Abstract
Study Objectives
The S-stereoisomer found in racemic albuterol may have associated proinflammatory properties. We tested the hypothesis that airway inflammation as assessed by exhaled nitric oxide is no different in patients with COPD when using racemic albuterol relative to levalbuterol or placebo.
Measurements
Twelve mild to moderate COPD patients were assigned to five days each of nebulized racemic albuterol, levalbuterol, and saline placebo. Before and after each course of treatment, airway inflammation was assessed via exhaled nitric oxide breath testing. Secondary functional outcomes that were measured included spirometry, a functional assessment utilizing a six-minute walk, and symptoms score using the University of California, San Diego Shortness of Breath Questionnaire.
Results
There was no statistically significant difference in pre and post FeNO levels within and between treatment groups (p = 0.121). There were also no significant differences within or between treatment groups for the secondary outcome measurements of FEV1 (p = 0.913), functional assessment utilizing a six-minute walk (p = 0.838) and the symptom scores using Shortness of Breath Questionnaire (p = 0.500).
Conclusion
We found no difference in mild to moderate COPD patients treated with racemic albuterol, levalbuterol or placebo for measurement of exhaled nitric oxide or the secondary outcomes that were measured.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease state characterized by airflow limitation that is not fully reversible and progressive chronic inflammation throughout the airways, parenchyma, and pulmonary vasculature (CitationGOLD 2005). Various bronchodilator medications, to include the β2-agonists, are commonly used in treating COPD. β2-agonists bind to the β2-receptor and relax airway smooth muscle reducing airflow obstruction. They are recommended both on a regular schedule and on an as needed basis (CitationGOLD 2005).
Albuterol, a short-acting β2-agonist, is available as a racemic preparation that is composed of (R) and (S)-isomers in a 50:50 mixture or as a single (R)-isomer (levalbuterol). The (R)-isomer is predominantly responsible for the bronchodilator effect and side effects of tachycardia, tremor, and nervousness (CitationHandley 2001). The (S)-isomer, considered inert due to its weak binding to the β2-adrenoceptor, has been reported to have potentially deleterious effects (CitationHandley 1999, Citation2001; CitationPage and Morley 1999) to include the promotion of bronchoconstriction and bronchial hyperresponsiveness (CitationMazzoni et al 1994; CitationWang et al 1994; CitationGauvreau et al 1997; CitationTempleton et al 1998). In addition, the (S)-isomer has been reported to have various proinflammatory effects including alterations in cytokine production (Gavreau et al 1997; CitationFrieri et al 2000; CitationCho et al 2001; CitationBaramki et al 2002), enhanced production of histamine (CitationCho et al 2001), immune cell proliferation/activation (CitationManolitsas et al 1995; CitationMorley et al 1995; CitationGauvreau et al 1997; CitationVolcheck et al 1998; CitationBaramki et al 2002), and increased nitric oxide release in stimulated small airway epithelial cells (CitationFrieri et al 2000).
As chronic inflammation can contribute to accelerated loss of lung function, the use of levalbuterol has been considered due to concern about the inflammatory potential of the (S)-isomer (CitationCostello 1999). Although information exists suggesting that the (S)-isomer may induce inflammation in the setting of asthma, to our knowledge, there are no studies investigating the presence of inflammatory response in the setting of COPD. In this study, we tested the hypothesis that airway inflammation, as measured by exhaled nitric oxide, is no different in COPD patients when sequentially comparing nebulized racemic albuterol, levalbuterol, or placebo.
Airway inflammation was assessed by measurement of the fraction of exhaled nitric oxide (FeNO), a marker of airway inflammation. Relative to baseline levels or normal controls, elevated FeNO levels have been documented in the setting of COPD (CitationAgusti et al 1999; CitationCorradi et al 1999; CitationAnsarin et al 2001; CitationKharitonov and Barnes 2004), chronic bronchitis (CitationDelen et al 2003) and COPD exacerbations (CitationMaziak et al 1998; CitationAgusti et al 1999). Additionally, inhaled corticosteroids have been shown to reduce baseline levels of elevated FeNO in stable COPD (CitationZietkowski et al 2005), suggesting that FeNO may serve as a surrogate measure of chronic perseverant airway inflammation at baseline that is ameliorated through the use of inhaled corticosteroids.
Methods
The study was conducted at Wilford Hall Medical Center and Brooke Army Medical Center in San Antonio, Texas. Patients 18 years of age and older, who had at least a 10 pack year smoking history with no recent tobacco use within the six months preceding protocol enrollment, and mild to moderate COPD defined by a baseline forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of less than 0.70 and an FEV1 greater than 50% predicted were enrolled in the study. The Brooke Army Medical Center Institutional Review Board approved the study, and all patients gave written informed consent.
Twelve patients were enrolled for the study. They were randomized, but not blinded, to complete three separate five-day treatments. The treatment groups consisted of albuterol (2.5 mg four times daily), levalbuterol (0.63 mg three times a day with one saline placebo dose), and 5 ml of saline placebo four times daily. All medications were administered through a nebulizer machine (PARI LC nebulizer, Midlothian, VA) that the patients took home with them. Each patient received instruction in how to properly use the nebulizer device and administer the medication. They were also instructed to complete a diary at home that recorded their use of medication. There was a minimal two day washout period between treatment groups during which the patients abstained from medications intended to treat COPD. All patients were provided with an albuterol metered dose inhaler (MDI) which could be used for “rescue” purposes at any time during the study. However, rescue inhaler use constituted ground for exclusion from further study participation. None of the participants used a rescue treatment throughout the course of the study.
Patients were excluded from the study if they had had an exacerbation of their COPD requiring an adjustment in their COPD medications, the addition of an oral corticosteroid, or the administration of antibiotics, within the 6 months prior to trial enrollment. Patients were also excluded if they had a history of hospitalization due to COPD. Further exclusion criteria included use of an inhaled corticosteroid regimen or phosphodiesterase inhibitor (ie, theophylline) within the previous one month, use of an oral β-blocker medication, long-acting inhaled β-agonist, antihistamine, or nasal corticosteroid within the preceding week of, or during, trial participation. Patients could not have evidence of a concurrent restrictive lung disease on pulmonary function testing, or 12% or greater improvement in FEV1 following administration of a bronchodilator. All patients who experienced a COPD exacerbation during the study interval were excluded from continuing the study (n = 1).
The primary outcome of the study was the measurement of FeNO. Based on a previous study (CitationAgusti et al 1999) in clinically stable COPD patients following exacerbation, we assumed a mean exhaled nitric oxide level of 15.8 ± 3.8 parts per billion at baseline. An increase of FeNO by 2.4 standard deviations was considered to be significant. Our study was powered at 80% to detect this difference with a level of confidence of 95%. Nine subjects acting as their own control were needed to detect a difference with the desired level of power and confidence.
FeNO measurements were made using a NIOX® nitric oxide monitoring system (Aerocrine AB, Sweden) using a flow rate of 50 ml/sec and reported as parts per billion (ppb). FeNO levels were measured both before and after completion of each treatment group and were carried out in accordance with the guidelines published by the American Thoracic Society (CitationATS 1999).
Secondary outcomes that were measured included spirometry, a functional assessment utilizing a six-minute walk, and symptom score using the University of California, San Diego Shortness of Breath Questionnaire (SOBQ) (CitationEakin et al 1998). A Sensorimedics 6200 machine was used for spirometry to record FEV1, FVC, and FEV1/FVC ratio. The six-minute walk test (6MWT) was conducted in accordance with the guidelines described by the American Thoracic Society in their 2002 Official Statement (CitationATS 2002). A MeterMan measuring wheel (Model 1212) was used to measure the distance covered by the patient in the six-minute period. A difference of 54 meters in the six-minute walk distance was considered to be clinically significant based on previous estimates (CitationRedelmeier et al 1997). The SOBQ was used with permission from the University of California, San Diego Medical Center’s Pulmonary Rehabilitation Program. Based on previous recommendations a change of 5 units for the SOBQ was considered to be a minimal clinically important difference (CitationKupferberg et al 2005). Secondary outcome measurements were obtained at the completion of each treatment group and compared with baseline measurements.
Data were analyzed using SPSS Sample Power, Version 2.0 (SPSS Inc, Chicago, IL). The distributions of the FeNO values were not significantly different from normal on the Kolmogorov-Smirnov test (p > 0.05) and were therefore analyzed with parametric testing. Paired t-test was used to assess differences in FeNO between times within treatments. Repeated measures of ANOVA using paired t-test on FeNO, FEV1, and six minute walk by treatment were used for between group comparisons. The Hyun-Feldt statistic was used to analyze the summed SOBQ scores for between group comparisons as the data were not normally distributed.
Results
Patient characteristic data is shown in . The average age of our patients was seventy years and ranged from fifty-five to eighty. There were nine males and three females. All patients had a baseline obstructive FEV1/FVC ratio less than 0.70. Baseline FEV1s were normal in four patients, mild in five, and moderate in three. Ten patients completed the study. One patient withdrew secondary to transportation issues. Another withdrew secondary to a COPD exacerbation that occurred while receiving placebo, which required treatment. No other patients reported use of albuterol for “rescue” purposes during the course of the study.
Table 1 Subject characteristics (n =12)
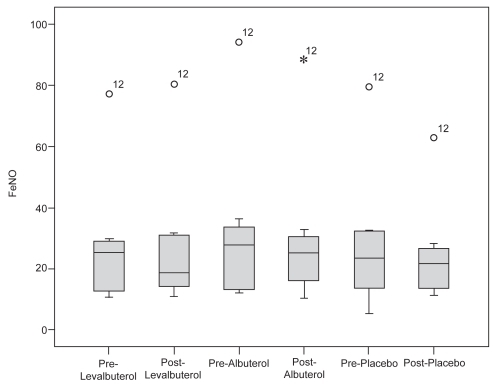
The mean pre- and post-treatment FeNO levels for all patients in each treatment group are listed in . The data are represented in an intention-to-treat format and include the two participants who were eventually excluded from the trial. Due to the constraints of the statistical methods, the remaining calculations only include the 10 subjects who completed the study. In an analysis of those patients, the mean FeNO levels and standard deviation were as follows: levalbuterol (pre- 27.3 ± 19.1 ppb; post- 26.1 ± 20.6 ppb), albuterol (pre- 30.6 ± 24.4 ppb; post- 28.9 ± 22.4 ppb), and placebo (pre- 26.9 ± 20.5 pbb; post- 23.8 ± 15.0 ppb). The between-group comparisons in FeNO are noted in the box plot in . There was no statistically significant difference in FeNO between treatment groups (p = 0.121). Patient number twelve was an outlier and upon re-review of the patient’s medical records and condition we were not able to explain the elevation in FeNO. This patient’s data were included in the analysis. Repeated analysis of treatment effect with subject number twelve excluded did not change the significance of our findings.
Table 2 Mean pre- and post-exhaled nitric oxide levels (parts per billion)
Figure 1 Box-plot of FeNO by treatment and time. The median is the dark line within each box. Each box is defined by the 25th and 75th percentiles so 50% of cases have values within the box. The error flags represent the largest and smallest observed values that are not outliers. No significant differences were noted between groups (p = 0.121).
Abbreviations: FeNO, fraction of exhaled nitric oxide.
Results for the secondary outcome measurements are listed in . There were no differences within or between treatment groups for FEV1 (p = 0.913), functional assessment utilizing the 6MWT (p = 0.838) and symptom scores using the SOBQ (p = 0.500). Complete SOBQ data were available for eight patients. There was a 6.25 and 4 unit increase in the SOBQ score with levalbuterol and albuterol respectively compared with baseline.
Table 3 Secondary outcome measures
Discussion
Given the associated proinflammatory potential of the (S)-isomer of albuterol, our study was designed to determine if airway inflammation, as measured by FeNO, was different in patients with COPD treated with albuterol compared with levalbuterol or placebo. We anticipated to measure a rise in FeNO if the (S)-isomer of albuterol was associated with induction of inflammation. In addition, we sought to determine if there were significant differences in racemic albuterol or levalbuterol relative to placebo in terms of improvement in spirometry, functional assessment utilizing a 6MWT, and symptom scores using the SOBQ.
We found no difference within or between treatment groups for measurement of FeNO, spirometry, functional capacity, or symptom scores. However, there was a 6.25 and 4 unit increase in the SOBQ score with levalbuterol and albuterol, respectively, compared to baseline. A five unit increase of the SOBQ score has previously been reported to represent a minimal clinically important difference suggesting levalbuterol may have provided a clinically significant improvement in dyspnea. However, these conclusions are limited by the open-label property of the study.
Of note, the amount of (R)-isomer in 2.5 mg of racemic albuterol is 1.25 mg, which is higher than the equivalent (R)-isomer dose of 0.63 mg used in the levalbuterol arm of the study. This could have limited our ability to find significant differences in the utilized functional secondary outcome measures. However, by using the higher dose of racemic albuterol, we administered a larger amount of (S)-isomer, which should have amplified any (S)-isomer associated difference in attendant airway inflammation, if present. No increase in airway inflammation, as measured by FeNO, was found with this higher dose of albuterol.
Our study had some limitations. Our study subjects’ baseline FeNO levels were higher than we initially assumed. This may have limited the sensitivity of the study. In addition, the small sample size may have limited our ability to detect a difference in FeNO levels if one existed. Also, the patients were not blinded to the treatment groups, which may have influenced the reporting of effort or subject-related measures such as walking effort or symptom score reporting. However, this should not have influenced the primary outcome measure of FeNO. A majority of our subjects had relatively mild disease, which may have limited our ability to detect clinically significant differences in the secondary outcomes that were measured. Lastly, the length of time on each therapy may not have been enough to cause a significant change in airway inflammation that could be detected by FeNO.
The prospects for improved therapy in COPD by the use of levalbuterol have been discussed previously (CitationCostello 1999). Despite the theoretical advantages there have been few studies addressing the potential usefulness of levalbuterol in the management of COPD. In a study by CitationTruitt and colleagues (2003), levalbuterol demonstrated a clinical and economic advantage over racemic albuterol in the treatment of hospitalized patients with COPD and asthma, although the significance of this finding has been brought into question (CitationHendeles 2003). Another study by CitationDatta and colleagues (2003) found no advantage of using levalbuterol over conventional nebulized bronchodilators in single dose for as needed treatment in stable COPD.
In conclusion, our study found no evidence of increased airway inflammation with the use of racemic albuterol compared with levalbuterol as determined by measurement of FeNO. In addition, there were no significant differences between levalbuterol and racemic albuterol in the secondary outcomes that were measured. As a whole, levalbuterol does not appear to be more advantageous than racemic albuterol for the treatment of COPD. Without a clearly derived benefit, the routine use of levalbuterol for the management of COPD is not recommended at this time. Further studies with more subjects and a longer treatment period are needed to confirm these results.
Acknowledgement
We are grateful to Laverne Erskin RN for her support and John A Ward Ph.D. from the Research Physiologist Department of Clinical Investigations, Brooks Army Medical Center for his assistance with statistics for this study.
The authors did not receive grants or outside funding in support of their research or preparation of this manuscript. They did not receive payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Air Force, the Department of Defense, or the United States Government.
References
- AgustiANVillaverdeJMTogoresB1999Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary diseaseEur Respir J,14523810543270
- AnsarinKChatkinJMFerreiraIM2001Exhaled nitric oxide in chronic obstructive pulmonary disease: relationship to pulmonary functionEur Respir J17934811488329
- [ATS] American Thoracic Society1999Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and childrenAm J Resp Crit Care Med16021041710588636
- [ATS] ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories2002ATS statement: Guidelines for the six-minute-walk testAmer J Resp Crit Care Med1661111712091180
- BaramkiDKoesterJAndersonAJ2002Modulation of t-cell function by (R)- and (S)-isomers of albuterol: anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomerJ Allergy Clin Immunol1094495411897990
- ChoSHHarrtleroadJYOhCK2001(S)-albuterol increases the production of histamine and IL-4 in mast cellsInt Arch Allergy Immunol1244788411340331
- CorradiMMajoriMCaccianiGC1999Increased exhaled nitric oxide in patients with stable chronic obstructive pulmonary diseaseThorax54572510377199
- CostelloJ1999Prospects for improved therapy in chronic obstructive pulmonary disease by the use of levalbuterolJ Allergy Clin Immunol104S61810452790
- DattaDVitaleALahirsB2003An evaluation of nebulized levalbuterol in stable COPDChest124844912970007
- DelenFMSippelJMOsborneML2003Increased exhaled nitric oxide in chronic bronchitis: comparison with asthma and COPDChest11769570110712993
- EakinEGResnikoffPMPrewittLM1998Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire University of California, San DiegoChest113619249515834
- FrieriMPergolizzitRMillanC2000Cytokine, chemokine and nitric oxide (NO) release in stimulated small airway epithelial cells (SAEC) treated with β2-agonist enantiomers of albuterol [abstract]J Allergy Clin Immunol105S2923
- GauvreauGMJordanaMWatsonRM1997Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjectsAm J Respir Crit Care Med1561738459412549
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease2005Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Updated 2005 (Based on an April 1998 NHLBI/WHO Workshop) [online]Accessed on 23 August 2005 URL: http://www.goldcopd.com/download.asp?intId=231
- HandleyD1999The asthma-like pharmacology and toxicology of (S)-isomers of β agonistsJ Allergy Clin Immunol104S697610452791
- HandleyD2001Single-isomer β-agonistsPharmacotherapy21S217
- HendelesL2003Levalbuterol is not more cost-effective than albuterol for COPD [letter]Chest1241176812970057
- KharitonovSABarnesPJ2004Effects of corticosteroids on noninvasive biomarkers of inflammation in asthma and chronic obstructive pulmonary diseaseProc Am Thorac Soc1191916113434
- KupferbergDHKaplanRMSlymenDJ2005Minimal clinically important difference for the USCD shortness of breath questionnaireJ Cardiopulm Rehabil26370716327533
- ManolitsasNDWangJDevaliaJL1995Am J Respir Crit Care Med1511925307767541
- MaziakWLoukidesSCulpittS1998Exhaled nitric oxide in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med15799810029517624
- MazzoniLNaefRChapmanID1994Hyperresponsiveness of the airways following exposure of guinea-pigs to racemic mixtures and distomers of β2-selective sympathomimeticsPulm Pharmacol7367767549224
- MorleyJMazzoniLChapmanID1995Rac-albuterol causes polymorphonuclear leukocyte accumulation in the pulmonary airways of the guinea-pig [abstract]Am J Respir Crit Care Med151A604
- PageCPMorleyJ1999Contrasting properties of albuterol stereoisomersJ Allergy Clin Immunol104S314110452786
- RedelmeierDABayoumiAMGoldsteinRS1997Interpreting small differences in functional status: the six minute walk test in chronic lung disease patientsAm J Respir Crit Care Med1551278829105067
- TempletonABChapmanIDChilversER1998Effects of s-albuterol on human isolated bronchusPulm Pharmacol Ther1169802957
- TruittTWitkoJHalpernM2003Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthmaChest1231283512527613
- VolcheckGWGleichGJKitaH1998Pro- and anti-inflammatory effects of beta adrenergic agonists on eosinophil response to IL-5 [abstract]J Allergy Clin Immunol101S35
- WangZBramleyAMMcNamaraA1994Chronic fenoterol exposure increases in vivo and in vitro airway responses in guinea pigsAm J Respir Crit Care Med14996058143062
- ZietkowskiZKucharewiczIBodzenta-LukaszykA2005The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary diseaseRespir Med998162415939243