Abstract
None of the drugs currently available for chronic obstructive pulmonary disease (COPD) are able to reduce the progressive decline in lung function which is the hallmark of this disease. Smoking cessation is the only intervention that has proved effective. The current pharmacological treatment of COPD is symptomatic and is mainly based on bronchodilators, such as selective β2-adrenergic agonists (short- and long-acting), anticholinergics, theophylline, or a combination of these drugs. Glucocorticoids are not generally recommended for patients with stable mild to moderate COPD due to their lack of efficacy, side effects, and high costs. However, glucocorticoids are recommended for severe COPD and frequent exacerbations of COPD. New pharmacological strategies for COPD need to be developed because the current treatment is inadequate.
Introduction
The objective of pharmacological treatment of chronic obstructive pulmonary disease (COPD) is to prevent and control symptoms, reduce the frequency and severity of exacerbations, and improve general health status and exercise tolerance. None of the classes of drugs currently used in the treatment of COPD are able to modify the progressive decline in lung function which is the hallmark of this disease (CitationAnthonisen et al 1994; CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000). Smoking cessation is currently the only intervention which has been shown to reduce the progression of COPD (CitationGICOPD 2001). To achieve this objective, behavioral therapy and pharmacological treatment such as the administration of bupropion (an antidepressant), and nicotine replacement therapy have proved useful (CitationJorenby et al 1999; CitationTashkin et al 2001). However, it is important to try to control symptoms of COPD with pharmacological treatment using the following general proposals (CitationGICOPD 2001):
There should be a stepwise increase in treatment, according to the severity of the disease. The step-down approach used in the chronic treatment of asthma is not applicable to COPD.
Treatment needs to be chronic and maintained at the same level for long periods of time, unless significant side effects or exacerbations occur.
Since individual patient response to the pharmacological treatment is variable, it is important to monitor pharmacological treatment closely and, if necessary, adjust it frequently.
Drugs currently recommended for the treatment of COPD are:
Bronchodilators (selective β2-agonists, anticholinergic antimuscarinic agents and methylxanthines);
glucocorticoids;
other types of medication (vaccines, antibiotics, α1-antitrypsin augmentation therapy, mucolytic agents, antioxidants, immunoregulators, antitussives and vasodilators).
These drugs will be presented in the order in which they would normally be prescribed for the treatment of patients with COPD, based on the level of severity of the disease. Current knowledge on inhibitors of phosphodiesterase type 4 (PDE4), a new class of drugs for COPD which are in the late phase III of clinical development (CitationLipworth 2005), will be presented. However, it must be emphasized that each treatment regimen needs to be patient-specific as the relationship between the severity of symptoms and the severity of lung function is influenced by other factors, such as the frequency and severity of exacerbations, the presence of complications, the presence of respiratory failure, the presence of other diseases, and general health status.
Treatment of stable chronic obstructive pulmonary disease
Bronchodilators
Bronchodilators are currently the mainstay of the treatment of COPD (CitationGICOPD 2001). Bronchodilators are selective short-acting β2-agonists such as salbutamol, metaproterenol, terbutaline, bambuterol, pirbuterol, isoetharine, bitolterol and fenoterol or selective long-acting β2-agonists such as salmeterol and formoterol; anticholinergic antimuscarinic agents such as ipratropium bromide, oxitropium bromide and tiotropium bromide, and methylxanthines such as theophylline. Short- and long-acting β2-agonists and antimuscarinic agents are generally administered by inhalation (aerosol, dry-powder or nebuliser solution). Bronchodilator therapy is most frequently delivered by pressurized metered-dose inhalers (MDIs) or dry-powder inhalers (DPIs). Because of the lower bioavailability in asthma patients, the dose delivered by DPIs should be doubled compared with that of MDIs (CitationRS 2003); while a study comparing ipratropium bromide delivered by MDIs and by DPIs in COPD patients found that there was no difference between these two types of inhalers (CitationCuvelier et al 2002). The use of a spacer device to improve drug delivery proves particularly useful for patients who have poor inhalation technique. In a study on patients with COPD, inhalation of salbutamol through MDIs with spacer and dry-powder inhalers produced similar bronchodilating effects (CitationIkeda et al 1999). However, further studies are necessary to establish deposition in the respiratory tract and the dose-effect relationship of drugs delivered by different inhalers in patients with COPD, as these are important factors in the choice of dosage. Short-acting β2-agonists such as salbutamol and terbutaline are also available for oral or parenteral delivery. Theophylline is generally administered orally, mostly frequently in controlled release preparations which prolong the pharmacological effect of the drug, even if they do not significantly eliminate the interindividual variability of the bioavailability. For chronic administration, bronchodilators are the mainstay of treatment of COPD as they prevent and improve symptoms (CitationChrystyn et al 1988; CitationVathenen et al 1988; CitationGross et al 1989; CitationRennard 2004). One of the main therapeutic effects of bronchodilators, at least in severe COPD, is improvement in the emptying of the lungs during expiration. This causes a reduction in dynamic hyperinflation at rest and during exercise with consequent improvement in exercise tolerance (CitationBelman et al 1996). However, it is not easy to predict the extent of this improvement based on an increase in forced expiratory volume in one second (FEV1) after a short period of bronchodilatory therapy (CitationBerger and Smith 1988; CitationHay et al 1992). The evaluation of the efficacy of bronchodilators is generally based on questionnaires concerning symptom variation. COPD may require inhalation of a short-acting β2-agonist on demand or, when airways obstruction is more severe, chronic administration of an antimuscarinic agent by inhalation or a long-acting β2-agonist (CitationRS 2003).
Selective β2-agonists
There are no significant differences between the various β2-agonists as far as selectivity for β2-adrenoceptors is concerned, with the exception of metaproterenol and iso-etharine, which are less selective (CitationUndem and 1 2001). Short-acting β2-agonists have a bronchodilatory effect within 1 to 5 minutes and which lasts for up to 4 hours (CitationRennard 2004). Long-acting inhaled β2-adrenergic agonists such as salmeterol and formoterol have a prolonged bronchodilatory effect for approximately 12 hours. Unlike salmeterol which has a slow onset of action, formoterol has a rapidly occurring bronchodilatory effect similar to that of short-acting β2-agonists (CitationUndem and Lichtenstein 2001; CitationRennard 2004). Selective β2-agonists mainly work by stimulating the β2-adrenoceptors on the airway smooth muscle cells. The formation of the drug-receptor complex activates a stimulatory protein (Gs) which binds to guanosine triphosphate (GTP) with activation of the adenylate cyclase which leads to an increase in intracellular cyclic adenosine monophosphate (cAMP) levels which, in turn, activates a cAMP-dependant protein kinase (PKA) (CitationJohnson and Coleman 1995). Activation of the latter causes myosin light chain kinase phosphorylation with a reduction in the affinity of this enzyme for the calcium–calmodulin complex, a reduction in the formation of active myosin light chain kinase, a reduction in myosin phosphorylation and, finally, a reduction in the interaction of actin and myosin filaments with consequent bronchodilation. In addition, PKA stimulation causes a reduction in intracellular calcium ion levels because of increased extrusion of calcium from the cell, reduction in the entry of calcium into the cell, and increase in the uptake of calcium by the smooth endoplasmic reticulum, which is the site at which calcium ions are deposited within the cell.
The reduction in the concentration of intracellular calcium causes a reduction in the formation of the calcium–calmodulin complex which produces the previously described effects. In addition to this main bronchodilation mechanism of selective β2-agonists, other mechanisms may, in theory, contribute to reducing airway obstruction. Stimulation of β2-adrenoceptors also increases potassium channel conduction with hyperpolarization and relaxation of the airway smooth muscle (CitationKume et al 1994). This effect is, in part, independent from the increase in cAMP levels (CitationKume et al 1994). The activation of β2-receptors on inflammatory cells such as mast cells, basophils, eosinophils, neutrophils and lymphocytes cause an increase in cAMP levels and subsequent inhibition of the release of inflammatory mediators such as leukotrienes, histamine and cytokines (CitationHughes et al 1983; CitationBarnes 1999). β2-agonists increase mucociliary clearance, decrease microvascular permeability and may inhibit phospholipase A2 with subsequent reduction in the synthesis of leukotrienes, prostaglandins and thromboxane A2, which are important inflammatory mediators (CitationSeale 1988). The importance of these mechanisms on the therapeutic effect of β2-agonists in humans, particularly in COPD, is not known.
Inhalation limits the absorption and therefore the systematic side effects of β2-agonists. However, only about 10% of the aerosol enters the respiratory tract while the remainder is swallowed and may be absorbed in the intestinal tract with consequent systemic side effects. Furthermore, aerosolized particles less than 1μm in diameter reach the alveoli and may be absorbed in the pulmonary capillaries. The main side effect of selective β2-agonists administered by inhalation at clinical doses is muscular tremor. However, this tends to diminish in intensity during prolonged treatment. This phenomenon is known as drug tolerance. It is not know if drug tolerance is due to the down regulation of the β2-adrenoceptors on the muscle cytoskeleton or to adaptation phenomena in the central nervous system. Tachycardia is uncommon in clinical doses of β2-agonists administered by inhalation. The absorption of β2-agonists may cause tachycardia by stimulating the cardiac β1-adrenoceptors, particularly at higher doses when relative selectivity for β2-adrenoceptors is lost. Although there are fewer cardiac β2-adrenoceptors, their stimulation may also contribute to tachycardia (CitationUndem and Lichtenstein 2001). This type of side effect is particularly relevant in patients with ischemic heart disease or pre-existing arrhythmias. In these patients, β2-agonists need to be administered with caution, even when inhaled. Bronchodilation may cause ventilation/perfusion mismatch with a consequent drop in the arterial partial pressure of oxygen. This is generally transitory and of little importance. Other side effects of systemically delivered β2-agonists, such as hypokalemia, increase in the plasma concentrations of glucose, lactose, and free fatty acids are much less common when β2-agonists are delivered by inhalation.
Antimuscarinic drugs
Antimuscarinics are delivered in the same way as bronchodilators. Antimuscarinics are quaternary ammonium derivatives which, after inhalation, undergo <1% absorption from the pulmonary or gastrointestinal tract. The parasympathetic nervous system has a role in the regulation of the bronchial tone. Vagal fibres activate nicotinic and M1 muscarinic receptors on the parasympathetic ganglia of the respiratory tract; short postganglionic fibers release acetylcholine which stimulates the M3 muscarinic receptors on the airway smooth muscle cells with consequent increase in motility. In addition, the airway submucosal glands have M3 muscarinic receptors. M3 muscarinic receptor stimulation increases bronchial secretion. Inflammatory mediators such as eicosanoids, histamine, and bradykinin may further produce parasympathetic reflexes which partly explains their bronchoconstriction effect. Antimuscarinic bronchodilators are non-selective antagonists of cholinergic muscarinic receptors. Their effect on airway obstruction is mainly due both to M3 muscarinic receptor antagonism in the airway smooth muscle cells, with consequent bronchodilation, and to M3 muscarinic receptor antagonism in the cells of the submucosal glands, with a reduction in the basal and stimulated cholinergic parasympathetic activity with consequent reduction in airway obstruction. Unlike atropine, antimuscarinic bronchodilators do not have an inhibitory effect on mucociliary clearance. The reason for this difference is not known. The effectiveness of antimuscarinic drugs depends on the role that cholinergic vagal tone has in the pathophysiology of bronchial obstruction. Antimuscarinic bronchodilators are generally considered to be more effective for COPD than for asthma. This could be partly due to the different pathophysiological role of the parasympathetic vagal system in these two diseases. The aerosol inhalation of ipratropium has a maximum effect 30–60 minutes after administration; its duration of action is 3 to 6 hours, making administration of the drug necessary 3 to 4 times a day. Ipratropium generally produces the same moderate bronchodilation as obtained with maximum doses of β2-agonists (CitationUndem and Lichenstein 2001). Oxitropium produces similar pharmacological effects to ipratropium and can be administered twice a day. Tiotropium bromide, a new long-acting anticholinergic drug, which can be administered just once a day, has similar or greater efficacy compared with other bronchodilators (CitationCalverley 2000) and is useful in combination with formoterol for COPD treatment (Citationvan Noord et al 2006). A randomized, prospective, double-blind, placebo-controlled, multicenter trial to assess whether adding salmeterol or salmeterol–fluticasone to chronic therapy with tiotropium would provide additional clinical benefit to patients with moderate to severe COPD is currently being carried out (CitationAaron et al 2004). Tiotropium improves sleeping arterial oxygen saturation (CitationMcNicholas et al 2004) and, in combination with pulmonary rehabilitation, exercise tolerance in patients with COPD (CitationCasaburi et al 2005). Tiotropium bromide is indicated in the maintenance treatment of COPD, but it is not effective in relieving acute bronchospasms. However, as is the case with asthmatic patients, some authors raised doubts about chronic administration of bronchodilators which could be associated with worsening COPD (Citationvan Schayck et al 1991). Because of their negligible absorption, antimuscarinics rarely cause systemic side effects and are generally well tolerated. Possible side effects include dry mouth, nausea, constipation, and headache (CitationRS 2003). Antimuscarinic bronchodilators should be administered with caution in glaucoma, benign prostatic hypertrophy and urinary obstruction (CitationRS 2003; CitationRennard 2004). Cases of acute angle-closure glaucoma have been reported with nebulized ipratropium, particularly when given with nebulized salbutamol.
Theophylline
Theophylline, a methylxanthine, is one of the least expensive bronchodilators. Given its very low solubility in water, theophylline is administered intravenously as aminophylline. Aminophylline is a theophylline and ethylenediamine mixture, which is 20 times more soluble than theophylline alone. The bronchodilatory effect of theophylline is due both to relatively non-selective inhibition of cyclic neucleotide phosphodiesterases and to competitive antagonism of adenosine receptors (CitationBarnes 2003). At least eleven phosphodiesterase isozymes have been identified (CitationSoderling and Beavo 2000). The inhibition of phosphodiesterase isozymes type III and IV causes relaxation of the airway smooth muscle cells in vitro (CitationTorphy et al 1993). Selective phosphodiesterase-4 inhibitors for COPD are currently in clinical trials (CitationLipworth 2005). Adenosine receptor antagonism also contributes to the brochodilatory effect of theophylline (CitationFeoktistov et al 1998). In addition to bronchodilation, theophylline may have anti-inflammatory effects at lower plasma concentrations in the respiratory tract as shown by the reduction both in the number of neutrophils and in the interleukin-8 concentration in induced sputum from COPD patients, where the same effects cannot be obtained after inhalation of high glucocorticoid doses (CitationBarnes 2003). In vitro studies have shown that low doses of theophylline may enhance the anti-inflammatory effect of corticosteroids and antagonize the “resistance” to corticosteroids caused by smoking in COPD patients by activating histone deacetylases (CitationBarnes 2003, Citation2006).
Theophylline is generally administered orally in conventional form or, more frequently, through sustained-release preparations or intravenously (like aminophylline) (CitationUndem and Lichtenstein 2001). Sustained-release preparations are available for administration every 8, 12, or 24 hours. Intravenous administration of theophylline must be given very slowly, over at least a 20 minute period (CitationRS 2003), because of the risk of serious toxic effects such as arrhythmias and convulsions. Inhalation of theophylline is not effective and intramuscular injection is not possible because of its irritant effect at the site of injection. Absorption of theophylline from conventional formulations is fast and complete with a plasma concentration peak within two hours of administration. Absorption of theophylline from sustained-release preparations has a large interindividual variability, for this reason, dosage must be changed to suit the individual patient. Theophylline is mainly eliminated by hepatic metabolism (less than 15% is excreted in the urine unchanged) (CitationUndem and Lichtenstein 2001). There is wide variation in the rate of elimination of theophylline. The half-life of elimination is on average 8 to 9 hours in adults and about 3.5 hours in children (CitationUndem and Lichtenstein 2001). The half-life is increased in heart or liver failure, viral infections, in the elderly and with concomitant administration of some drugs such as cimetidine, ciprofloxacin, erythromycin, fluvoxamine, and oral contraceptives. The half-life of elimination of theophylline is decreased in smokers and in chronic alcoholics, and with concomitant administration of drugs such as phenytoin, carbamazepine, rifampicin, and barbiturates (CitationRS 2003). In addition to bronchodilation, theophylline may increase cardiac contractility, have a psychostimulant or diuretic effect, and increase diaphragmic contractility. A possible therapeutic importance for COPD has been attributed to this last effect. However, theophylline has some disadvantages including its low therapeutic index which limits its administration. For this reason, and because of the large interindividual variability in its bioavailability, therapeutic monitoring of plasma theophylline concentrations is required. This involves taking regular blood samples after the theophylline concentrations reach a steady-state to ensure that the plasma theophylline concentration is within the therapeutic range for plasma theophylline concentrations for which the majority of patients obtain therapeutic effects without toxic effects, even though side effects are found with therapeutic concentrations. The therapeutic range for theophylline is between 10–20 μg/ml. Toxic side effects are associated with theophylline concentrations of more than 25 μg/ml. The risk of serious toxic effects, such as arrhythmias and convulsions, is high with theophylline concentrations of more than 40 μg/ml. At therapeutic plasma concentrations the main side effects of theophylline include anorexia, nausea, vomiting, insomnia, agitation, palpitation, and hypotension (CitationUndem and Lichtenstein 2001).
Glucocorticoids
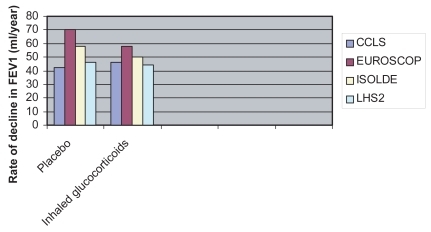
Inhaled glucocorticoids currently available include beclomethasone dipropionate, budesonide, flunisolide, fluticasone propionate and triamcinolone acetonide. There are no significant differences between these drugs in terms of their efficacy and tolerability (CitationUndem and Lichtenstein 2001; CitationRS 2003). Mometasone and ciclesonide, two new glucocorticoids in advanced clinical trials, seem to have a longer duration of action with the possibility of once a day administration. Ciclesonide is a prodrug which is activated by a pulmonary esterase, with the possibility of increased selectivity of action and reduced systemic side effects resulting from glucocorticoid absorption. Glucocorticoids do not cause relaxation of the airway smooth muscle and therefore have no effect on acute bronchoconstriction. Glucocorticoids bind to specific cytoplasmic receptor proteins, which, in turn bind to regulatory proteins such as heat shock proteins and an immunophyllin (CitationUndem and Lichtenstein 2001). The glucocorticoid–receptor interaction causes a receptor conformational change which leads to regulatory protein detachment, dimerization of the glucocorticoid receptor complexes and translocation to the nucleus where the drug-receptor complex binds to specific regulatory DNA sequences (glucocorticoid response elements, GRE), which modulate the expression of the adjacent genes. The time required for gene expression and protein synthesis explains the delayed effect of glucocorticoids which generally occurs several hours after administration. The anti-inflammatory mechanisms of action of glucocorticoids are largely due to the inhibition of gene expression which encodes for proinflammatory cytokines in the airway inflammatory cells (CitationUndem and Lichtenstein 2001). Part of the anti-inflammatory effect of glucocorticoids may be due to the induction of lipocortin. Lipocortin is a protein which inhibits phospholipase A2. Phospholipase A2 enzymes cause cleavage of arachidonic acid from phospholipids in the plasma membrane. The arachidonic acid is the substrate for the synthesis of leukotrienes, prostaglandins, and thromboxane A2, which are important inflammatory mediators. Although glucocorticoids are generally effective in asthma, the anti-inflammatory effect of glucocorticoids in COPD patients remains controversial and seems to be very limited (CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000; CitationLHSRG 2000). This seems to reflect a pathophysiological difference between COPD and asthma. It has been suggested, on the basis of in vitro studies, that oxidative stress and cigarette smoke, which both play an important role in the pathophysiology of COPD, induce resistance to the action of glucocorticoids through mechanisms which involve the acetylation of histones. Histones are nucleic proteins that are important regulators in gene expression (CitationBarnes 2000, Citation2003). Furthermore, the variable effects of glucocorticoids on airway inflammation may be due to the heterogeneity of the disease and the limited reproducibility of markers of inflammation (Keatings et al 1997; CitationConfalonieri et al 1998). What is certain is that glucocorticoids do not modify the natural history of COPD, as measured by the rate of decline in FEV1 (). Four large randomized placebo-controlled multicenter clinical trials (European Respiratory Society study on chronic obstructive pulmonary disease [EUROSCOP], Copenhagen City Lung Study, Inhaled Steroids in Obstructive Lung Disease in Europe [ISOLDE], and Lung Health Study 2 [CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000; CitationLHSRG 2000]) all found that inhaled glucocorticoids had no significant effects on the progressive decline in FEV1 in patients with mild and moderate to severe COPD. One of these studies (CitationBurge et al 2000) in patients with more severe COPD showed a reduction in the frequency of exacerbations (from 1.33 to 0.99 per year, a reduction of 25%). Other clinical trials with both fluticasone and budesonide showed that inhaled glucocorticoids reduce COPD exacerbation rate (CitationCalverley, Pauwels, et al 2003; CitationJones et al 2003; CitationSzafranski et al 2003), although the mechanism for this effect is currently unknown. However, an earlier study on a smaller sample showed a reduction in severity, but not in the frequency of exacerbations with inhaled glucocorticoids (CitationPaggiaro et al 1998). From these clinical trials it is concluded that inhaled glucocorticoids should only be administered to patients with severe to very severe COPD (post-bronchodilator FEV1 ≤50% of the predicted value) with frequent exacerbations requiring treatment with antibiotics or oral glucocorticoids (CitationGICOPD 2001). The dose of glucocorticoids required to reduce the frequency of exacerbations in these patients with COPD is not known (CitationMacNee and Calverley 2003).
Figure 1 Rate of decline in forced expiratory volume in one second (FEV1) after administration of placebo or inhaled glucocorticoids in four randomized controlled multicenter clinical trials: Copenhagen City Lung Study (CCLS), European Respiratory Society study On Chronic Obstructive Pulmonary disease (EUROSCOP), Inhaled Steroids in Obstructive Lung Disease (ISOLDE), and Lung Health Study 2 (LHS2). Inhaled glucocorticoids did not have a significant effect on the rate of decline in FEV1 in any of the clinical trials.
Improvement in airflow in patients with COPD after chronic treatment with inhaled glucocorticoids is very modest (CitationPauwels et al 1999; CitationBurge et al 2000). However, some studies reported further improvement in airflow when glucocorticoids are added to long-acting β2-agonist bronchodilators (CitationMahler et al 2002; CitationCalverley, Pauwels, et al 2003; CitationSzafranski et al 2003).
One study reported that withdrawal of fluticasone propionate from combined salmeterol–fluticasone treatment in patients with COPD resulted in acute and persistent deterioration in lung function and dyspnoea and in increase in mild exacerbations (CitationWouters et al 2005). However, median percentage predicted FEV1at start of the 3 month run-in period was about 50% implying that a considerable number of patients included in this study had moderate COPD (FEV1 ≥50% of the predicted value) for which inhaled glucocorticoids are not indicated (CitationGICOPD 2001). On the other hand, all the patients in this study had a history of at least 2 COPD exacerbations in the last year treated with oral glucocorticoids and/or antibiotics which suggest chronic treatment with inhaled glucocorticoids (CitationGICOPD 2001).
The effect of inhaled glucocorticoids on mortality for COPD is controversial and mainly based on retrospective data (CitationSin and Tu 2001; CitationSoriano et al 2002; CitationFan et al 2003; CitationSuissa 2003; CitationSin et al 2005). Prospective studies assessing the effect of inhaled glucocorticoids and long-acting β2-agonists, alone or in combination, on mortality in patients with COPD are in progress (CitationRennard 2004; CitationVestbo 2004).
Oral glucocorticoids are not generally recommended for the treatment of stable COPD due to their limited therapeutic effects and numerous systemic side effects.
Administration of inhaled glucocorticoids for patients with stable COPD must again be carefully considered because of possible side effects. Oropharyngeal candidiasis, due to the local immunosuppressive effect of glucocorticoids, is a common side effect. Oropharyngeal candidiasis is avoidable to a certain extent by using large volume spacers or rinsing the oropharynx with water after drug delivery. Even when delivered locally, such as by aerosolized inhaler, glucocorticoids may be partly absorbed with consequent systemic side effects due to their high lipid solubility. Particularly notable side effects include suppression of endogenous cortisol synthesis by inhibiting the hypothalamic–pituitary–adrenal axis, particularly at doses higher than 1500 μg/day (CitationUndem and Lichtenstein 2001); the possibility of osteoporosis even at doses of 500 μg/day (CitationUndem and Lichtenstein 2001); a slightly increased risk of glaucoma, particularly during long-term treatment at high doses, and of cataract (CitationUndem and Lichtenstein 2001). Glucocorticoids, particularly at high doses, should be inhaled using a large-volume spacer which increases the profound deposition of the drug and reduces drug deposition in the oropharynx, with a consequent reduction in the incidence of candidiasis. However, in the absence of proven efficacy and because of possible side effects, including systemic side effects, and high costs, there is no rational pharmacological basis for the use of inhaled glucocorticoids for all patients with stable COPD, and their use should be limited just to the cases previously described (CitationGICOPD 2001).
Clinical considerations
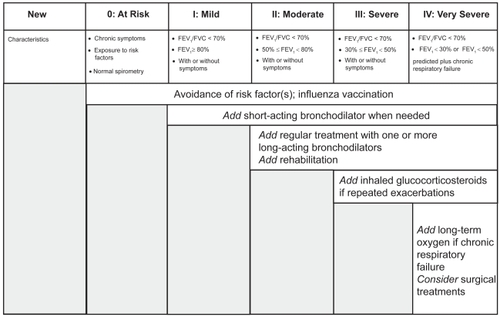
The choice between β2-agonists, anticholinergic antimuscarinic agents, theophylline or drug combinations depends on individual patient response to the treatment. Pharmacological therapy at each stage of stable COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines is shown in (). Bronchodilator combinations may improve drug efficacy and reduce side effects, compared with an increase in the dosage of a single bronchodilator (CitationCOMBIVENT 1994).
Figure 2 Pharmacological treatment of stable COPD based on GOLD guidelines. Adapted from CitationGICOPD (2001).
For patients with COPD, long-acting β2-agonists delivered by inhalation, such as salmeterol and formoterol, significantly improve symptoms, exercise tolerance and general health status (CitationCazzola et al 1995; CitationUlrik 1995; CitationBoyd et al 1997). Both β2-agonists and long-acting anticholinergics reduce the frequency of exacerbations in COPD (CitationRennard et al 2001; CitationCasaburi et al 2002) although the mechanism(s) of this effect is currently unknown. Possible anti-inflammatory effects of long-acting β2-agonists (CitationJohnson and Rennard 2001) might explain the reduced frequency of exacerbations of COPD (CitationMahler et al 1999; CitationRennard et al 2001; CitationCalverley, Pauwels, et al 2003). These bronchodilators do not seem to cause tachyphylaxis, namely rapid reduction in the intensity of the pharmacological effect due to continued or repeated administration, and are well tolerated. Although bronchodilator treatment in COPD is generally started with a single agent, the use bronchodilators in combination is suggested in the current guidelines as this strategy can result in improved bronchodilator and clinical effect (CitationGICOPD 2001; CitationCelli and McNee 2004). However, the interindividual variability of the bronchodilating effect can be high. The efficacy of combining either short or long-acting β2-agonists with anticholinergics (CitationCOMBIVENT 1994; Citationvan Noord et al 2000, Citation2006), anticholinergics and theophylline (CitationBleecker and Britt 1991; CitationBellia et al 2002), and all three classes of bronchodilators is known (CitationNishimura et al 1995). A fixed inhaled combination of albuterol and ipratropium is available.
High doses of nebulized bronchodilators are still widely prescribed for severe COPD. The British Thoracic Society (BTS) guidelines on nebulizer therapy (CitationNPG 1997) recommend that a respiratory physician should evaluate the effectiveness of nebulizer therapy. Before trying chronic nebulizer therapy, it is advisable to evaluate the efficacy of high dose bronchodilator treatment delivered by metered dose inhaler (MDI) with a spacer. The administration of theophylline for stable COPD remains controversial. Theophylline causes bronchodilation in COPD (CitationMurciano et al 1989; CitationMcKay et al 1993) with a variable effect on exercise tolerance and symptoms (CitationMurciano et al 1984; CitationCooper et al 1987; CitationChrystyn 1988; CitationMulloy and McNicholas 1993). Theophylline has a slow onset of action and it is generally recommended for maintenance treatment, rather than for the rapid treatment of symptoms. In the major guidelines for the treatment of COPD, theophylline is considered a third line bronchodilator after inhaled anticholinergics and β2 agonists (CitationBarnes 2006). Nevertheless, theophylline is a useful treatment in patients with severe COPD as its withdrawal leads to significant clinical worsening of the disease (Kirsten DK 1993). The most recent and largely selective phosphodiesterase inhibitors, such as inhibitors of phosphodiesterase type 4 isoenzyme, slightly improve lung function in COPD (CitationCompton et al 2001) and reduce the frequency of exacerbations (CitationEdelson et al 2001; CitationRabe et al 2005; CitationRennard et al 2006) (see below). However, further studies are needed to adequately evaluate the efficacy and tolerability of this new class of drugs.
Long-acting bronchodilators administered once or twice daily can improve symptoms to the same extent or more compared with chronic administration of short-acting bronchodilators. For this reason it is possible to consider the use of long-acting bronchodilators for the treatment of COPD patients requiring chronic treatment to improve symptoms. Theophylline may be recommended for patients with persistent symptoms instead of long-acting bronchodilator treatment. However, because of the disadvantages outlined above, administration of theophylline should be continued only if its efficacy on the symptoms has been demonstrated. The role of theophylline in the treatment of COPD is currently being re-evaluated based on important pharmacological evidence which has allowed the molecular mechanism of action of theophylline to be clarified (CitationBarnes 2003).
Inhaled glucocorticoids are generally prescribed for COPD without demonstration of their effectiveness. However, COPD patients generally have no, or a negligible, response to glucocorticoids (CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000; CitationLHSRG 2000; CitationRS 2003). Some patients may improve after glucocorticoid treatment, but it is likely that these patients have concomitant asthma (CitationBarnes 2000). A two-week trial of oral glucocorticoids was proposed to identify patients with a documented spirometric response, assuming that an acute response is predictive of a chronic response (GISCOPD 2001). However, the oral administration of glucocoticoids for two weeks does not generally allow identification of those patients who will respond to inhaled glucocorticoids (CitationSenderovitz et al 1999; CitationBurge et al 2000, Citation2003). Therefore, a trial of glucocorticoids is not generally recommended for COPD (GISCOPD 2001), unless required to exclude asthma (CitationBarnes 2000). Glucocorticoids do not reduce airway inflammation in COPD (CitationBarnes 2000) and various long-term studies showed that glucocorticoids are not able to reduce the progression of COPD (CitationPauwels et al 1999; CitationVestbo et al 1999; CitationBurge et al 2000; CitationLHSRG 2000). For this reason, and taking into account the risk of systemic side effects, particularly at high doses, glucocorticoids should not generally be administered for COPD (CitationBarnes 2000), though they are recommended for the treatment of exacerbations. Furthermore, inhaled glucocorticoids are recommended for symptomatic COPD patients who have a documented spirometric response to these drugs or for patients with FEV1 ≤50% of the predicted value and frequent exacerbations (two or more per year), which require antibiotics or oral glucocorticoids, according to the GOLD guidelines (GISCOPD 2001). However, these recommendations are not universally agreed (CitationBarnes 2000), as the effect of inhaled glucocorticoids at high doses on the frequency of exacerbations in patients with severe COPD is controversial (CitationPaggiaro et al 1998; CitationBurge et al 2000). Furthermore, the cost of glucocorticoids as well as their pharmacological profile, as discussed above, does not justify indiscriminate administration of inhaled glucocorticoids in COPD. Fixed combinations of inhaled glucocorticoids and long-acting β2-agonists, namely fluticasone–salmeterol (CitationDransfiel and Bailey 2004) and budesonide–formoterol, were approved for use in COPD in the US and other countries based on clinical trials showing their effectiveness for health status and exacerbations of COPD (CitationCalverley, Boonsawat, et al 2003; CitationCalverley, Pauwels, et al 2003; CitationSzafranski et al 2003). However, there were conflicting results when the different combination therapies were compared with the mono-component alone (CitationNannini et al 2004). More studies to definitively establish the effects of combination therapy in a single inhaler are required, including double-dummy trials on the comparative effects with separate adiministration of the two drugs (CitationNannini et al 2004).
Other drugs
Vaccines
Vaccination can reduce severe complications and mortality from influenza in elderly patients, including those with COPD. Vaccination is recommended once in the autumn or, once in the autumn and once in the winter each year (CitationNichol et al 1994; CitationHak et al 1998). Pneumococcal vaccines have been used in COPD patients and can reduce complications of pneumonia in elderly patients, but there is no evidence to support its general use in COPD patients.
Antibiotics
Numerous large-scale controlled studies have shown that prophylactic antibiotics and chronic antibiotic administration have no effect on the frequency of exacerbations in COPD (CitationMacNee and Calverley 2003). Prophylactic antibiotics are also ineffective in winter (CitationMacNee and Calverley 2003). Current available data does not support the effectiveness of prophylactic antibiotics against bacterial infections or exacerbations of COPD.
Mucolytic agents
Mucolytic agents such as ambroxol, erdosteine, carbocysteine, and iodinated glycerol produce a reduction in the frequency of exacerbations in chronic bronchitis compared with a placebo (CitationPoole and Black 2000). Since these studies are of relatively short duration, between two to six months, and in patients with mild to moderate COPD (FEV1 >50% of the predicted value), the systematic administration of mucolytic drugs in COPD is not currently recommended.
Antioxidants
Oxidant stress plays an important role in the pathophysiology of COPD. Therefore, administration of antioxidant agents is an interesting treatment strategy. Although antioxidant and mucolytic drug N-acetylcysteine has been shown to reduce the frequency of exacerbations of COPD in most clinical trials (CitationGrandjean et al 2000), a recent 3 year randomized placebo-controlled multicenter trial has shown that N-acetylcysteine is ineffective at prevention of deterioration in lung function and prevention of exacerbations in patients with COPD (CitationDecramer et al 2005). On the basis of current data, N-acetylcysteine is not recommended for COPD (MacNee and Calverley). The details of various antioxidants trials are discussed elsewhere (CitationRahman 2006).
Drugs in phase III of clinical development
Phosphodiesterase-4 inhibitors
Selective inhibitors of phosphodiesterase (PDE) type 4 increase intracellular concentrations of cyclic AMP by inhibiting its breakdown with consequent airway smooth muscle relaxation and anti-inflammatory effects on effector cells involved in the pathophysiology of COPD (CitationLipworth 2005). PDE4 enzymes include at least four isoenzymes (PDE4A, PDE4B, PDE4C, and PDE4D) (CitationSoderling and Beavo 2000). Inhibition of PDE4B is mainly responsible for the anti-inflammatory effects of PDE4 inhibitors, whereas inhibition of PDE4D causes nausea which is one of the main adverse effects of these drugs (CitationLipworth 2005). Cilomilast and roflumilast, the two main orally active PDE4 inhibitors, are in the late phase III of clinical development for COPD (CitationLipworth 2005). Placebo-controlled studies for up to 6 months with cilomilast 15 mg twice daily and roflumilast 500 mg once daily have shown small, although significant, improvements in lung function tests in patients with COPD (CitationCompton et al 2001; CitationRabe et al 2005). PDE4 inhibitors also reduce the rate of COPD exacerbations compared with placebo (CitationRabe et al 2005; CitationRennard et al 2006). The effect of PDE4 inhibitors on health status in patients with COPD is variable possibly due to differences in the duration of treatment (CitationCompton et al 2001; CitationRabe et al 2005; CitationRennard et al 2006). Cilomilast at a dose of 15 mg twice daily for 12 weeks reduces CD4+ T-lymphocyte subsets and CD68+ macrophages in bronchial biopsies from patients with COPD (CitationGamble et al 2003). However, whether these changes in airway inflammation are related to clinical outcomes in patients with COPD is unknown (CitationLipworth 2005). The main adverse effects of PDE4 inhibitors are nausea, diarrhea, and abdominal pain (CitationLipworth 2005). Roflumilast is more tolerated than cilomilast as it has similar selectivity for PDE4 isoenzymes, whereas cilomilast is 10 times more selective for PDE4D than other isoenzymes (CitationManning et al 1999; CitationHatzelmann and Schudt 2001). Long-term studies are required to definitively establish the effects of PDE4 inhibitors on clinical outcomes, including lung function, exacerbation rate, and health status in patients with COPD.
Treatment of exacerbations of COPD
According to a recent definition, exacerbations of COPD are “a variation in symptoms above the normal day to day variation which causes a change in a patient’s medication” (CitationRodriguez-Roisin 2000). Exacerbations of COPD worsen general health status (CitationSeemungal et al 1998) and are considerably expensive. The number of exacerbations is related to disease severity. Patients with moderate to severe COPD have one or two exacerbations per year (CitationSeemungal et al 1998; CitationBurge et al 2000). Many factors determine the type of treatment used and where the treatment is carried out, for example, in an outpatient or hospital setting.
The severity of the exacerbations is related to the severity of the disease. In patients with mild COPD, exacerbations are associated with increased breathlessness accompanied by cough and sputum production. They may often be managed out of hospital. Exacerbations of severe COPD are often associated with respiratory failure which may be fatal and require hospital admission on a general ward or intensive care unit. Exacerbations are caused by bacterial or viral infections in around 50% of cases (CitationMonso et al 1995; CitationSoler et al 1998; CitationSeemungal et al 2000). Other factors such as air pollution and temperature may cause exacerbations (CitationMacNee 2002). However, it is not possible to identify a cause in about one third of exacerbations of COPD (CitationConnors et al 1996). One of the main problems is the decision to treat exacerbations of COPD at home or in hospital. This decision is influenced by the severity of symptoms, the severity of COPD, and whether hospital admission is required. Recent trials have shown that about 30% of patients admitted to hospital with exacerbations of COPD could be adequately treated at home with immediate or early discharge and nurse-led home care (CitationSkwarska et al 2000; CitationCotton et al 2000; CitationDavies et al 1999, Citation2000). Patients prefer this form of treatment, which has been widely adapted to facilitate the discharge of those patients initially requiring hospitalization (CitationCotton et al 2000; CitationOjoo et al 2002). However, a clinical trial showed that home care did not improve the health of the patients whilst increasing costs compared with admission to hospital (CitationShepperd, Harwood, Gray, et al 1998; CitationShepperd, Harwood, Jenkinson, et al 1998).
Home treatment
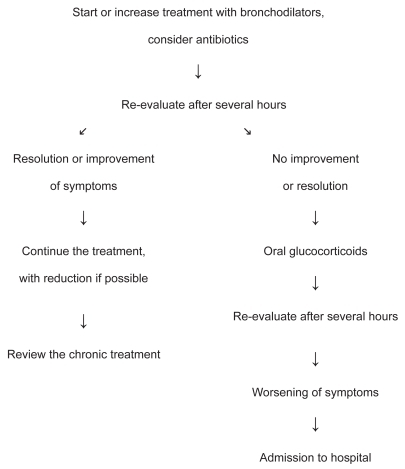
The algorithm shown in , which is based on a stepwise therapeutic approach (CitationSachs 1981; CitationATS 1995; CitationSiafakas et al 1995; CitationCelli 1996; CitationShepperd, Harwood, Jenkinson, et al 1998), may be used in the home treatment of exacerbations of COPD using bronchodilators, if necessary delivered by nebulizer, or antibiotics if symptoms indicate a bacterial infection, and oral glucocorticoids in moderate to severe exacerbations. The dose and/or frequency of bronchodilator delivery must be increased or bronchodilator treatment must be started if these drugs have not been used previously. Administration of high doses of antimuscarinics on demand delivered for a number of days using a nebulizer may be useful, even though chronic nebulizer treatment is not generally recommended after an exacerbation (CitationGICOPD 2001). Systemic glucocorticoids are effective for exacerbations of COPD as they facilitate clinical improvement and lung function (CitationThompson et al 1996; CitationNiewoehner et al 1999; CitationCotton et al 2000) and are recommended for patients with moderate to severe exacerbations of COPD when the baseline FEV1is less than 50% of the predicted value (CitationGICOPD 2001). The optimum dose and duration of treatment is not known, but administration of oral prednisolone at 40mg per day for ten days is generally effective (CitationGICOPD 2001). Nebulized budesonide is as effective as oral glucocorticoids in improving lung function. However, it is not clear whether the increased costs of inhaled glucocorticoids are justified in all cases (CitationMaltais et al 2002). Antibiotic treatment, which does not generally require parenteral administration, is recommended if two or more of the following symptoms are present: increasing dyspnoea, increasing sputum purulence, or increasing sputum volume (CitationAnthonisen et al 1987). Antibiotics should be chosen which are effective against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. These are the bacteria that most frequently cause exacerbations of COPD. However, the choice of antibiotics must be based on the antibiogram (CitationGICOPD 2001).
Figure 3 Algorithm for the home treatment of an exacerbation of COPD (adapted from Global Initiative for Chronic Obstructive Lung Disease. 2001. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. National Institutes of Health. National Heart, Lung, and Blood Institute. Publication number 2701).
Hospital treatment
The criteria for necessary hospital treatment, whether during admission to the emergency department, or during hospitalization, for exacerbations of COPD are described in the GOLD guidelines (CitationGICOPD 2001). Hospital treatment is increasingly focused on the management of lung function and associated complications, particularly considering the increased number of early discharges (CitationMacNee and Calverley 2003). Firstly, it is necessary to establish whether the exacerbation of COPD may be life-threatening and if this is the case the patients must be admitted to intensive care. The risk of mortality from exacerbations of COPD is connected with the level of respiratory acidosis, the presence of concomitant diseases, and the need for assisted ventilation (CitationConnors et al 1996). In the absence of these characteristics, the risk of mortality is low, even though patients with severe COPD require hospitalization (CitationGICOPD 2001). In these cases the effectiveness of home treatment is poor (CitationShepperd, Harwood, Gray, et al 1998). However, the results are better if home treatment follows a careful assessment in the emergency department and is combined with adequate doctor- or nurse-administered home care (CitationGravil et al 1998). Controlled oxygen therapy is the cornerstone of hospital treatment of exacerbations of COPD (CitationGICOPD 2001). It is relatively easy to achieve adequate oxygenation (PaO2 > 60 mmHg or SaO2 > 90%) in uncomplicated exacerbations, but CO2 retention can occur (CitationGICOPD 2001). β2-agonists are the main bronchodilators used for the treatment of exacerbations of COPD (CitationSiafakas et al 1995; CitationCelli 1996; CitationBTS 1997). If rapid bronchodilation does not occur with these drugs, an antimuscarinic agent may be administered, even if the effectiveness of this combination is controversial (CitationFernandez et al 1994; CitationMoayyedi et al 1995). The role of theophylline in the treatment of exacerbations of COPD is also controversial (CitationGICOPD 2001). Theophylline leads to modest improvements in lung function without gas exchange deterioration (CitationBarbera et al 1992; CitationMahon et al 1999). Administration of intravenous theophylline can be considered in more severe exacerbations (CitationGICOPD 2001) although the available data do not support the use of methylxanthines for the treatment of exacerbations of COPD (Barr et al 2003). However, this requires close monitoring of the plasma concentrations to avoid the occurrence of dose-dependent toxic effects (CitationLloberes et al 1988; CitationEmerman et al 1990; CitationBarbera et al 1992). Oral or intravenous glucocorticoids are recommended with bronchodilator therapy and, if necessary, antibiotics and oxygen therapy, for treatment in hospital of exacerbations of COPD (CitationThompson et al 1996; CitationDavies et al 1999; CitationNiewoehner et al 1999; CitationGICOPD 2001). The optimum dose of glucocorticoids is not known, but high doses increase the risk of significant side effects. Generally 30 mg to 40 mg of oral prednisolone is administered for 10 to 14 days (CitationGICOPD 2001). Prolonged treatment does not improve efficacy, while increasing the risk of side effects. Antibiotics are only effective when exacerbations also occur with sputum purulence and increased sputum volume and/or fever, indicating a bacterial infection (CitationAnthonisen et al 1987). Antibiotics must be effective against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. These are the bacterial microorganisms most frequently involved in exacerbations of COPD. However, the choice of antibiotics must be based on the antibiogram. Toxicity due to excessive oxygen therapy in the emergency department is still a prevalent problem which could be resolved by increasing patient awareness of the risk (CitationPlant et al 2000a). In cases of severe exacerbations of COPD (stage III) mechanical ventilation is required which may be invasive (conventional) or non-invasive with the aim of reducing morbidity and mortality and improving symptoms. Non-invasive positive pressure ventilation (NIPPV) is an effective alternative to conventional mechanical ventilation in the intensive care unit and is even applicable in the general ward with specialized nursing staff (CitationBrochard et al 1995; CitationPlant et al 2000a). NIPPV reduces patient morbidity and length of stay in hospital by preventing the onset of nosocomial pneumonia, but it is less effective than intermittent positive pressure ventilation in patients with a pH persistently <7.30 (CitationPlant et al 2000b).
Conclusions
Pharmacological treatment of COPD is currently symptomatic and is based on inhaled bronchodilators such as anticholinergics, selective β2-adrenergic agonists and theophylline. In the absence of proven efficacy and due to higher costs and side effects, glucocorticoids are not generally recommended for stable COPD, though they are effective in the treatment of exacerbations. In the absence of drugs which reduce progressive decline in lung function, which is the hallmark of this disease, cessation of cigarette smoking, the main risk factor for COPD, is the most effective measure. Numerous studies are being carried out to define the pathophysiology of COPD, about which still relatively little is known. It is hoped that these studies will allow new and effective drugs to be discovered and developed, as the current pharmacological treatment of COPD is inadequate.
Acknowledgments
This report has been partly produced using faculty funds 2005–2006 from the Catholic University of the Sacred Heart in Rome.
References
- AaronSDVandemheenKFergussonD2004The Canadian Optimal Therapy for COPD trial: design, organization and patient recruitmentCan Respir J11581515611808
- [ATS] American Thoracic Society1995Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med152S771217582322
- AnthonisenNRConnettJEKileyJP1994Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA27214975057966841
- AnthonisenNRManfredaJWarrenCP1987Antibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med1061962043492164
- BarberaJAReyesARocaJ1992Effect of intravenously administered aminophylline on ventilation/perfusion inequality during recovery from exacerbations of chronic obstructive pulmonary diseaseAm Rev Respir Dis1451328331595998
- BarnesPJ1999Effect of β-agonists on inflammatory cellsJ Allergy Clin Immunol104S101710452784
- BarnesPJ2000Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med161342410673166
- BarnesPJ2003Theophylline: new perspectives for an old drugAm J Respir Crit Care Med1678131812623857
- BarnesPJ2006Theophylline for COPDThorax61742416936233
- BelliaVForesiABiancoS2002Efficacy and safety of oxitropium bromide, theophylline and their combination in COPD patients: a double-blind, randomised, multicentre studyRespir Med96881912418585
- BelmanMJBotnickWCShinJW1996Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med153967758630581
- BergerRSmithD1988Effect of inhaled metaproterenol on exercise performance in patients with stable “fixed” airway obstructionAm Rev Respir Dis13862493202416
- BleeckerERBrittEJ1991Acute bronchodilating effects of ipratropium bromide and theophylline in chronic obstructive pulmonary diseaseAm J Med91247
- BoydGMoriceAHPounsfordJC1997An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD)Eur Respir J10815219150318
- BrochardLMancheboJWysockiM1995Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary diseaseN Engl J Med333817227651472
- [BTS] The COPD Guidelines Group of the Standards of Care Committee of the BTS1997BTS guidelines for the management of chronic obstructive pulmonary diseaseThorax52Suppl 5S128
- BurgePSCalverleyPMJonesPW2000Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ3201297130310807619
- BurgePSCalverleyPMJonesPW2003Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE studyThorax58654812885977
- CalverleyPM2000The future for tiotropiumChest1172 Suppl67S69S10673479
- CalverleyPMBoonsawatWCsekeZ2003Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J229121914680078
- CalverleyPPauwelsRVestboJ2003Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised control trialLancet3614495612583942
- CasaburiRKukafkaDCooperCB2005Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest1278091715764761
- CasaburiRMahlerDAJonesPW2002A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J192172411866001
- CazzolaMMateraMGSantangeloG1995Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response studyRespir Med89357627638371
- CelliBR1996Current thoughts regarding treatment of chronic obstructive pulmonary diseaseMed Clin North Am805896098637305
- CelliBRMcNeeW2004Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J239324615219010
- ChrystynHMulleyBAPeakeMD1988Dose response relation to oral theophylline in severe chronic obstructive airways diseaseBMJ2971506103147048
- COMBIVENT Inhalation Aerosol Study Group1994In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter TrialChest1051411198181328
- ComptonCHGubbJNiemanRInternational Study Group2001Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging studyLancet3582657011498212
- ConfalonieriMMainardiEDella PortaR1998Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary diseaseThorax5358359797758
- ConnorsAFJrDawsonNVThomasC1996Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments)Am J Respir Crit Care Med154959678887592
- CooperCBDavidsonACCameronIR1987Aminophylline, respiratory muscle strength and exercise tolerance in chronic obstructive airway diseaseBull Eur Physiopathol Respir2315223297214
- CottonMMBucknallCEDaggKD2000Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trialThorax55902611050257
- CuvelierAMuirJFBenhamouD2002Dry powder ipratropium bromide is as safe and effective as metered-dose inhaler formulation: a cumulative dose-response study in chronic obstructive pulmonary disease patientsRespir Care471596611812272
- DaviesLAngusRMCalverleyPM1999Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trialLancet3544566010465169
- DaviesLWilkinsonMBonnerS2000“Hospital at home” versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial”BMJ3211265811082090
- DecramerMRutten-van MolkenMDekhuijzenPN2005Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trialLancet36515526015866309
- DransfieldMTBaileyWC2004Fluticasone propionate/salmeterol for the treatment of chronic-obstructive pulmonary diseaseExpert Opin Pharmacother518152615264996
- EdelsonJDComptonCNiemanR2001Cilomilast Ariflo a potent, selective phosphodiesterase-4 inhibitor for treatment of patient with chronic obstructive pulmonary disease: results of 6-month trialAm J Respir Crit Care Med163A771
- EmermanCLConnorsAFLukensTW1990Theophylline concentrations in patients with acute exacerbations of COPDAm J Emerg Med8289922363749
- FanVSBrysonCLCurtisJR2003Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalisation: time-dependent analysisAm J Respir Crit Care Med16814889414525798
- FeoktistovIPolosaRHolgateST1998Adenosine A2B receptors: a novel therapeutic target in asthma?Trends Pharmacol Sci19148539612090
- FernandezAMunozJde la CalleB1994Comparison of one versus two bronchodilators in ventilated COPD patientsIntensive Care Med201992028014286
- GambleEGrootendorstDCBrightlingCE2003Anti-inflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1689768212816740
- [GICOPD] Global Initiative for Chronic Obstructive Lung Disease2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseNational Institutes of Health, National Heart, Lung, and Blood Institute Publication number 2701
- GrandjeanEMBerthetPRuffmannR2000Efficacy and oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trialsClin Ther222092110743980
- GravilJHAl-RawasOACottonMM1998Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment serviceLancet351185359652670
- GrossNJPettyTLFriedmanM1989Dose response to ipratropium as a nebulized solution in patients with chronic obstructive pulmonary disease. A three-center studyAm Rev Respir Dis1391188912523681
- HakEvan EssenGABuskensE1998Is immunising all patients with chronic lung disease in the community against influenza cost effective? Evidence from a general practice based clinical prospective cohort study in Utrecht, The NetherlandsJ Epidemiol Community Health5212059578860
- HatzelmannASchudtC2001Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitroJ Pharmacol Exp Ther2972677911259554
- HayJGStonePCarterJ1992Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary diseaseEur Respir J5659641628722
- HughesJMSealeJPTempleDM1983Effect of fenoterol on immunological release of leukotrienes and histamine from human lung in vitro: selective antagonism by beta-adrenoceptor antagonistsEur J Pharmacol95239456197312
- IkedaANishimuraKKoyamaH1999Comparison of the bronchodilator effects of salbutamol delivered via a metered-dose inhaler with spacer, a dry-powder inhaler, and a jet nebulizer in patients with chronic obstructive pulmonary diseaseRespiration661192310202314
- JohnsonMColemanRABusseWWHolgateST1995Mechanisms of action of adrenoceptor β2 agonistsAsthma and rhinitisCambridgeBalckwell Sci Pub127895
- JohnsonMRennardS2001Alternative mechanisms for long-acting β2-agonists in COPDChest1202587011451847
- JonesPWWillitsLRBurgePS2003Disease severity and effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbationsEur Respir J21687312570111
- JorenbyDELeischowSJNidesMA1999A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessationN Engl J Med3406859110053177
- KeatingsVMJatakanonAWorsdellYMEffects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPDAm J Respir Crit Care Med15554289032192
- KirstenDKWegnerREJorresRA1994Effects of theophylline withdrawal in severe chronic obstructive pulmonary diseaseChest104110178404175
- KumeHHallIPWashabauRJ1994Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanismsJ Clin Invest9337197904270
- LipworthBJ2005Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary diseaseLancet3651677515639300
- [LHSRG] The Lung Health Study Research Group II2000Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary diseaseN Engl J Med3431902911136260
- LloberesPRamisLMontserratJM1988Effect of three different bronchodilators during an exacerbation of chronic obstructive pulmonary diseaseEur Respir J153692971565
- MacNeeW2002Acute exacerbations of COPD. Consensus conference on management of chronic obstructive pulmonary diseaseJ R Coll Physicians Edinb321626
- MacNeeWCalverleyPM2003Chronic obstructive pulmonary disease. 7: Management of COPDThorax58261512612309
- MahlerDADonohueJFBarbeeRA1999Efficacy of salmeterol xinafoate in the treatment of COPDChest1159576510208192
- MahlerDAWirePHorstmanD2002Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16610849112379552
- MahonJLLaupacisAHodderRV1999Theophylline for irreversible chronic airflow limitation: a randomized study comparing n of 1 trials to standard practiceChest11538489925061
- MaltaisFOstinelliJBourbeauJ2002Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trialAm J Respir Crit Care Med16569870311874817
- ManningCDBurmanMChristensenSB1999Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4BBr J Pharmacol1281393810602317
- McKaySEHowieCAThomsonAH1993Value of theophylline treatment in patients handicapped by chronic obstructive lung diseaseThorax48227328497820
- McNicholasWTCalverleyPMLeeA2004Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPDEur Respir J238253115218993
- MoayyediPCongletonJPageRL1995Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary diseaseThorax5083477570433
- MonsoERuizJRosellA1995Bacterial infection in chronicobstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brushAm J Respir Crit Care Med1521316207551388
- MulloyEMcNicholasWT1993Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary diseaseAm Rev Respir Dis148103068214921
- MurcianoDAubierMLecocguicY1984Effects of theophylline on diaphragmatic strength and fatigue in patients with chronic obstructive pulmonary diseaseN Engl J Med311349536738652
- MurcianoDAuclairMHParienteR1989A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary diseaseN Engl J Med320152152498658
- NanniniLCatedCJLassersonTJ2004Combined corticosteroid and long acting β-agonist in one inhaler for chronic obstructive pulmonary diseaseCochrane Database Syst Rev3CD00379415266502
- NicholKLMargolisKLWuorenmaJ1994The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the communityN Engl J Med331778848065407
- NiewoehnerDEErblandMLDeupreeRH1999Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study GroupN Engl J Med3401941710379017
- NishimuraKKoyamaHIkedaA1995The additive effect of theophylline on high-dose combination of inhaled salbutamol and ipratropium bromide in stable COPDChest107718237874943
- [NPG] The Nebuliser Project Group of the British Thoracic Society Standards and Care Committee1997BTS Guidelines on current best practice for nebuliser treatmentThorax52Suppl 2S1106
- OjooJCMoonTMcGloneS2002Patients’ and carers’ preferences in two models of care for acute exacerbations of COPD: results of a randomised controlled trialThorax57167911828049
- PaggiaroPLDahleRBakranI1998Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study GroupLancet351773809519948
- PauwelsRALofdahlCGLaitinenLA1999Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary DiseaseN Engl J Med34019485310379018
- PlantPKOwenJLElliottMW2000aOne year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non-invasive ventilation and oxygen administrationThorax55550410856313
- PlantPKOwenJLElliottMW2000bEarly use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicenter randomised controlled trialLancet3551931510859037
- PoolePJBlackPN2000Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary diseaseCochrane Database Syst Rev2CD00128710796634
- RabeKFBatemanEDO’DonnellD2005Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trialLancet3665637116099292
- RahmanI2006Antioxidant therapies in COPDInt J COPD11531
- RennardSI2004Treatment of stable chronic obstructive pulmonary diseaseLancet36479180215337408
- RennardSIAndersonWZuWallackR2001Use of a long-acting inhaled β2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16310879211316640
- RennardSISchachterNStrekM2006Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4Chest129566616424413
- Rodriguez-RoisinR2000Toward a consensus definition for COPD exacerbationsChest117Suppl 2398401S10669681
- [RS] Respiratory system2003British National Formulary45th edLondonPharmaceutical Pr13165
- SachsFL1981Chronic bronchitisClin Chest Med279897030600
- SealeJP1988Whither β-adrenoceptor agonists in the treatment of asthmaProg Clin Biol Res263367772454492
- SeemungalTADonaldsonGCPaulEA1998Effect of exacerbation on quality of life in patients with chronic obstructive pulmonay diseaseAm J Respir Crit Care Med1571418229603117
- SeemungalTAHarper-OwenRBhowmikA2000Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary diseaseEur Respir J166778311106212
- SenderovitzTVestboJFrandsenJ1999Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. The Danish Society of Respiratory MedicineRespir Med937151810581660
- ShepperdSHarwoodDGrayA1998Randomised controlled trial comparing hospital at home care with inpatient hospital care. II: cost minimisation analysisBMJ316179169624069
- ShepperdSHarwoodDJenkinsonC1998Randomised controlled trial comparing hospital at home care with inpatient hospital care. I: three month follow up of health outcomesBMJ3161786919624068
- SiafakasNMVermeirePPrideNB1995Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J813984207489808
- SinDDTuJV2001Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med164580411520719
- SinDDWuLAndersonJA2005Inhaled corticosteroids and mortality in chronic obstructive pulmonary diseaseThorax60992716227327
- SkwarskaECohenGSkwarskiKM2000Randomized controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary diseaseThorax559071211050258
- SoderlingSHBeavoJA2000Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functionsCurr Opin Cell Biol12174910712916
- SolerNTorresAEwigS1998Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilationAm J Respir Crit Care Med15714985059603129
- SorianoJBVestboJPrideNB2002Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practiceEur Respir J208192512412670
- SuissaF2003Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studiesAm J Respir Crit Care Med168495312663327
- SzafranskiWCukierARamirezA2003Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J21748112570112
- TashkinDKannerRBaileyW2001Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trialLancet3571571511377644
- ThompsonWHNielsonCPCarvalhoP1996Controlled trial of oral prednisone in outpatients with acute COPD exacerbationAm J Respir Crit Care Med154407128756814
- TorphyTJUndemBJCieslinskiLB1993Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscleJ Pharmacol Exp Ther2651213238389856
- UlrikCS1995Efficacy of inhaled salmeterol in the management of smokers with chronic obstructive pulmonary disease: a single centre randomised, double blind, placebo controlled, crossover studyThorax5075047570409
- UndemJBLichtensteinLMHardmanJGLimbirdLEGoodman GilmanA2001Drugs used in the treatment of asthmaThe pharmacological basis of therapeutics10th ednNew YorkMcGraw-Hill73354
- van NoordJAAumannJLJanssensE2006Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPDChest1295091716537846
- van NoordJAde MunckDRBantjeTA2000Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropiumEur Respir J158788510853852
- van SchayckCPDompelingEvan HerwaardenCL1991Bronchodilator treatment in moderate asthma or chronic bronchitis: continuous or on demand? A randomised controlled studyBMJ3031426311837744
- VathenenASBrittonJREbdenP1988High-dose inhaled albuterol in severe chronic airflow limitationAm Rev Respir Dis13885052462383
- VestboJSorensenTLangeP1999Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trialLancet35318192310359405
- VestboJTORCH Study Group2004The TORCH (towards a revolution in COPD health) survival study protocolEur Respir J242061015332386
- WoutersEFPostmaDSFokkensB2005Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trialThorax60480715923248