnell, Fluge, et al 2004). Cycle exercise endurance testing, combined with measurements of exertional dyspnea, ventilation, and dynamic operating lung volumes, has been shown to be reliable, reproducible, and responsive to the intervention (CitationOga et al 2000; CitationPuente-Maestu et al 2003; CitationO’Donnell, Fluge, et al 2004).
Heliox
Helium is gas that is less dense and more viscous than air. Its lower density can decrease airway resistance in the absence of any anatomical change. Therefore, the greatest theoretical benefit of heliox would be achieved by decreasing turbulent flow in large airways and at branch points in the tracheobronchial tree.
Heliox is unlikely to be of substantial benefit in adults with asthma. A systematic analysis pooled results from seven trials enrolling nearly 400 patients and found no significant improvement in recovery of pulmonary function in patients with acute asthma who were treated with heliox (CitationRodrigo et al 2001, Citation2003). One study examined the effects of heliox (80:20) on gas exchange, breathing pattern, respiratory mechanics, and gas distribution in patients with stable, but severe, COPD (CitationSwidwa et al 1985). Heliox was not associated with significant changes in minute ventilation or breathing pattern, however a 15% reduction in FRC which increased back to baseline after a return to air breathing was observed. On the other hand another small trial found no change in DH among COPD patients (CitationPecchiari et al 2004). In addition, the high cost and limited availability of heliox make its clinical use more difficult. In another study, researchers used an esophageal balloon in recently extubated patients without significant lung disease to quantify intrathoracic pressure swings and estimate the work of breathing (CitationJaber et al 2001). Fifteen of 18 patients exhibited a drop in their work of breathing, although gas exchange parameters were unchanged. The patients also reported decreased dyspnea while breathing heliox.
The clinical effects of heliox in the absence of positive pressure ventilation have not been well studied. Fifty normoxic COPD with an acute exacerbations were prospectively randomized in the emergency room to receive either heliox (70:30), or air as the driving gas for updraft nebulization of a mixture of salbutamol and ipratropium bromide The base-line FEV1 was 44% There were no significant differences in the change of FEV1 between the two groups by either the 1 h or 2 h time point. Lung volume measurements were not made, but the improvement in forced expiratory flow over the middle half of the FVC (FEF25%–75%) was significantly greater in the heliox group, the authors considered that this improvement had no clinical significance, but measures of dyspnea or recovery were not provided (CitationdeBoisblanc et al 2000). A retrospective review of 81 patients presenting to the emergency department with COPD and hypercarbic respiratory insufficiency found a significant reduction in the rates of intubation (8% vs 50%) and in-hospital mortality (3% vs 24%) in the patients who received heliox as compared with control patients (CitationGerbeaux et al 2001).
Several trials have compared noninvasive positive pressure ventilation (NPPV) combined with either air or heliox in patients with exacerbations of COPD. In one well-designed trial, patients with acute exacerbations of COPD were treated with low or high pressure NPPV in combination with heliox or oxygen-supplemented air (CitationJaber et al 2000). Differences in breathing pattern, work of breathing, and gas exchange were measured in nine patients using an esophageal balloon technique. Heliox was not associated with changes in breathing pattern, whereas high pressure NPPV produced significant increases in tidal volume and minute ventilation. Heliox was associated with a reduction in PaCO2 and improvement in all measured indices of respiratory effort and work. The beneficial effects on the measured variables were further increased when high pressure NPPV was combined with heliox, compared with the low pressure/air group. The authors suggest that the addition of heliox to NPPV may allow a larger number of patients to benefit from NPPV. In addition, heliox may allow the use of lower levels of pressure support, which could reduce complications and patient discomfort resulting from high pressures and flow rates (CitationJaber et al 2000). A crossover study monitored the effects of NPPV plus either heliox (70:30) or oxygen (FiO2 0.30) on gas exchange and dyspnea in 19 patients with severe COPD (CitationJolliet et al 1999). The use of heliox decreased PaCO2, reduced dyspnea, and favorably changed the breathing pattern of patients. Peak inspiratory flow rates were higher, while inspiratory time and the ratio of inspiratory time to respiratory cycle length were both decreased. All of these findings suggest a reduced work of breathing. A third series of 23 intubated patients with COPD and respiratory failure found that the administration of heliox significantly reduced intrinsic PEEP, trapped lung volume, and peak and mean airway pressures (CitationTassaux et al 2000). Similar findings were noted in a second small study of 12 patients recovering from acute exacerbations of COPD. In this randomized prospective crossover trial, heliox decreased the resistive work of breathing and intrinsic positive end-expiratory pressure (auto-PEEP) without changing the breathing pattern (CitationDiehl et al 2003).
One study examined the impact of heliox on pulmonary function in ten aging runners with very mild COPD during cardiopulmonary exercise testing. Heliox was associated with an increase in minute ventilation, in the absence of any change in the metabolic cost of breathing (CitationBabb et al 2003). Another work studied 12 patients with severe COPD (FEV1 38[10]% of predicted) comparing heliox (79:21) with air. Exercise endurance increased significantly and peak ventilation time with heliox. This was associated with a significant reduction in lung DH and dyspnea at isotime. The reduction in dyspnea correlated significantly with the increase in IC induced by heliox (CitationPalange et al 2004).
Increasing elastic recoil
Although there are no currently available treatments to return the lung to its normal structure once it has been damaged by the inflammatory process (like COPD), this may indeed be possible in the future using stem cells (CitationOrtiz et al 2003; CitationSuratt et al 2003). Stem cells may indeed improve the compliance characteristics of the lung. However, at this time lung volume-reduction surgery (LVRS; a surgical or bronchoscopic procedure that involves resection or exclusion of the most severely affected regions of diseased lung tissue) in patients with diffuse emphysema, may improve lung function by increasing elastic recoil of the lung, the effective pressure driving maximal expiratory flow airway, and the conductance of the airways by augmenting the radial traction of their alveolar attachments. Hence it may improve flow proportionately at all areas of the lung and secondarily reduce hyperinflation (CitationBrantigan et al 1959; CitationRogers et al 1968; CitationFessler and Permutt 1998; CitationIngenito et al 2001). This surgery was initially described in the late 1950s (CitationBrantigan et al 1959). The persistent air leaks that limited early success have been diminished considerably by the recent surgical advances, which has increased interest in this procedure and led to its greater availability (CitationWakabayashi et al 1991; CitationCooper et al 1995; CitationKeenan et al 1996; CitationHazelrigg et al 1997; CitationBrenner et al 2004).
Several randomized trials have compared LVRS with optimal medical treatment (CitationGeddes et al 2000; CitationFishman et al 2003; CitationMiller, Berger, et al 2005) and by far the largest of these was the National Emphysema Treatment Trial (NETT) (CitationFishman et al 2003). NETT enrolled over 1200 patients with severe emphysema who underwent baseline assessment followed by six months of mandatory pulmonary rehabilitation. The patients were then randomly assigned to surgical or continued medical therapy, with plans for three-year follow-up. In this study high risk patients were defined as having: FEV1 < 20% and either diffusing capacity of the lung for carbon monoxide (DLCO) < 20% predicted or homogeneous changes on chest computer tomography (). The 30-day mortality was 16% in the surgical group compared with no deaths in the medically managed group (CitationNETT 2001). In the remainder of the study, while there was a significant improvement in exercise capacity in the surgical group (the same has been found in another study [CitationDolmage et al 2004]), there was no difference in the total mortality rate at the end of follow-up with surgical or medical therapy (9% vs 10% per year with medical therapy), however, the 90-day mortality was significantly higher with surgery (5.2% vs 1.5%) (CitationFishman et al 2003). Only the subset of patients with upper lobe predominant emphysema and low exercise capacity (24%) had a significant reduction in total mortality with surgery (7% vs 15% per year, risk ratio 0.47). In this study, low exercise capacity was defined as less than 40% of the gender–specific predicted work rate (40 W in men and 25 W in women) (CitationFishman et al 2003).
Table 3 Subjects likely to benefit for lung volume surgery
Recently LVRS has been attempted by bronchoscopically placing one-way valves in airways associated with areas of severe emphysema and hyperexpansion. These valves allow air and secretions to move from alveoli to the central airways, but prevent air from entering the distal airspaces. Over time, this results in collapse of the affected regions. Experience in humans is limited, but one series of 19 patients who underwent unilateral valve placement noted decreased air-trapping and improvement in exercise tolerance one month after the procedure (CitationHopkinson et al 2005).
LVRS produces less functional improvement than lung transplant (CitationGaissert et al 1996), but since patients who undergo LVRS are not automatically excluded from receiving a subsequent lung transplant both treatments might be sequentially applied (CitationNathan et al 2004).
Large bullae which can potentially benefit from surgical resection are uncommon clinically. An important challenge for the clinician is to select the patient for bullectomy who can have the greatest benefit with the lowest morbidity and mortality. The most common indication for bullectomy is severe dyspnea in the setting of a large bulla occupying at least 30%–50% of the hemithorax. Another indication is history of a pneumothorax. The physiologic outcome after surgery is determined largely by the size of the bulla and the severity of the underlying emphysema, and patients with severe generalized emphysema tend to do poorly (CitationNickoladze 1992). The surgical risk is increased when the FEV1 is less than 40% of predicted, and the presence of severe dyspnea, a markedly reduced FEV1, hypercapnia, or cor pulmonale makes the risk of surgery almost prohibitive (CitationGunstensen and McCormack 1973).
Relieving the inspiratory threshold load
Pursed-lip breathing
We have reviewed the effects of PLB above. In addition to reducing respiratory rate, it likely reduces dynamic airways compression by generating an extratoracic resistance and thus creates PEEP.
Positive end-expiratory pressure
Intrinsic PEEP is frequent in mechanically ventilated patients with obstructive airways disease. Several studies indicate that in patients with flow limitation by dynamic airway collapse, the application of small amounts of external PEEP can be beneficial since intrinsic PEEP can account for about one-third of the total work of breathing (CitationCoussa et al 1993). Hyperinflation may not be affected when applied PEEP is below 85% of the measured auto-PEEP (CitationRanieri et al 1993), however not all patients with auto-PEEP and DH have expiratory flow limitation (CitationArmaganidis et al 2000) and care has to be taken to reduce the impact of a narrow diameter or kinked endotracheal tube, inspisated secretions, exhalation/PEEP valves, and asynchrony due to pain or agitation. The incidence of expiratory flow limitation has been reported to be as high as 93% upon initiation of mechanical ventilation, but it is reduced by half over time with therapy (CitationAlvisi et al 2003). It seems to be more likely when patients are supine instead of semirecumbent (CitationValta et al 1994).
A recent meta-analysis of 7 studies including a total of 65 patients with COPD shows a modest beneficial effect of ventilatory support on exercise tolerance as well (van ‘t CitationHul et al 2002), probably in part because continuous positive airway pressure (CPAP) unloads inspiratory muscles from the inspiratory threshold load imposed by intrinsic PEEP (CitationPepe and Marini 1982; CitationHaluszka et al 1990; CitationPetrof et al 1990; CitationLougheed et al 1995). In severe COPD patients CPAP of 0.5kPa resulted in a significant reduction in dyspnea and an increase in exercise endurance time (CitationO’Donnell, Sanii, Giesbrecht, et al 1988; CitationO’Donnell, Sanii, Younes, et al 1988), however excessive CPAP increases the perception of breathing effort (CitationO’Donnell, Sanii, Giesbrecht, et al 1988) and hence it should be titrated individually. Inspiratory pressure support (IPS) a form of pressure-targeted mechanical ventilation which each breath is patient triggered and supported has been shown to improve exercise tolerance (CitationKeilty et al 1994; CitationMaltais et al 1995; CitationKyroussis et al 2000; CitationPolkey et al 2000; Citationvan’t Hul et al 2004) and reduce lactate production (CitationPolkey et al 2000). Proportional assisted ventilation, a mode of partial ventilatory assistance adapted to the intensity and timing of spontaneous ventilatory pattern by providing inspiratory pressure in proportion to a patient’s spontaneous effort, has been shown also to increase exercise tolerance (CitationDolmage and Goldstein 1997; CitationBianchi et al 1998). The role of pressure support in severely limited COPD patients is not yet clear. In a recent double-blind controlled study of 29 patients with moderate-severe COPD and ventilatory limitation during an incremental exercise test, 37 patients were exercised with either IPS 1kPa (19 subjects) versus 0.5kPa (18 subjects) in the control group. Statistically significant differences were found in favor of the IPS in shuttle walking distance, cycle endurance, and reduction in minute ventilation during exercise (van’t CitationHul et al 2004).
Measuring flow limitation and dynamic hyperinflation
Spirometry
Spirometry is a central parameter in the diagnosis and staging of COPD, yet it is poorly correlated with dyspnea and exercise capacity (CitationHay et al 1992; CitationBauerle et al 1998) and has limited individual value to predict the increase in exercise tolerance in patients with severe COPD (CitationTobin et al 1984; CitationO’Donnell et al 1998), however resting IC seems to be better index of exercise capacity (CitationDiaz et al 2000) and changes in IC are better indicator of the reduction of dyspnea and exercise tolerance (CitationRamirez-Venegas et al 1997; CitationHadcroft and Calverley 2001; CitationBoni et al 2002; CitationCelli et al 2003; CitationO’Donnell, Fluge, et al 2004; CitationStevenson et al 2005), furthermore the use of IC as an adjunct criterion for a bronchodilator test apparently increases the sensitivity to detect functional changes clinically relevant (CitationNewton et al 2002; CitationParker et al 2005). Although there is no defined criterion to evaluate changes in IC (CitationPellegrino et al 2005), changes after bronchodilators have been usually larger than 15% (CitationRamirez-Venegas et al 1997; CitationHadcroft and Calverley 2001; CitationBoni et al 2002; CitationCelli et al 2003; CitationO’Donnell, Fluge, et al 2004). Other useful indexes of air trapping are FRC, RV, and their relationship with TLC (CitationDiez Herranz 1995), although they are more complex to obtain and not as widely available as spirometry.
Flow volume loops
The conventional method used to detect expiratory flow limitation using tidal breathing was one proposed by CitationHyatt and Wilcox (1963). It consists of superimposing a flow–volume loop of a tidal breath within a maximum flow–volume curve. Expiratory flow limitation is not present when the patient breathes below the maximal expiratory flow–volume curve (CitationPride 1999). This method to detect expiratory flow limitation has several methodological deficiencies such as intrathoracic gas compression artefacts (which only can be detected with a constant pressure body plethysmograph (CitationIngram and Schilder 1966), incorrect alignment of tidal with maximal expiratory flow–volume curve considering the TLC as a fixed reference point, which is not always valid (CitationYounes and Kivinen 1984; CitationD’Angelo et al 1993). Respiratory mechanics and the intrathoracic pressure swinging are different during the tidal and maximal expiratory efforts and exercise may result in changes in airways tone (CitationBeck et al 1994). Another important limitation of the conventional method is that it requires patient cooperation.
The negative expiratory pressure technique
The negative expiratory pressure technique (NEP) method is based on the principle that the increase in pressure caused by NEP should result in increased expiratory flow. By contrast, in flow-limited subjects, application of NEP should not change the expiratory flow (CitationKoulouris et al 1995). For technical details we refer the reader elsewhere. This method does not require FVC maneuvers, collaboration on the part of the patient, or use of a body plethysmograph, and can be used during spontaneously breathing subjects during exercise (CitationCalverley and Koulouris 2005).
Measurement of dynamic hyperinflation
Since TLC remains essentially unaltered during exercise (CitationStubbing et al 1980; CitationYounes and Kivinen 1984) changes in EELV during exercise can be reliably tracked by serial IC measurements (). Exercise IC measurements are reproducible, provided care is taken with their measurement. In one study of 29 patients the variability coefficient was 17% at rest and approximately 20% during exercise with intra-class correlation coefficients of 0.77 and 0.73 respectively (CitationO’Donnell et al 1998). In another study of 15 patient esophageal pressures were found reproducible during IC maneuvers during exercise at different work-rates, the reduction in IC during exercise correlates well with esophageal balloon-derived measurements of dynamic compliance (CitationO’Donnell, Chau, et al 1997). In another study, three methods of measuring IC were tested in 10 severe COPD subjects. IC calculated, after correction of the expiratory part of the signal, as the difference between the mean EELV of the six breaths that preceded the IC prompt and the peak inspiratory volume was found to be the most reproducible method (CitationDolmage and Goldstein 2002). In an unpublished multicentric study of 463 patients with moderate to severe COPD, the intra-class correlation of exercise IC measurements during serial exercise tests exceeded 0.85 (CitationO’Donnell, He, et al 2004). The extent of the reduction in IC with exercise in COPD is variable. In a population of 105 patients with moderate to severe COPD, IC at end-exercise was reduced by 20% of its already-reduced resting value (CitationO’Donell et al 2001). In another smaller group of 27 severe COPD patients a similar reduction was found at maximum ventilation at the end of high intensity exercise (CitationPuente-Maestu et al 2005). Similar changes were found in severe COPD in 15 severe COPD (CitationYan et al 1997). However, guidelines for standardization of IC measurement have not yet been issued. Recently a new complex method called optoelectronic plethysmography has been developed for research purposes (CitationAliverti et al 2004)
Conclusion
Flow limitation and DH are crucial pathophysiological mechanisms in the development of exercise intolerance, dyspnea, and respiratory failure in COPD patients. As the disease advances with progression of symptoms and impairment, the flow limitation and DH proportionally worsens. Several methods of treatment may palliate its impact of flow limitation and DH on the patient, including physical therapy, bronchodilators, ventilatory support, oxygen, heliox, or surgery. Measurement of DH may be done indirectly by spirometry or by more direct approaches that have known limitations or are not widely available. Clinical use is limited by the lack of a standard method to measure DH or flow limitation during exercise.
References
- [ACCP/AACVPR] Pulmonary Rehabilitation Guidelines Panel, American College of Chest Physicians, American Association of Cardiovascular and Pulmonary Rehabilitation1997Pulmonary rehabilitation: joint ACCP/AACVRR evidence based guidelinesChest1121363969367481
- AgostoniEHyattRE1986Static behaviour of the respiratory systemHandbook of physiology The respiratory system, sect 3III1Bethesda, MDAmerican Physiological Society113130
- AldrichTKShapiroSMShermanMS1989Alveolar pressure and airway resistance during maximal and submaximal respiratory effortsAm Rev Respir Dis1408999062802377
- AlivertiAStevensonNDellacaRL2004Regional chest wall volumes during exercise in chronic obstructive pulmonary diseaseThorax592101614985554
- AlvisiVRomanelloABadetM2003Time course of expiratory flow limitation in COPD patients during acute respiratory failure requiring mechanical ventilationChest12316253212740283
- ArmaganidisAStavrakaki-KallergiKKoutsoukouA2000Intrinsic positive end-expiratory pressure in mechanically ventilated patients with and without tidal expiratory flow limitationCrit Care Med2838374211153623
- BabbTGDeLoreyDSWyrickBL2003Ventilatory response to exercise in aged runners breathing He-O2 or inspired CO2J Appl Physiol946859312531912
- BabbTGViggianoRHurleyB1991Effect of mild-to-moderate airflow limitation on exercise capacityJ Appl Physiol70223302010380
- BarachAL1955Breathing exercises in pulmonary emphysema and alied chronic respiratory diseaseArch Phys Med Reahab3637990
- BauerleOChruschCAYounesM1998Mechanisms by which COPD affects exercise toleranceAm J Respir Crit Care Med15757689445279
- BeckKCOffordKPScanlonPD1994Bronchoconstriction occurring during exercise in asthmatic patientsAm J Respir Crit Care Med14935278306029
- BeehKMBeierJKornmannO2003Long-term repeatability of induced sputum cells and inflammatory kers in stable, moderately severe COPDChest1237788312628878
- BelleeFGrassinoA1982Effect of pressure and timing of contraction on human diaphragm fatigueJ Appl Physiol53119057174413
- BelleeFGrassino1983A Force reserve of the diaphragm in patients with chronic obstructive pulmonary diseaseJ Appl Physiol558156411667
- BelmanMJBotnickWCShinJW1996Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med153967758630581
- BianchiLFoglioKPaganiM1998Effects of proportional assist ventilation on exercise tolerance in COPD patients with chronic hypercapniaEur Resp J114227
- BianchiRGigliottiFRomagnoliI2004Chest wall kinematics and breathlessness during pursed-lip breathing in patients with COPDChest1254596514769725
- BlackLFHyattRE1969Maximal respiratory pressures: normal values and relationship to age and sexAm Rev Respir Dis996967025772056
- BoniECordaLFranchiniD2002Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at restThorax575283212037229
- BowenJBVottoJJThrallRS2000Functional Status and Survival Following Pulmonary RehabilitationChest11869770310988191
- BrantiganOCMuellerEKressMB1959A surgical approach to pulmonary emphysemaAm Rev Respir Dis8019420213670425
- BrennerMHannaNMMina-AraghiR2004Innovative approaches to lung volume reduction for emphysemaChest1262384815249467
- CalverleyPMAKoulourisNG2005Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiologyEur Respir J251869915640341
- CasaburiRGosselinkRRamerM1999American Thoracic Society/European Respiratory Society skeletal muscle dysfunction in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med159S1S4010194189
- CasaburiRPorszaszJBurnsMR1997Am J Respir Crit Care Med1551541519154855
- CasaburiR2001Special considerations for exercise trainingACSM resource manual for guidelines for exercise testing and prescription4th edACSM34652
- CasanovaCCoteCde TorresJP2005Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med171591715591470
- CelliBZuWallackRWangS2003Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest1241743814605043
- CelliBRCoteCGMarinJM2004The body mass index, airflow obstruction, dyspnoea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med35010051214999112
- CherniackRMHodsonA1963Compliance of the Chest wall in chronic bronchitis and emphysemaJ Appl Physiol187071114020594
- CitterioGAgostoniEDel SantoA1981Decay of inspiratory muscle activity in chronic airway obstructionJ Appl Physiol511388977319872
- CooperJDTrulockEPTriantafillouAN1995Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary diseaseJ Thorac Cardiovasc Surg109106167815786
- CosioMGHaleKANiewoehnerDE1980Morphologic and morphometric effects of prolonged cigarette smoking on the small airwaysAm Rev Respir Dis122265217416603
- CoussaMLGuerinCEissaNT1993Partitioning of work of breathing in mechanically ventilated COPD patientsJ Appl Physiol751711198282624
- D’AngeloEPrandiEMilic-EmiliJ1993Dependence of maximal flow-volume curves on time-course of preceding inspirationJ Appl Physiol75115598226524
- DeanNCBrownJKHimelmanRB1992Oxygen may improve dyspnoea and endurance in patients with chronic obstructive pulmonary disease and only mild hypoxemiaAm Rev Respir Dis14694151416422
- deBoisblancBPDeBleiuxPResweberS2000Randomized trial of the use of heliox as a driving gas for updraft nebulization of bronchodilators in the emergent treatment of acute exacerbations of chronic obstructive pulmonary diseaseCrit Care Med2831778011008978
- Di MarcoJMilic-EmiliJBoveriP2003Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPDEur Respir J21869412570114
- DiazOVillafrancaCGhezzoH2000Exercise tolerance in COPD patients with and without tidal expiratory flow limitation at restEur Respir J162697510968502
- DiehlJLMercatAGuerotE2003Helium/oxygen mixture reduces the work of breathing at the end of the weaning process in patients with severe chronic obstructive pulmonary diseaseCrit Care Med3114152012771612
- Diez HerranzA1995RV/TLC% ratio: alternative criteria of normalityEur Respir J81812138586145
- DoddDSBrancatisanoTEngelLA1984Chest wall mechanics during exercise in patients with severe chronic air-flow obstructionAm Rev Respir Dis1293386230971
- DolmageTEGoldsteinRS1997Proportional assist ventilation and exercise tolerance in subjects with COPDChest111948549106574
- DolmageTEGoldsteinRS2002Repeatability of inspiratory capacity during incremental exercise in patients with severe COPDChest1217081411888950
- DolmageTEWaddellTKMaltaisF2004The influence of lung volume reduction surgery on exercise in patients with COPDEur Respir J232697414979502
- DonahoeMRogersRMWilsonDO1989Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis140385912764376
- EltayaraLBecklakeMRVoltaCA1996Relationship between chronic dyspnoea and expiratory flow limitation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1541726348970362
- FesslerHEPermuttS1998Lung volume reduction surgery and airflow limitationAm J Respir Crit Care Med157715229517581
- FishmanAMartinezFNaunheimKNational Emphysema Treatment Trial Research Group2003A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med34820597312759479
- GaissertHATrulockEPCooperJD1996Comparison of early functional results after volume reduction or lung transplantation for chronic obstructive pulmonary diseaseJ Thorac Cardiovasc Surg1112963068583802
- GallagherCG1994Exercise limitation and clinical exercise testing in chronic obstructive pulmonary diseaseClin Chest Med15305268088095
- GarrodRDallimoreKCookJ2005An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patientsChron Respir Dis2677216279153
- GeddesDDaviesMKoyamaH2000Effect of lung-volume-reduction surgery in patients with severe emphysemaN Engl J Med3432394510911005
- GerardiDALovettLBenoit-ConnorsML1996Variables related to increased mortality following out-patient pulmonary rehabilitationEur Respir J943158730000
- GerbeauxPGainnierMBoussugesA2001Use of heliox in patients with severe exacerbation of chronic obstructive pulmonary diseaseCrit Care Med292322411801835
- GigliottiFColiCBianchiR2003Exercise training improves exertional dyspnoea in patients with COPD: evidence of the role of mechanical factorsChest123179480212796152
- GigliottiFColiCBianchiR2005Arm exercise and hyperinflation in patients with COPD: effect of arm trainingChest12812253216162710
- GottliebDJStonePJSparrowD1996Urinary desmosine excretion in smokers with and without rapid line of lung function: the Normative Aging StudyAm J Respir Crit Care Med154129058912738
- GreenMPrideMBScaddingJGCummingGThrubeckWM1997Normal respiratory mechanicsScientific foundations of respiratory medicineLondonWilliam Heinemann Medical books11329
- GrimbyGBunnJMeadJ1968Relative contribution of rib cage and abdomen to ventilation during exerciseJ Appl Physiol24159665637678
- GrossDLaddHWRileyEJ1980The effect of training on strength and endurance of the diaphragm in quadriplegiaAm J Med6827357350802
- GunstensenJMcCormackRJ1973The surgical management of bullous emphysemaJ Thorac Cardiovasc Surg6592054704241
- HaasFSalazar-SchichiJAxenKCasaburiRPettyTL1993Desensitization to dyspnoea in chronc obstructive pulmonary diseasePrinciples and practice of pulmonary rehabilitationPhiladelphiaWB Saunders Company24151
- HadcroftJCalverleyPM2001Alternative methods for assessing bronchodilator reversibility in chronic obstructive pulmonary diseaseThorax567132011514693
- HaluszkaJChartrandDAGrassinoAE1990Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis141119472111105
- HamiltonALKillianKJSummersE1996Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disordersChest1101255638915230
- HayJGStonePCarterJ1992Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary diseaseEur Respir J5659641628722
- HazelriggSRBoleyTMNaunheimKS1997Effect of bovine pericardial strips on air leak after stapled pulmonary resectionAnn Thorac Surg63157359205150
- HenkeKGSharrattMPegelowD1988Regulation of end-expiratory lung volume during exerciseJ Appl Physiol64135463356631
- HiragaTMaekuraROkudaY2003Prognostic predictors for survival in patients with COPD using cardiopulmonary exercise testingClin Physiol Funct Imaging23324914617262
- HopkinsonNSTomaTPHansellDM2005Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysemaAm J Respir Crit Care Med1714536015579725
- HyattREWilcoxRE1963The pressure-flow relationships of the intrathoracic airway in manJ Clin Invest42293913955989
- HyattRE1961The interrelationships of pressure, flow, and volume during various respiratory maneuvers in normal and emphysematous subjectsAm Rev Respir Dis836768313717137
- HyattRE1983Expiratory flow limitationJ Appl Physiol55186350246
- Im HofVWestPYounesM1986Steady-state response of normal subjects to inspiratory resistive loadJ Appl Physiol601471813710967
- IngenitoEPLoringSHMoyML2001Interpreting improvement in expiratory flows after lung volume reduction surgery in terms of flow limitation theoryAm J Respir Crit Care Med16310748011316638
- IngramRHJrSchilderDP1966Effect of gas compression on pulmonary pressure, flow, and volume relationshipJ Appl Physiol21182165929308
- IngramRHJrSchilderDP1967Effect of pursed lips expiration on the pulmonary pressure-flow relationship in obstructive lung diseaseAm Rev Respir Dis9638186039092
- JaberSCarlucciABoussarsarM2001Helium-oxygen in the postextubation period decreases inspiratory effortAm J Respir Crit Care Med164633711520728
- JaberSFodilRCarlucciA2000Noninvasive ventilation with helium-oxygen in acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med161119120010764311
- JohnsonBDReddanWGSeowKC1991Mechanical constraints on exercise hyperpnea in a fit aging populationAm Rev Respir Dis143968772024852
- JollietPTassauxDThouretJM1999Beneficial effects of helium: oxygen versus air: oxygen noninvasive pressure support in patients with descompensated chronic obstructive pulmonary diseaseCrit Care Med272422910579259
- JollyEAguirreLJorgeE1996Acute effect of lorazepam on respiratory muscles in stable patients with chronic obstructive pulmonary diseaseMedicina (B Aires)5647289239882
- KeatingsVMCollinsPDScottDM1996Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthmaAm J Respir Crit Care Med15353048564092
- KeenanRJLandreneauRJSciurbaFC1996Unilateral thoracoscopic surgical approach for diffuse emphysemaJ Thorac Cardiovasc Surg111308158583803
- KeiltySEPonteJFlemingTA1994Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary diseaseThorax4999067974316
- KervioGCarreFVilleNS2003Reliability and intensity of the six-minute walk test in healthy elderly subjectsMed Sci Sports Exerc351697412544651
- KillianKJJonesNL1994Mechanisms of exertional dyspnoeaClin Chest Med15247578088091
- KillianKJLeblancPTinDH1992Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitationAm Rev Respir Dis146935401416421
- KimMJDruzWSDanonJ1976Mechanics of the canine diaphragmJ Appl Physiol4136982965306
- KnoxAJMorrisonJFMuersMF1998Reproducibility of walking test results in chronic obstructive airways diseaseThorax43388923194867
- KoulourisNGValtaPLavoieA1995A simple method to detect expiratory flow limitation during spontaneous breathingEur Respir J8306137758567
- KyroussisDPolkeyMIHamnegardCH2000Respiratory muscle activity in patients with COPD walking to exhaustion with and without pressure supportEur Respir J156495510780754
- LeithDEBradleyM1976Ventilatory muscle strength and endurance trainingJ Appl Physiol4150816985393
- LevisonHCherniackRM1968Ventilatory cost of exercise in chronic obstructive pulmonary diseaseJ Appl Physiol252175661150
- LieskerJJWijkstraPJTen HackenNH2002A systematic review of the effects of bronchodilators on exercise capacity in patients with COPDChest12159760811834677
- LightRWMuroJRSatoRI1989Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis139126332492170
- LightRWStansburyDWWebsterJS1996Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPDChest109975818635380
- LiuANMohammedAZRiceWR1999Perforin-independent CD8(+) T-cell-mediated cytotoxicity of alveolar epithelial cells is preferentially mediated by tumor necrosis factor-alpha: relative insensitivity to Fas ligandAm J Respir Cell Mol Biol208495810226053
- LougheedMDWebbKAO’DonnellDE1995Breathlessness during induced hyperinflation in asthma: role of the inspiratory threshold loadAm J Respir Crit Care Med152911207663804
- MahlerDABrentBNLokeJ1984Right ventricular performance and central circulatory hemodynamics during upright exercise in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis13072296388444
- MaltaisFHamiltonAMarciniukD2005Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest12811687816162703
- MaltaisFLeBlancPJobinJ1997Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med155555619032194
- MaltaisFReissmannHGottfriedSB1995Pressure support reduces inspiratory effort and dyspnoea during exercise in chronic airflow obstructionAm J Respir Crit Care Med1511027337697226
- ManWDMustfaNNikoletouD2005Effect of salmeterol on respiratory muscle activity during exercise in poorly reversible COPDThorax59471615170026
- MannixETManfrediFFarberMO1999Elevated O2 cost of ventilation contributes to tissue wasting in COPDChest1157081310084480
- MarhsallR1962Relationships between stimulus and work of breathing at different lung volumesJ Appl Physiol1791721
- MarinJMCarrizoSJGasconM2001Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1631395911371407
- McGregorMBecklakeMR1961The relationship of oxygen cost of breathing to respiratory mechanical work and respiratory forceJ Clin Invest409718013773979
- MeadJTurnerJMMacklemPT1967Significance of the relationship between lung recoil and maximum expiratory flowJ Appl Physiol22951086017658
- Milic-EmiliJ2004Provocative hypothesis: does mechanical injury the peripheral airways play a role in the genesis of COPD in smokers?J Chron Obstruc Pulm Dis118
- MillerJDBergerRLMalthanerRA2005Lung volume reduction surgery vs medical treatment: for patients with advanced emphysemaChest12711667715821191
- MillerJDPegelowDFJacquesAJ2005Effects of augmented respiratory muscle pressure production on locomotor limb venous return during calf contraction exerciseJ Appl Physiol9918021516051714
- Montes de OcaMRassuloJCelliBR1996Respiratory muscle and cardiopulmonary function during exercise in very severe COPDAm J Respir Crit Care Med154128498912737
- MuellerRChanezPCampbellAM1996Different cytokine patterns in bronchial biopsies in asthma and chronic bronchitisRespir Med9079858730325
- MuellerREPettyTLFilleyGF1970Ventilation and arterial blood gas changes induced by pursed lips breathingJ Appl Physiol2878495419502
- MyersJPrakashMFroelicherV2002Exercise capacity and mortality among men referred for exercise testingN Engl J Med34679380111893790
- NathanSDEdwardsLBBarnettSD2004Outcomes of COPD lung transplant recipients after lung volume reduction surgeryChest12615697415539729
- NederAJonesPWNeryLE2000Determinants of the exercise endurance capacity in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16249750410934077
- [NETT] National Emphysema Treatment Trial Research Group2001Patients at high risk of death after lung-volume-reduction surgeryN Engl J Med34510758311596586
- NewtonMFO’DonnellDEForkertL2002Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflationChest12110425011948031
- NickoladzeGD1992Functional results of surgery for bullous emphysemaChest101119221729056
- NieldMAroraADracupK2003Comparison of breathing patterns during exercise in patients with obstructive and restrictive ventilatory abnormalitiesJ Rehabil Res Dev404071415080225
- NiewoehnerDEKleinermanJRiceDB1974Pathologic changes in the peripheral airways of young cigarette smokersN Engl J Med29175584414996
- NishimuraKIzumiTTsukinoM2002Dyspnoea is a better predictor of 5-year survival than airway obstruction in patients with COPDChest12114344012006425
- NishiyamaOTaniguchiHKondohY2005Factors in maintaining long-term improvements in health-related quality of life after pulmonary rehabilitation for COPDQual Life Res1423152116328910
- O’DonnellDEChauLKLBertleyJC1997Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanismsAm J Respir Crit Care Med155109159001298
- O’DonnellDEHeZLamM2004Reproducibility of measurements of inspiratory capacity, dyspnoea intensity and exercise endurance in multicentre trials in COPDEur Respir J24323s
- O’DonnellDERevillSWebbKA2001Dynamic hyperinflation and exercise intolerance in COPDAm J Respir Crit Care Med164770711549531
- O’DonnellDEWebbKACalverleyPMacNeeWRennardS2003Chapter 18: ExerciseChronic obstructive lung disease2nd edLondonEdward Arnold Ltd24365
- O’DonnellCEFlugeTGerkenF2004Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J238324015218994
- O’DonnellDEBainDJWebbKA1997Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitationAm J Respir Crit Care Med15553059032190
- O’DonnellDED’ArsignyCFitzpatrickM2002Exercise hypercapnia in advanced chronic obstructive pulmonary disease. The role of lung hyperinflationAm J Respir Crit Care Med166663812204862
- O’DonnellDELamMWebbKA1998Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1581557659817708
- O’DonnellDESaniiRGiesbrechtG1988Effect of continuous positive airway pressure on respiratory sensation in patients with chronic obstructive pulmonary disease during submaximal exerciseAm Rev Respir Dis1381185913059891
- O’DonnellDESaniiRYounesM1988Improvement in exercise endurance in patients with chronic airflow. Limitation using continuous positive airway pressureAm Rev Respir Dis1381510143059897
- O’DonnellDEVoducNFitzpatrickM2004Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary diseaseEur Respir J24869415293609
- O’DonnellDEWebbKA1993Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflationAm Rev Respir Dis148135178239175
- OgaKNishimuraKTsukinoM2000The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. a comparison of three different exercise testsAm J Respir Crit Care Med161189790110852763
- OgaTNishimuraKTsukinoM2002Relationship between different indices of exercise capacity and clinical measures in patients with chronic obstructive pulmonary diseaseHeart Lung313748112487016
- OgaTNishimuraKTsukinoM2003Analysis of factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health statusAm J Respir Crit Care Med1675444912446268
- OrtizLAGambelliFMcBrideC2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effectsProc Natl Acad Sci U S A10084071112815096
- Oswald-MammosserMApprillMBachezP1991Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous typeRespiration58304101792422
- Oswald-MammosserMKesslerRMassardG1998Effect of lung volume reduction surgery on gas exchange and pulmonary hemodynamics at rest and during exerciseAm J Respir Crit Care Med158102059769254
- PalangePValliGOnoratiP2004Effect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patientsJ Appl Physiol97163715234959
- ParkerMVoducNAaronSD2005Physiological changes during symptom recovery from moderate exacerbations of COPDEur Respir J26420816135722
- PauwelsRABuistASCalverleyPMA2001Am J Respir Crit Care Med16312567611316667
- PecchiariMPelucchiAD’AngeloE2004Effect of heliox breathing on dynamic hyperinflation in COPD patientsChest12520758215189924
- PeinadoVIBarberaJAAbateP1999Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary diseaseAm J Respir Crit Care Med15916051110228134
- PellegrinoRViegiGBrusascoV2005Interpretative strategies for lung function testsEur Respir J269486816264058
- PellegrinoRVillosioCMilaneseU1999Breathing during exercise in subjects mild-to-moderate airflow obstruction: effects of physical trainingJ Appl Physiol169770410562611
- PepePEMariniJJ1982Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effectAm Rev Respir Dis126166707046541
- PesciABalbiBMajoriM1998Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary diseaseEur Respir J1238069727789
- PetoRSpeizerFECochraneAL1983The relevance in adults of airflow obstruction, but not of mucous hypersecretion, to mortality from chronic lung diseaseAm Rev Respir Dis1284915006614643
- PetrofBJCalderiniEGottfriedSB1990Effect of CPAP on respiratory effort and dyspnoea during exercise in severe COPDJ Appl Physiol69179882203722
- Pinto-PlataVMCoteCCabralH2004The 6-min walk distance: change over time and value as a predictor of survival in severe COPDEur Respir J23283314738227
- PolkeyMIHamnegardCHHughesPD1998Influence of acute lung volume change on contractile properties of human diaphragmJ Appl Physiol85132289760323
- PolkeyMIHawkinsPKyroussisD2000Inspiratory pressure support prolongs exercise induced lactataemia in severe COPDThorax55547910856312
- PoonCSYounesMGallagherCG1987Effects of expiratory resistive load on respiratory motor output in conscious humansJ Appl Physiol631837453121575
- PorszaszJEmtnerMGotoS2005Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at sub-maximal intensities in patients with COPDChest12820253416236851
- PotterWAOlafssonSHyattRE1971Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung diseaseJ Clin Invest50910195547281
- PrideNBGreenMScaddingJGCummingGThrubeckWM1997Abnormalities of respiratory mechanicsScientific foundations of respiratory medicineLondonWilliam Heinemann Medical Bks66988
- PrideNBMacklemPTFishmanAP1986Lung mechanics in diseaseHandbook of physiology, Section 3, Volume III, Part 2: The respiratory systemBethesda MDAmerican Physiological Society65992
- PrideNBHughesJMBPrideNB1999Tests of forced expiration and inspirationLung function tests: physiological principles and clinical applicationsLondonWB Saunders325
- Puente-MaestuLGarcíaJMartínez-AbadY2005Dyspnoea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in COPDChest1286515616100150
- Puente-MaestuLMartínezYPedrazaF2006A controlled trial of the effects of leg training on breathing pattern and dynamic hyperinflation in severe COPDLung1841596716902841
- Puente-MaestuLSantaCruzAVargasT2003Effects of training on the tolerance to high-intensity exercise in patients with severe COPDRespiration703677014512671
- Puente-MaestuLSánzMLSánzP2000Comparison of effects of supervised versus self-monitored training programs in patients with chronic obstructive pulmonary diseaseEur Respir J155172510759446
- Ramirez-VenegasAWardJLentineT1997Salmeterol reduces dyspnoea and improves lung function in patients with COPDChest112336409266866
- RanieriVMDambrosioMBrienzaN1996Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failureEur Respir J91283928804950
- RanieriVMGiulianiRCinnellaG1993Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilationAm Rev Respir Dis1475138420430
- RodrigoGRodrigoCPollackC2001Helium-oxygen mixture for nonintubated acute asthma patients (Cochrane Review)Cochrane Database Syst Rev1CD00288411279771
- RodrigoGJRodrigoCPollackCV2003Use of helium-oxygen mixtures in the treatment of acute asthma: a systematic reviewChest123891612628893
- RogersRMDuBoisABBlakemoreWS1968Effect of removal of bullae on airway conductance and conductance volume ratiosJ Clin Invest472569795725275
- RossiAGanassiniAPoleseG1997Pulmonary hyperinflation and ventilator-dependent patientsEur Respir J101663749230263
- RoussosCMacklemPT1982The respiratory musclesN Engl J Med307786977050712
- Ruiz de Oña LacastaJMGarcia de PedroJPuente-MaestuL2004Effects of muscle training on breathing pattern in patients with severe chronic obstructive pulmonary diseaseArch Bronconeumol4020314718117
- SaettaMBaraldoSCorbinoL1999Am J Respir Crit Care Med1607111710430750
- SaettaMTuratoGMaestrelliP2001Cellular and structural bases of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1631304911371392
- SaksenaFBBurrowsB1966Thoracic compliance in chronic obstructive lung diseaseJ Lab Clin Med68427325922754
- SalviSBlombergARudellB1999Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteersAm J Respir Crit Care Med159702910051240
- SchumakerGLEpsteinSK2004Managing acute respiratory failure during exacerbation of chronic obstructive pulmonary diseaseRespir Care497668215222909
- ShapiroSDSeniorRM1999Matrix metalloproteinases: matrix degradation and moreAm J Respir Cell Mol Biol201100210340927
- SharpJTVan LithPVej NughprayoonC1968The thorax in chronic obstructive lung diseaseAm J Med443946
- ShindohCHidaWKikuchiY1994Oxygen consumption of respiratory muscles in patients with COPDChest10579078131542
- SimilowskiTYanSGauthierAP1991Contractile properties of the human diaphragm during chronic hyperinflationN Engl J Med325917231881417
- SomfayAPorszaszJLeeSM2001Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patientsEur Respir J18778411510809
- SpahijaJde MarchieMGrassinoA2005Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnoea at rest and during exercise in COPDChest1286405016100149
- StevensonNJCalverleyPM2004Effect of oxygen on recovery from maximal exercise in patients with chronic obstructive pulmonary diseaseThorax596687215282386
- StevensonNJWalkerPPCostelloRW2005Lung mechanics and dyspnoea during exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med17215101616166620
- StockleyRA1999Neutrophils and protease/antiprotease imbalanceAm J Respir Crit Care Med160S49S5210556170
- StocksJQuanjerPH1995Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of the European Respiratory SocietyEur Respir J84925067789503
- StubbingDGPengellyLDMorseJL1980Pulmonary mechanics during exercise in normal malesJ Appl Physiol49506107204174
- SurattBTCoolCDSerlsAE2003Human pulmonary chimerism after hematopoietic stem cell transplantationAm J Respir Crit Care Med1683182212724127
- SwidwaDMMontenegroHDGoldmanMD1985Helium-oxygen breathing in severe chronic obstructive pulmonary diseaseChest8779053996069
- TakayamaTShindohCKurokawaY2003Effects of lung volume reduction surgery for emphysema on oxygen cost of breathingChest12318475212796159
- TassauxDJollietPRoeselerJ2000Effects of helium-oxygen on intrinsic positive end-expiratory pressure in intubated and mechanically ventilated patients with severe chronic obstructive pulmonary diseaseCrit Care Med282721810966241
- ThomanRLStokerGLRossJC1966The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis9310065901381
- ThurlbeckWCherniackN1991Pathology of chronic airflow obstructionChronic obstructive pulmonary diseasePhiladelphiaWB Saunders320
- ThurlbeckWMPettyTL1985Chronic airflow obstruction. Correlation of structure and functionChronic obstructive pulmonary diseaseNew YorkMarcel Dekker129204
- TiepBLBurnsMKaoD1986Pursed lips breathing training using ear oximetryChest90218213731893
- TobinMJHughesJAHutchisonDC1984Effects of ipratropium bromide and fenoterol aerosols on exercise toleranceEur J Respir Dis6544166236095
- TojoNIchiokaMChidaM2005Pulmonary exercise testing predicts prognosis in patients with chronic obstructive pulmonary diseaseIntern Med4420515704658
- ValtaPCorbeilCLavoieA1994Detection of expiratory flow limitation during mechanical ventilationAm J Respir Crit Care Med1501311177952558
- van’t HulAJKwakkelGGosselinkR2002The acute effects of non-invasive ventilatory support during exercise on exercise endurance and dyspnoea in patients with COPD: a systematic reviewJ Cardiopulm Rehabil22290712202851
- van’t HulAGosselinkRHollanderP2004Acute effects of inspiratory pressure support during exercise in patients with COPDEur Respir J23344014738228
- VestboJPrescottELangePlthe Copenhagen City Heart Study Group1996Association of chronic mucus hypersecretion with FEV1 line and chronic obstructive pulmonary disease morbidityAm J Respir Crit Care Med153153058630597
- VogiatzisIGeorgiadouOGolematiS2005Patterns of dynamic hyperinflation during exercise and recovery in patients with severe chronic obstructive pulmonary diseaseThorax60723915964912
- VogiatzisINanasSKastanakisE2004Dynamic hyperinflation and tolerance to interval exercise in patients with advanced COPDEur Respir J243859015358696
- VogiatzisINanasSRoussosC2002Interval training as an alternative modality to continuous exercise in patients with COPDEur Respir J20121912166558
- Von EssenSGO’NeillDPMcGranaghanS1995Neutrophilic respiratory tract inflammation and peripheral blood neutrophilia after grain sorghum dust extract challengeChest1081425337587452
- WakabayashiABrennerMKayalehRA1991Thoracoscopic carbon dioxide laser treatment of bullous emphysemaLancet33788131672970
- WoodcockAAGrossERGeddesDM1981Oxygen relieves breathlessness in “pink puffers”Lanceti90796112324
- WoodcockAAGrossERGellertA1981Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gasesN Engl J Med3051611166796885
- YamamotoCYonedaTYoshikawaM1997Airway inflammation in COPD assessed by sputum levels of interleukin-8Chest112505109266891
- YanSKaminskiDSliwinskiP1997Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary diseaseAm J Resp Crit Care Med1565599230726
- YounesMKivinenG1984Respiratory mechanics and breathing pattern during and following maximal exerciseJ Appl Physiol571773826511552
- YounesMRiddleW1981A model for the relation between respiratory neural and mechanical outputs. I. TheoryJ Appl Physiol51963787298440
- YounesMWhippBJWassermanK1991Determinants of thoracic excursion during exerciseExercise pulmonary physiology and physiopathologyNew YorkMarcel Dekker165