Abstract
The importance of the underlying local and systemic oxidative stress and inflammation in chronic obstructive pulmonary disease (COPD) has long been established. In view of the lack of therapy that might inhibit the progress of the disease, there is an urgent need for a successful therapeutic approach that, through affecting the pathological processes, will influence the subsequent issues in COPD management such as lung function, airway clearance, dyspnoea, exacerbation, and quality of life. N-acetylcysteine (NAC) is a mucolytic and antioxidant drug that may also influence several inflammatory pathways. It provides the sulfhydryl groups and acts both as a precursor of reduced glutathione and as a direct reactive oxygen species (ROS) scavenger, hence regulating the redox status in the cells. The changed redox status may, in turn, influence the inflammation-controlling pathways. Moreover, as a mucolytic drug, it may, by means of decreasing viscosity of the sputum, clean the bronchi leading to a decrease in dyspnoea and improved lung function. Nevertheless, as successful as it is in the in vitro studies and in vivo studies with high dosage, its actions at the dosages used in COPD management are debatable. It seems to influence exacerbation rate and limit the number of hospitalization days, however, with little or no influence on the lung function parameters. Despite these considerations and in view of the present lack of effective therapies to inhibit disease progression in COPD, NAC and its derivatives with their multiple molecular modes of action remain promising medication once doses and route of administration are optimized.
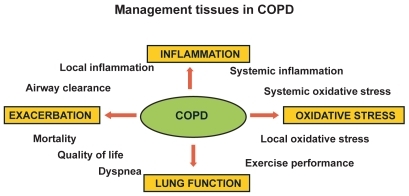
Management issues in COPD
Lung function, dyspnoea and quality of life
Chronic obstructive pulmonary disease (COPD) is a condition defined as a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of lungs to noxious particles or gases, primarily caused by cigarette smoking (CitationCelli et al 2004). In Europe, COPD is ranked as the third most common cause of death (CitationPauwels et al 2001).
The lung function and consequently patient quality of life worsens as disease progresses (CitationSeemungal et al 1998; CitationHogg 2004). The standard way of assessing the progression of COPD (CitationBurrows and Earle 1969) is an annual decline in forced expiratory volume in one second (FEV1), which is considered to be the best single correlate of mortality (CitationCelli et al 2004) and is used as the main parameter in the evaluation of many other COPD aspects. In comparison with the normal subject where the FEV1 decline reaches 20–30 mL/year, in COPD patients it reaches 60 mL/year (CitationAnthonisen et al 1994). This accelerated decline in lung function is closely associated with an increased number of neutrophils in the sputum (CitationStanescu et al 1996) and hence with higher level of airway inflammation. This, on the other hand, is proportional to the rise in the colonizing bacterial load as well as its changes (CitationWilkinson et al 2003).
Cross-sectional studies have shown that quality of life deteriorates as lung function declines (CitationStahl et al 2005). Some groups, however, reported that the health status and health-related quality of life (HRQOL) do not strongly correlate with the lung function parameters such as FEV1 (CitationAntonelli-Inc et al 2003), but relate more to exercise performance, mobility, and mortality (CitationJones et al 1989; CitationFan et al 2002). Since any improvement in physical performance relates to an improvement in quality of life scores and dyspnoea perception (CitationVerrill et al 2005), the limitation of a functional status is considered very highly predictive of survival (ZuWallack et al 2004). Furthermore, dyspnoea correlates more closely with an impact on HRQOL than FEV1. Therefore, dyspnoea and HRQOL have become an important focus for therapy as dyspnoea is the predominant complaint for which patients with COPD seek medical attention (CitationHajiro et al 1999).
Exacerbations
Patients with COPD are prone to periodic acute exacerbations (AE) of their disease with worsening of the respiratory symptoms and inflammatory parameters (CitationDrost et al 2005). The frequency of these exacerbations varies, depending on the definition and severity (CitationWedzicha and Donaldson 2003). AE contributes to a diminished HRQOL and consequently to the reduced health status, frequent hospitalizations, and eventually increased morbidity (CitationDonaldson et al 2002). Moreover, the exacerbations lead to the increased healthcare costs with exacerbations associated with a hospitalization accounting for 90% of the total costs of exacerbations (CitationOostenbrink and Rutten-van Molken 2004).
Furthermore, the number of exacerbations per year correlates with disease severity (CitationSiafakas et al 1995). An increased frequency of these episodes may hasten disease progression and accelerate the decline in the lung function since a physiologic recovery after an exacerbation is usually incomplete resulting in the decrease in quality of life and a tendency to future exacerbations (CitationDonaldson et al 2002). Also more pronounced physiologic changes during AE are related to a longer exacerbation recovery time. Additionally, it has been reported that lung function together with health status are the most important risk factors for the re-admission that also relate to the psychological status of the patients (CitationGudmundsson et al 2005). Therefore the prevention of an acute exacerbation and improving the health status should be considered as one of the most important therapeutic targets.
Oxidative stress and inflammation
There is increasing evidence that an oxidant/antioxidant imbalance, in favor of oxidants, occurs in COPD (CitationRahman et al 1996; CitationMacNee 2000). The main reason for this is cigarette smoke which is a complex mixture of more than 4700 chemical compounds, including high concentrations of free radicals and other oxidants (CitationChurch and Pryor 1985). Components of the lung matrix (eg, collagen, elastin) can be directly damaged by the cigarette smoke (CitationCantin and Crystal 1985). In the breath, the oxidants generated by cigarette smoking or released from leukocytes and epithelial cells, are increased in stable patients and those with an acute exacerbation of COPD (CitationDekhuijzen et al 1996; CitationMaziak et al 1998). There is also evidence that a systemic oxidative stress is present in the COPD patients. This is reflected by an increased production of superoxide anion from the peripheral blood neutrophils in stable (CitationNoguera et al 1998) and exacerbated COPD patients (CitationRahman et al 1996). Oxidative stress is inextricably linked to the inflammatory response. It becomes clear that the inflammatory process potentiates as COPD progresses (CitationHogg 2004) and exerts damage which is irreversible. This damage involves a complex remodeling process resulting in emphysema and fibrosis of the small airways. Tobacco smoking is not only a major source of the oxidants but is also a major factor initiating the local inflammatory response (CitationChurg et al 2002).
During acute exacerbation of COPD (AE), except for changes in the overall health status, there are differences observed in the inflammatory and oxidative stress status of the patients. The increased number of inflammatory cells and the accompanying elevated cytokine levels in stable COPD patients tend to rise even further during AE (CitationDrost et al 2005), reflecting the worsening of the underlying chronic inflammation in the lungs. These acute-upon-chronic episodes augment the damage exerted by chronic processes and may diminish the chances of full recovery.
The management points in COPD are shown in .
Role of mucolytics
For patients suffering from COPD, asthma, and cystic fibrosis, mucus hypersecretion is considered to be a risk factor for increased morbidity. While chronic mucus hypersecretion may not impair the lung function of the healthy individual, in a case of obstructive lung disorder, excessive mucus production accompanied by an inadequate mucus clearance may further hinder the air passage by clogging the already obstructed airways (CitationJeffery and Li 1997). The mucus is a complex mixture of proteins, lipids, water, and electrolytes (CitationSnider et al 1984; CitationRobinson et al 1989) that, under normal conditions, maintains the moisture of the airway epithelium. It is produced by goblet, mucous, and serous cells (CitationLundgren and Shelhamer 1990) and stored until a secretory signal is given. The mucus secretion may be stimulated by mediators produced by macrophages, lymphocytes, and epithelium (CitationCohan et al 1991; CitationGollub et al 1992). Furthermore, in the view that neutrophils are the key cells in COPD, there is emerging evidence that they play a key role in epidermal growth factor receptor (EGFR)-mediated mucin production through releasing tumor necrosis factor-alpha (TNF-α) and hence inducing EGFR expression (CitationTakeyama et al 1999; CitationKim and Nadel 2004). Moreover, the differentiation of the mucus cells as well as secretion of the mucus from airway glands are induced by neutrophil elastase (CitationBreuer et al 1987).
Nevertheless, by entrapping and removing foreign materials, the mucus forms a basic defense system of the respiratory system. In the large airways, mucus hypersecretion causes coughing and sputum production. In the peripheral airways, because of the smaller diameter, the formed mucus plugs are difficult to remove and may block the peripheral airway completely. This, in turn, may result in gas trapping with increased total lung capacity (TLC) and decreased forced vital capacity (FVC). In COPD, mucus hypersecretion is associated with disease exacerbation (CitationPoole and Black 2003), accelerated decline in FEV1 (CitationVestbo et al 1996) and inflammatory cell infiltration (CitationWedzicha and Donaldson 2003). Moreover, the remaining sputum hinders the accessibility of inhaled medication to the peripheral airways. Therefore the mucus clearance and sterility maintenance are of importance in COPD. There is a large number of medications available that are meant to change the properties of airway secretion or block its production or release, or both. The mucolytics are responsible for the disruption of the mucous gel, generally by altering the degree of the cross-linking or the interactions between molecules in the gel. They include N-acetylcysteine (NAC) and related compounds, dornase–α, F-actin de-polymerizing agents and nondestructive mucolytics, like hypertonic saline and oligosaccharide agents and were previously reviewed (CitationKing and Rubin 2002).
Current treatments for COPD are symptomatic and focus on the bronchodilatation. No effective medication currently exists that may influence the progress of the disease. Therefore, mucolytics like NAC may form an interesting therapeutic approach. Classical mucolytics, like NAC and other thiol reducing agents, degrade the three-dimensional network that forms the mucus by reducing the disulphide bonds (S-S) to a sulfhydryl (SH) bond (-SH) that no longer participates in the cross-linking. They may act on the mucus elasticity and viscosity as well as modulate its production and secretion (CitationLivingstone et al 1990; CitationKing and Rubin 2002). NAC has been reported to reduce the viscosity of sputum in both cystic fibrosis and COPD, facilitating the removal of pulmonary secretions (CitationVentresca et al 1989). Moreover, by maintaining the airway clearance, it prevents bacterial stimulation of mucin production and hence mucus hypersecretion (CitationAdler et al 1986).
NAC however, has also its disadvantages concerning its accessibility of the peripheral airways and risk of bronchospasm in hyperreactive patients. Nevertheless, the superiority of NAC over the other mucolytics may be in its anti-inflammatory and antioxidant properties and its mucolytic actions.
NAC: pharmacology and pharmacokinetics
N-acetylcysteine is a thiol compound which has a chemical formula C5H9NO3S and a molecular weight of 163.2. It is rapidly absorbed following an oral dose of 600 mg with a peak of 4.6 μM after 60 min (CitationTsikas et al 1998). The plasma half-life has been reported to be 2.5 hours and no NAC is detectable 10–12 hours after administration (CitationDe Caro et al 1989). It has been estimated that the oral bioavailability of the intact NAC molecule was about 10% (CitationBorgstrom and Kagedal 1990). Following an oral dose, the majority of NAC is metabolized into another compound since accompanying increase in nonprotein and protein SH groups were found in plasma (CitationCotgreave, Berggren, et al 1987; CitationDe Caro et al 1989). Normalized maximal plasma concentration was reported to be significantly higher after a 600 mg dose than after a 200 mg dose. Moreover, increasing the dose also increased NAC bioavailability and time for maximal plasma concentration (CitationBorgstrom and Kagedal 1990). Yet, higher concentration in plasma may be achieved after the intravenous administration (CitationPrescott et al 1989; CitationCrouch and Rusho 2005). During absorption, NAC is rapidly metabolized to cysteine, whose thiol group has reducing and antioxidant properties and which is a direct precursor of the glutathione. Because of its ability to reduce disulphide bounds, NAC is widely used to reduce viscosity and elasticity of the mucus (CitationAruoma et al 1989). Moreover, NAC has the potential to interact directly with oxidants such as hydrogen peroxide, hydroxyl radical, and hypochloric acid (CitationAruoma et al 1989). To exert some effects in the lungs, concentration of NAC or its derivatives have to be sufficiently elevated in bronchial epithelium or in epithelial lining fluid. Nevertheless, no NAC was found in bronchoalveolar lavage fluid (BALF) after 2 weeks of NAC intake (200 mg three times daily [tid]) by healthy volunteers (CitationCotgreave, Eklund, et al 1987).
Antioxidant properties
As a source of SH groups in cells, NAC can stimulate glutathione (GSH) synthesis and play a role in the detoxification (CitationKelly 1998) and play an important role in the antioxidant defense. However, when administered orally it fails to reach the desired concentration in the lungs as 600 mg/day did not alter cysteine and glutathione in BALF of healthy volunteers. However, it significantly increased free and total plasma glutathione and cysteine (CitationCotgreave, Eklund, et al 1987). When 600 mg was administered to COPD patients an increase in plasma cysteine was observed with no difference in GSH levels. The higher dose was necessary (600 mg tid) in order to increase GSH levels in COPD patients (CitationBridgeman et al 1994). This observation may suggest that under the condition of permanent oxidative stress, 600 mg may not be sufficient to replenish the diminished GSH content. NAC can also act as direct scavenger of free radicals such as OH and H2O2 and O2 (CitationBenrahmoune et al 2000) and it can protect against O2 toxicity in the lung (CitationErzurum et al 1993).
Anti-inflammatory properties
Inflammatory response is strongly influenced by redox-sensitive nuclear transcription factor-κB (NF-κB) which regulates a variety of pro-inflammatory genes, and hence modulates the inflammatory response (CitationDesaki et al 2000). In vitro studies showed that a decrease in several inflammatory mediators was related to NF-κB activity inhibition (CitationLappas et al 2003). Due to the fact that NAC modulates the cellular redox status, it can affect several pathways leading to decrease in NF-κB activity (CitationHutter and Greene 2000). Namely, NAC may affect thioredoxin and glutaredoxin expression which form other parts of ubiquitously expressed thiol-reducing system next to glutathione. Thioredoxin increases DNA binding of NF-κB by reducing cysteine of the p50 subunit (CitationMatthews et al 1992) while glutaredoxin by sensing the changes in the redox state of GSH/GSSG may be involved in altering signal transduction pathways resulting in biological responses modulation (CitationSong et al 2002). By decreasing the release of thioredoxin and glutaredoxin, NAC may attenuate NF-κB binding to DNA (CitationHoppe et al 2003; CitationNakamura H et al 1996) resulting in an impaired inflammatory response (CitationPeltoniemi et al 2004). Except from its effects on transcription factor NF-κB, NAC influences several signaling pathways such as p38, ERK1/2, SAPK/JNK, c-Jun, and c-Fos, among others (CitationWuyts et al 2003). Clinical application of NAC in different disorders as well as its molecular actions have been reviewed previously (CitationWuyts et al 2003; CitationZafarullah et al 2003).
Role of NAC in COPD
Lung function
N-acetylcysteine was reported to improve the lung function when administered for a longer period of time. Namely, CitationPela and colleagues (1999) reported a significant improvement in FEV1 and maximal expiratory flow at 50% of forced vital capacity (MEF50) after 6 month treatment of stable COPD patients with NAC (600 mg/day). It supported the study of CitationAylward and colleagues (1980) who observed an improvement from 25 to 30% FEV1 (% predicted) in the NAC-treated group during the 4 weeks study period (CitationAylward et al 1980). On the other hand, several groups did not find any changes in FEV1 and FVC although some reduction of hyperinflation was obtained (CitationDecramer et al 2005; Citationvan Overveld et al 2005).
Exacerbation
As far as exacerbations are concerned, in a recent literature review, CitationPoole and Black (2003) found that regular use of mucolytics resulted in a significant reduction of exacerbations by 0.07 exacerbation/month and reduction of illness days of 0.56 days/month. Moreover, the study of CitationGerrits and colleagues (2003) found the correlation between the dose of NAC and the number of hospitalizations due to exacerbation. They concluded that NAC may decrease the risk of hospitalizations by about 30%. This reduction however needed minimal dose of 400 mg/day in order to reach protective effect (CitationGerrits et al 2003). It was also supported by study of CitationBoman and colleagues (1983), who showed a reduction in exacerbation rate of 40%, which was significantly different from 19% in placebo group. On the other hand, some studies only observed a trend towards a lower exacerbation rate (CitationParr and Huitson 1987) or no effect at all (CitationRasmussen and Glennow 1988). Nevertheless, those studies reported a significant reduction in a sick-leave days after 4 and 6 months of NAC treatment. The last large, 3-year study on NAC, the BRONCHUS trial, reported a significant decrease in exacerbation rate only with no concomitant steroid therapy (CitationDecramer et al 2005). The reports on NAC as add-on treatment during an acute exacerbation of COPD are scarce. The study of Black et al showed that the treatment of AE with NAC (600 mg twice daily) did not affect breathlessness, lung function, and oxygen saturation (CitationBlack et al 2004).
The mechanisms underlying the reduction in the number of exacerbations as well as the days of illness are not very well described. Because of the fact that no significant increase in GSH level was found in plasma and BALF while using low dose (600 mg daily) higher dosages were necessary (600 mg tid) to obtain sustained effect on the thiols level in plasma, it seems unlikely that the changes induced by lower dose of NAC may alter the exacerbation rate or the course of exacerbations. On the other hand, NAC was reported to influence sputum cytokine level (Citationvan Overveld et al 2005) as well as sputum chemoattractant properties (Citationvan Overveld et al 2000). Because of the fact that a correlation between exacerbation frequency and sputum IL-6 and IL-8 levels exists (CitationBhowmik et al 2000), NAC, by acting on baseline level of those cytokines, may play a role in limiting the inflammatory status and hence modulate the exacerbation frequency. Moreover, by decreasing the viscosity of the sputum, it may facilitate the airway clearance and hence influence the course of the exacerbation, since there is a relationship between bacterial colonization and exacerbation frequency and character (CitationPatel et al 2002). Effects of NAC on lung function and exacerbation rate are summarized in . The results from aforementioned reports indicate that low doses of NAC may have rather low antioxidant potential resulting in insignificant improvement of the lung function and limited influence on the exacerbation rate.
Table 1 Effects of NAC on clinical COPD outcomes
Inflammation and oxidative stress in COPD
N-acetylcysteine has been reported to influence several factors that are involved in the modulation of the inflammatory response. Namely, NAC decreased the chemoattractant properties of the sputum of COPD patients after 10 month treatment (Citationvan Overveld et al 2000) and modulated the inflammatory response after 10 weeks of intake (CitationSadowska et al 2005). Moreover, in healthy volunteers it decreased the formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated respiratory burst and chemotaxis (CitationSadowska et al 2006). Furthermore, in the study of CitationUrban and colleagues (1997), NAC improved the phagocytotic ability of neutrophils while decreasing their production of superoxide anion (SA) with no effects on migration already after 14 days of administration in healthy volunteers.
As far as oxidative stress is concerned, NAC decreased the exhaled H2O2 levels in COPD patients after long-term treatment (CitationKasielski and Nowak 2001; CitationDe Benedetto et al 2005). Moreover, when administered to healthy volunteers, it increased the glutathione peroxidase activity (GPx) after 2 weeks of oral treatment (CitationUrban et al 1997) and increased thiol level after 30 min and 3 hours after administration (CitationSzkudlarek et al 2004).
It did not, however, influence GSH and cysteine level in BALF and lung tissue (CitationBridgeman et al 1994).
Furthermore supplementation with NAC (200 mg × 4/day for 2 days + 800 mg on the test morning) increased the pre-exercise scavenging activity of healthy volunteer plasma (CitationSen et al 1994). When administered in higher doses (125 mg/kg) (intravenously) NAC significantly increased GSH content and attenuated its decline during intense intermittent exercise and led to lesser glutathione disulfide (GSSG) formation (CitationMedved et al 2003).
These reports strongly suggest that for effective anti-inflammatory and antioxidant action, higher doses are necessary as the low NAC concentration (as also observed in vitro) was not found satisfactory (CitationSadowska et al 2007). This could be due to pharmacokinetics of NAC and administration route, which is far from optimal as it is unlikely to achieve desired concentration in the lung compartment (CitationBridgeman et al 1994). The novel route (eg, inhalation) is currently under investigation for both NAC and its derivatives and it is discussed in the last section.
Patient-focused perspectives
Quality of life
FEV1 hardly correlates with dyspnoea, which is closely related to the patient’s quality of life and which can vary among the patients with the same degree of the airway obstruction (CitationWolkove et al 1989). Dyspnoea, on the other hand, may reflect more comprehensive information than airway obstruction in patients with COPD and should be taken into account while evaluating the successful treatment. NAC was reported to decrease the dyspnoea in COPD patients in comparison with placebo during the long-term treatment (CitationAylward et al 1980; CitationTattersall et al 1984). This may be partly caused by the mucolytic effects of NAC leading to increase in the sputum volume and pourability. Moreover, an improvement in the subjects’ clinical state after NAC treatment has been noted (CitationJackson et al 1984) as well as an improvement of bronchitis-related symptoms (CitationStey et al 2000) and increase in the patients’ well-being (CitationHansen et al 1994). Furthermore, in study of CitationPela and colleagues (1999), 65% of the treated patients reported improvement in quality of life in the NAC group (NAC on top of the current therapy) compared with 29% in placebo group.
As far as the influence of NAC on physical activity is concerned, it successfully improves the performance on fatiguing exercise when administered in high dosages. Namely, in the study by CitationMatuszczak and colleagues (2005), at the oral dose of 150 mg/kg, NAC delayed handgrip fatigue, increasing task performance by one-third compared with the baseline. Similarly, in the study by CitationMedved and colleagues (2004) (125 mg/kg intravenously), NAC increased time to fatigue. Furthermore, the same dose was reported to inhibit fatigue of human skeletal muscles caused by repetitive, low-frequency electrical stimulation (CitationReid et al 1994). Moreover, high doses of NAC (600 mg × 3/day) prevented the exercise-induced increase in plasma thiobarbituric reactive substances and O2 release and led to improved endurance time (CitationKoechlin et al 2004). It may be suggested that NAC in high doses may act on GSH content replenishment and may increase the task performance and have positive effect on the physical activity and hence quality of life.
Compliance
The compliance is concerned to be better following the 600 mg/day regimen (CitationPela et al 1999). For example, in the BRONCHUS study, a compliance of 94% in the NAC group was observed in comparison with 92% in the placebo group (CitationDecramer et al 2005). Nevertheless, a lower compliance observed at the regimen of 200 mg tid could be improved, following additional patient instructions (CitationParr and Huitson 1987).
Tolerability and side-effects
NAC is generally safe. The oral dose of 600 mg/day or less is usually very well tolerated with no apparent side-effects and no impact on the patient satisfaction (CitationJackson et al 1984; CitationParr and Huitson 1987; CitationStey et al 2000). A dose of 1800 mg/day administered for a period of 4 days in COPD patients (CitationKoechlin et al 2004) and for 5–6 weeks in the polycystic ovary syndrome was also well tolerated with no reported side-effects (CitationFulghesu et al 2002). Nevertheless, some trials mention some gastro-intestinal adverse reactions, which are not significantly different from placebo group and comprise of dyspepsia, diarrhea or heartburn (CitationAylward et al 1980). In the last study of CitationMatuszczak and colleagues (2005) where 150 mg/kg of NAC was administered orally, mild reactions were reported in most of the patients (erythema, sweating, altered sensations, gastrointestinal disturbances). Except for gastrointestinal disturbances that persisted up to 4 hours and light-headedness which disappeared after 3 hours, the remaining complaints disappeared 60 min after indigestion of NAC. The most common side-effects associated with high oral doses are nausea, vomiting, and other gastrointestinal disturbances, and therefore oral administration is contraindicated in persons with active peptic ulcer (CitationKoo et al 1986). On the other hand, at low doses NAC may have a positive influence on the bacterial colonization in gastritis (CitationHuynh et al 2004). Infrequently, anaphylactic reactions after intravenous administration due to histamine release occur and can consist of rash, angioedema, bronchospasm, tachycardia, and changes in blood pressure (CitationTenenbein 1984).
Cost-effectiveness
High prevalence of COPD carries with it serious financial consequences. According to the Belgisch Instituut voor Gezondheidseconomie, the costs of COPD increase together with the disease progression amounts to (in €/year/patient) 2977€ for Stage I, 4740€ for Stage II, and 8565€ for Stage III, and IV (De Backer pers comm).
Disease severity, on the other hand, correlates with the number of exacerbations per year (CitationSiafakas et al 1995). Since exacerbation is one of the important factors facilitating the progression of the disease and brings additional costs to the global expenditure, which are especially high in case of hospitalization or intensive care unit admission (CitationNiederman et al 1999), effective exacerbation prevention could be the major cost-reducing factor.
Grandjean and colleagues (2000) showed that NAC at dose 400 mg/day not only led to a significant reduction in the number of exacerbations but also resulted in a smaller percentage of sick-leave days and lower hospitalization rate, what leads to a direct (NAC treatment, AE management, hospitalization) and indirect (sick-leave days) cost reduction.
Conclusion: optimal usage, place in therapy, and future perspectives
Based on the accessible literature, NAC in COPD has been studied mostly at rather low dosages with variable outcomes. The short-term, low-dose studies suggest that NAC may not be a drug of choice to enhance the GSH antioxidant potential of the lungs, since the effects are poor. While its effect on the oxidative stress markers seems to be influenced even at low dosages, the higher doses may ensure more rapid response. On the other hand, even long-term administration at low doses does not always have the anti-inflammatory potential but substantially higher doses are necessary as in vitro studies have already demonstrated. As far as exacerbation rate, the number of sick-leave days and quality of life are concerned; the use of NAC seems to be promising as the majority of studies give favorable reports even at 600 mg/day dose.
Because of the fact that higher doses seem to be more effective in inflammation and oxidative stress modulation with little side-effects, a change in the administration pattern may be of importance.
Furthermore, evaluating novel methods of drug delivery (inhaled NAC) (CitationSzkudlarek et al 2004) and use of NAC derivatives with better bioavailability may result in more effective treatment. For example, Nacystelyn (NAL), a lysine salt of NAC, has mucolytic and antioxidant properties. Its mucolytic activity is approximately equal to the sum of the activities of its two components, namely acetylcysteine and lysine, and its antioxidant properties are comparable with those of NAC (CitationVan Antwerpen et al 2005). Moreover, because of its neutral pH, NAC can be delivered into the airways in inhaled form (CitationGillissen et al 1997; CitationAntonicelli et al 2004). The inhaled form, tested in cystic fibrosis patients, was well tolerated and positively influenced airway mucus clearance (CitationApp et al 2002).
Another, newly developed form of NAC is N-acetylcysteine amide (NACA). Because of its neutral carboxyl group, NACA is lipophilic, cell permeating and has strong antioxidant and protective effects (CitationGrinberg et al 2005). Nevertheless, more studies are necessary to assess the effectiveness of those derivatives in lung disorders in vivo.
References
- AdlerKBHendleyDDDavisGS1986Bacteria associated with obstructive pulmonary disease elaborate extracellular products that stimulate mucin secretion by explants of guinea pig airwaysAm J Pathol125501143099581
- AnthonisenNRConnettJEKileyJP1994Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA27214975057966841
- Antonelli-IncImperialeCBelliaV2003Do GOLD stages of COPD severity really correspond to differences in health status?Eur Respir J22444914516133
- AntonicelliFBrownDParmentierM2004Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant NacystelynAm J Physiol Lung Cell Mol Physiol286L13192715136298
- AppEMBaranDDabI2002Dose-finding and 24-h monitoring for efficacy and safety of aerosolized Nacystelyn in cystic fibrosisEur Respir J1929430211866009
- AruomaOIHalliwellBHoeyBM1989The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acidFree Radic Biol Med659372546864
- AylwardMMaddockJDewlandP1980Clinical evaluation of acetyl-cysteine in the treatment of patients with chronic obstructive bronchitis: a balanced double-blind trial with placebo controlEur J Respir Dis Suppl1118197011835
- BenrahmouneMTherondPAbedinzadehZ2000The reaction of superoxide radical with N-acetylcysteineFree Radic Biol Med297758211053779
- BhowmikASeemungalTARSapsfordRJ2000Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbationsThorax551142010639527
- BlackPNMorgan–DayAMcMillanTE2004Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]BMC Pulm Med41315581425
- BomanGBackerULarssonS1983Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary DiseasesEur J Respir Dis64405156350033
- BorgstromLKagedalB1990Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to manBiopharm Drug Dispos1113162328298
- BreuerRChristensenTGLuceyEC1987An ultrastructural morphometric analysis of elastase-treated hamster bronchi shows discharge followed by progressive accumulation of secretory granulesAm Rev Respir Dis1366987033651126
- BridgemanMMMarsdenMSelbyC1994Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissueThorax4967058066561
- BurrowsBEarleRH1969Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patientsN Engl J Med2803974045763088
- CantinACrystalRG1985Oxidants, antioxidants and the pathogenesis of emphysemaEur J Respir Dis Suppl1397172995106
- CelliBRMacNeeWAgustiA2004Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J239324615219010
- ChurchDFPryorWA1985Free-radical chemistry of cigarette smoke and its toxicological implicationsEnviron Health Perspect64111263007083
- ChurgAZayKShayS2002Acute Cigarette Smoke-Induced Connective Tissue Breakdown Requires both Neutrophils and Macrophage Metalloelastase in MiceAm J Respir Cell Mol Biol273687412204900
- CohanVLScottALDinarelloCA1991Interleukin-1 is a mucus secretagogueCell Immunol136425341873825
- CotgreaveIABerggrenMJonesTW1987Gastrointestinal metabolism of N-acetylcysteine in the rat, including an assay for sulfite in biological systemsBiopharm Drug Dispos8377863620596
- CotgreaveIAEklundALarssonK1987No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluidEur J Respir Dis707373817074
- CrouchBIRushoWJ2005Intravenous administration of N-AcetylcysteineAnn Emerg Med4620716046960
- De BenedettoFAcetoADraganiB2005Long-term oral N-acetylcysteine reduces exhaled hydrogen peroxide in stable COPDPulm Pharmacol Ther1841715607126
- De CaroLGhizziACostaR1989Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteersArzneimittelforschung3938262757663
- DecramerMRutten-Van MolkenMDekhuijzenP2005Effects of NAC on outcome of COPD. The bronchitis randomized on NAC cost-utility study (BRONCHUS)Lancet
- DekhuijzenPNAbenKKDekkerI1996Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med154813168810624
- DesakiMTakizawaHKasamaT2000Nuclear factor-kappa b activation in silica-induced interleukin 8 production by human bronchial epithelial cellsCytokine1212576010930308
- DonaldsonGCSeemungalTABhowmikA2002Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax578475212324669
- DrostEMSkwarskiKMSauledaJ2005Oxidative stress and airway inflammation in severe exacerbations of COPDThorax6029330015790984
- ErzurumSCDanelCGillissenA1993In vivo antioxidant gene expression in human airway epithelium of normal individuals exposed to 100% O2J Appl Physiol751256628226538
- FanVSCurtisJRTuSP2002Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseasesChest1224293612171813
- FulghesuAMCiampelliMMuzjG2002N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndromeFertil Steril7711283512057717
- GerritsCMHeringsRMLeufkensHG2003N-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary diseaseEur Respir J21795812765423
- GillissenAJaworskaMOrthM1997Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitroRespir Med91159689135855
- GollubEGGoswamiSKSperberK1992Isolation and characterization of a macrophage-derived high molecular weight protein involved in the regulation of mucus-like glycoconjugate secretionJ Allergy Clin Immunol896967021545090
- GranjeanEMBerthetPHRuffmannR2000Cost-effectiveness analysis of oral NAC as preventive treatment in chronic bronchitisPharmacol Res42395010860633
- GrinbergLFibachEAmerJ2005N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stressFree Radic Biol Med381364515589382
- GudmundssonGGislasonTJansonC2005Risk factors for re-hospitalisation in COPD: role of health status, anxiety and depressionEur Respir J264141916135721
- HajiroTNishimuraKTsukinoM1999A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPDChest1161632710593787
- HansenNCSkriverABrorsen-RiisL1994Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitisRespir Med8853157972979
- HoggPJ2004Pathophysiology of airflow limitation in chronic obstructive pulmonary diseaseLancet3647092115325838
- HoppeGChaiYCSearsJ2003Endogenous oxidoreductase expression is induced by aminoglycosidesArch Biochem Biophys414192312745250
- HutterDGreeneJJ2000Influence of the cellular redox state on NF-kappaB-regulated gene expressionJ Cell Physiol183455210699965
- HuynhHQCouperRTTranCD2004N-acetylcysteine, a novel treatment for Helicobacter pylori infectionDig Dis Sci4918536115628716
- JacksonIMBarnesJCookseyP1984Efficacy and tolerability of oral acetylcysteine (Fabrol) in chronic bronchitis: a double-blind placebo controlled studyJ Int Med Res121982066376210
- JefferyPKLiD1997Airway mucosa: secretory cells, mucus and mucin genesEur Respir J101655629230262
- JonesPWBaveystockCMLittlejohnsP1989Relationships between general health measured with the sickness impact profile and respiratory symptoms, physiological measures, and mood in patients with chronic airflow limitationAm Rev Respir Dis1401538432604285
- KasielskiMNowakD2001Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary diseaseRespir Med954485611421501
- KellyGS1998Clinical applications of N-acetylcysteineAltern Med Rev3114279577247
- KimSNadelJA2004Role of neutrophils in mucus hypersecretion in COPD and implications for therapyTreat Respir Med31475915219174
- KingMRubinBK2002Pharmacological approaches to discovery and development of new mucolytic agentsAdv Drug Deliv Rev5414759012458156
- KoechlinCCouillardACristolJP2004Does systemic inflammation trigger local exercise-induced oxidative stress in COPD?Eur Respir J235384415083751
- KooMWOgleCWChoCH1986Effects of verapamil, carbenoxolone and N-acetylcysteine on gastric wall mucus and ulceration in stressed ratsPharmacology32326343725888
- LappasMPermezelMRiceGE2003N-acetylcysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity and nuclear factor-κB deoxyribonucleic acid-binding activity in human fetal membranes in vitroJ Clin Enocrinol Metab8817239
- LivingstoneCRAndrewsMAJenkinsSM1990Model systems for the evaluation of mucolytic drugs: acetylcysteine and S-carboxymethylcysteineJ Pharm Pharmacol427381972405
- LundgrenJDShelhamerJH1990Pathogenesis of airway mucus hyper-secretionJ Allergy Clin Immunol853994172406321
- MacNeeW2000Oxidants/antioxidants and COPDChest117303 S17S10669665
- MatthewsJRWakasugiNVirelizierJL1992Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62Nucleic Acids Res203821301508666
- MatuszczakYFaridMJonesJ2005Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exerciseMuscle Nerve32633816025522
- MaziakWLoukidesSCulpittS1998Exhaled nitric oxide in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med15799810029517624
- MedvedIBrownMJBjorkstenAR2004NAC enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individualsJ Appl Physiol9714778515194675
- MedvedIBrownMJBjorkstenAR2003N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humansJ Appl Physiol9415728212496140
- NakamuraHYoshimuraKMcElvaneyNG1996Netrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces IL-8 gene expression in a human bronchilal epithelial cellsJ Clin Invest891478841569186
- NiedermanMSMcCombsJSUngerAN1999Treatment cost of acute exacerbations of chronic bronchitisClin Ther215769110321424
- NogueraABusquetsXSauledaJ1998Expression of adhesion molecules and G proteins in circulating neutrophils in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med158166489817722
- OostenbrinkJBRutten-van MolkenMP2004Resource use and risk factors in high-cost exacerbations of COPDRespir Med988839115338802
- ParrGDHuitsonA1987Oral Fabrol (oral N-acetyl-cysteine) in chronic bronchitisBr J Dis Chest8134183329530
- PatelISSeemungalTAWilksM2002Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbationsThorax577596412200518
- PauwelsRABuistASCalverlyPMA2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summaryAm J Respir Crit Care Med16312567611316667
- PelaRCalcagniAMSubiacoS1999N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPDRespiration6649550010575333
- PeltoniemiMKaarteenaho-WiikRSailyM2004Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivoHum Pathol351000715297967
- PoolePJBlackPN2003Preventing exacerbations of chronic bronchitis and COPD: therapeutic potential of mucolytic agentsAm J Respir Med23677014719989
- PrescottLFDonovanJWJarvieDR1989The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosageEur J Clin Pharmacol3750162598989
- RahmanIMorrisonDDonaldsonK1996Systemic oxidative stress in asthma, COPD, and smokersAm J Respir Crit Care Med1541055608887607
- RasmussenJBGlennowC1988Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitisEur Respir J135153294038
- ReidMStokicDSKochSM1994NAC inhibits muscle fatigue in humansJ Clin Invest942468747989604
- RobinsonNPKyleHWebberSE1989Electrolyte and other chemical concentrations in tracheal airway surface liquid and mucusJ Appl Physiol662129352745281
- SadowskaAMManuelYKDe BackerWA2007Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A reviewPulm Pharmacol Ther2092216458553
- SadowskaAMManuelYKVertongenT2006Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: In vivo and in vitro studyPharmacol Res532162516384711
- SadowskaAMvan OverveldFJGoreckaD2005The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: modulation by inhaled steroids and antioxidantRespir Med991419
- SeemungalTADonaldsonGCPaulEA1998Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1571418229603117
- SenCKRankinenTVaisanenS1994Oxidative stress after human exercise: effect of N-acetylcysteine supplementationJ Appl Physiol76257077928885
- SiafakasNMVermeirePPrideNB1995Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J813984207489808
- SniderGLLuceyECChristensenTG1984Emphysema and bronchial secretory cell metaplasia induced in hamsters by human neutrophil productsAm Rev Respir Dis129155606561016
- SongJJRheeJGSuntharalingamM2002Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2J Biol Chem277465667512244106
- StahlELindbergAJanssonSA2005Health-related quality of life is related to COPD disease severityHealth Qual Life Outcomes35616153294
- StanescuDSannaAVeriterC1996Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophilsThorax51267718779129
- SteyCSteurerJBachmannS2000The effect of oral NACin chronic bronchitis: a quantitative systematic reviewEur Respir J162536210968500
- SzkudlarekUZdziechowskiAWitkowskiK2004Effect of inhaled N-acetylcysteine on hydrogen peroxide exhalation in healthy subjectsPulm Pharmacol Ther171556215123225
- TakeyamaKDabbaghKLeeHM1999Epidermal growth factor system regulates mucin production in airwaysProc Natl Acad Sci U S A963081610077640
- TattersallABBridgmanKMHuitsonA1984Irish general practice study of acetylcysteine (Fabrol) in chronic bronchitisJ Int Med Res12961016373444
- TenenbeinM1984Hypersensitivity-like reactions to N-acetylcysteineVet Hum Toxicol26Suppl 2356523726
- TsikasDSandmannJIkicM1998Analysis of cysteine and N-acetylcysteine in human plasma by high-performance liquid chromatography at the basal state and after oral administration of N-acetyl-cysteineJ Chromatogr B Biomed Sci Appl70855609653946
- UrbanTAkerlundBJarstrandC1997Neutrophil function and glutathione-peroxidase (GSH-px) activity in healthy individuals after treatment with N-acetyl-L-cysteineBiomed Pharmacother51388909452788
- Van AntwerpenPBoudjeltiaKZBavarS2005Thiol containing molecules interact with MPO/H2O2/chloride system to inhibit LDL oxidationBiochem Biophys Res Commun33782816171780
- van OverveldFJDemkowUGoreckaD2005New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteineJ Physiol Pharmacol56Suppl 41354216204787
- van OverveldFJVermeirePADe BackerWA2000Induced sputum of patients with chronic obstructive pulmonary disease (COPD) contains adhesion-promoting, therapy-sensitive factorsInflamm Res4981310778915
- VentrescaGPCicchettiFerrariBragaPC1989Drugs in bronchial mucologyNew YorkRaven Pr77102
- VerrillDBartonCBeasleyW2005The effects of short-term and long-term pulmonary rehabilitation on functional capacity, perceived dyspnea, and quality of lifeChest1286738316100153
- VestboJPrescottELangeP1996Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study GroupAm J Respir Crit Care Med153153058630597
- WedzichaJADonaldsonGC2003Exacerbations of chronic obstructive pulmonary diseaseRespir Care4812041314651761
- WilkinsonTMPatelISWilksM2003Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1671090512684248
- WolkoveNDajczmanEColaconeA1989The relationship between pulmonary function and dyspnea in obstructive lung diseaseChest961247512582829
- WuytsWAVanaudenaerdeBMDupontLJ2003Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cellsRespir Med978111712854631
- ZafarullahMLiWQSylvesterJ2003Molecular mechanisms of N-acetylcysteine actionsCell Mol Life Sci20036062012613655
- ZuWallackRLHaggertyMCJonesPClinically meaningful outcomes in patients with chronic obstructive pulmonary diseaseAm J Med117Suppl 12A49S59S15693643