Abstract
Objective
The purpose of this study was to compare peripheral muscle oxygenation in persons with chronic obstructive pulmonary disease (COPD) to healthy control persons, during submaximal exercise.
Methods
Eight persons with COPD (forced expiratory volume in one second [FEV1] = 1.00 ± 0.27 L) and eight healthy control persons (FEV1 = 1.88 ± 0.55L) performed a submaximal graded exercise test (GXT), and completed 4 min of constant load exercise (CON) at 50% of peak GXT. Measurements included oxygen uptake, heart rate, arterial oxygen saturation and peripheral muscle oxygenation (%StO2) at rest, during exercise, and recovery.
Results
Significantly greater workloads were attained for controls compared with COPD for peak GXT and CON. No significant differences in %StO2 were observed between groups at: rest (GXT: 29.5 ± 22.8 vs 30.4 ± 17.3%; CON: 33.3 ± 15.4 vs 35.1 ± 17.2%); peak GXT (29.4 ± 19.4 vs 26.5 ± 15.9%); 4 min of CON (25.9 ± 13.5 vs 34.5 ± 21.8%); and recovery (GXT: 46.6 ± 29.1 vs 44.3 ± 21.7%; CON: 40.9 ± 21.5 vs 44.5 ± 23.2%).
Conclusion
These results suggest that peripheral skeletal muscle oxygenation is not compromised in COPD during submaximal exercise, and limitations in exercise capacity are most likely a result of muscle disuse and poor lung function.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by multiple physiological problems such as impaired ventilation and gas exchange, cardiovascular and peripheral muscle deconditioning, muscular wasting and weakness, and dypsnea (CitationSala et al 1999; CitationSimon et al 2001; CitationReusch 2002; CitationOkamoto et al 2003; CitationSaey et al 2003; CitationRichardson et al 2004). Limitations in ventilation (including airflow obstruction, and lung hyperinflation leading to mechanical disadvantage of the ventilatory muscles) and gas exchange have been considered to be the primary causes of exercise intolerance in COPD (CitationATS and ERS 1999). However, research has recently focused on the limitations in skeletal muscle function that may further contribute to exercise intolerance (CitationRichardson et al 2004). The primary focus of the research on skeletal muscle function in persons with COPD has attempted to determine whether the limitations in skeletal muscle function are a result of muscle dysfunction associated with COPD pathology, or muscle disuse (CitationATS and ERS 1999).
Studies examining the role of skeletal muscle in the pathophysiology of COPD have primarily compared individuals with COPD to control participants who are regularly active (CitationMaltais et al 1998; CitationOkamoto et al 2003), or have not utilized a control group for comparison purposes (CitationSimon et al 2001; CitationSaey et al 2003). Thus, it is difficult to assess the effects COPD is having on skeletal muscle function. Previous research by CitationSimon and colleagues (2001) demonstrated that patients with COPD have limitations in blood flow directed to peripheral muscles and that oxygen extraction during exercise is limited. This was attributed to a redistribution of cardiac output and oxygen from lower limb exercising muscles to ventilatory muscles. CitationCasaburi et al (1991), Maltias et al (1998), CitationOkamoto et al (2003), and CitationSaey et al (2003) have also provided evidence that skeletal muscle dysfunction may be responsible for exercise impairment through a variety of mechanisms including an early onset of lactic acidosis, contractile fatigue, and an impaired re-oxygenation of peripheral muscle. In contrast to these findings, CitationRichardson and colleagues (2004) demonstrated that the muscle metabolic capacity of persons with COPD and healthy, age matched sedentary controls is similar, and suggested that muscular mechanical efficiency is reduced in persons with COPD and may be the primary factor limiting skeletal muscle function.
In summary, it is difficult to assess if the limitations in skeletal muscle function in persons with COPD results from muscle dysfunction associated with COPD pathology or muscle disuse because control subjects have not been closely matched to COPD subjects in previous studies. The aim of this study was to further investigate if the limitations in skeletal muscle function in persons with COPD results from muscle dysfunction associated with COPD pathology or muscle disuse. In the present study we compared peripheral muscle oxygenation, monitored by near infrared spectroscopy (NIRS), during incremental and constant load aerobic exercise, in persons with COPD against healthy control persons, who were matched by age, weight, and gender.
Methods
Participants
Eight persons with COPD and eight healthy age, weight, and gender matched-control persons completed this study. Although a quantitative assessment of activity levels was not done, all subjects were interviewed through personal communications about current activity levels, and we attempted to closely match subjects by their verbal description of their activity levels. Participants’ activity levels were matched for type, duration and intensity of exercise performed, as well as activities of daily living that they completed on a day to day basis (ie, laundry, vacuuming, grocery shopping). The diagnosis of COPD was based on current clinical evaluation by the participant’s doctor and from pulmonary function testing during participation in the study. Participants were not suffering from any acute illness at the time of study and were free from any other medical conditions that would limit their ability to perform the exercise testing. Participants were all nonsmokers at the time of the study with the exception of one COPD participant. The participant was asked to abstain from smoking 24hrs prior to any testing and this was confirmed verbally with the investigator upon arrival to the laboratory. Written informed consent, which was approved by the human subjects committee at Florida Agricultural and Mechanical University, was obtained from each individual prior to participation.
Incremental exercise testing
Participants visited the laboratory on two separate occasions. During the first visit, participants performed a submaximal (~70% of estimated maximal heart rate), symptom-limited, graded exercise test (GXT) on an electronically braked cycle ergometer (SensorMedics, Ergoline 800S™, Yorba Linda, CA). Participants rested quietly for 5 min prior to the start of exercise so that baseline resting measurements could be obtained. The GXT utilized a ramp protocol, starting at 0 W for the 1st min and then increased by 7 W every minute thereafter for persons with COPD, and by 15 W for control participants. Participants were constantly monitored to maintain their cadence between 50–60 rpm. Exercise was terminated upon attainment of 70% estimated maximal heart rate and participants remained quietly seated for the next 5 min for the collection of post exercise measurements.
Constant load exercise testing
During a second visit to the lab, participants performed a 6 min steady state exercise test (constant load) at 50% of the workload obtained during the submaximal GXT. Prior to the start of the test, participants rested for 5 min for the determination of resting baseline measurements and then began the exercise test. At the start of exercise, participants warmed up for 1 min at 0 W. The workload then increased to 25% of the workload obtained during the submaximal GXT for 1 min. Following this 2 min warm-up period, the workload was increased to 50% of the workload obtained during the submaximal GXT and participants maintained this workload for 6 min. Participants were constantly monitored to maintain their cadence between 50–60 rpm.
Oxygen uptake, heart rate and dyspnea measures
Oxygen uptake (VO2) was determined by breath-by-breath gas exchange analysis (SensorMedics, Vmax 229™ Metabolic Cart System, Yorba Linda, CA). Before each exercise test, the flow-volume sensor was calibrated with a 3-L calibration syringe and gas analyzers were calibrated using known gas concentrations. The metabolic data were averaged over the final 20 sec of each minute of collection. A 12-lead electrocardiogram (ECG) (Quniton, Q4500™, Bothall, WA) was utilized to continuously monitor heart rate (HR) and was supervised by a physician during all testing sessions. Dyspnea was subjectively rated during the last 10 seconds of each minute of exercise and recovery, using a modified Borg, 0 to 10 scale (CitationJones 1988).
Peripheral muscle oxygenation and arterial oxygenation
Peripheral muscle oxygenation (%StO2) was continuously estimated, noninvasively, through NIRS during rest, exercise, and recovery periods (Inspectra Tissue Spectrometer Model 325 System, Hutchinson Technology Inc., Hutchinson, MN). The principles of NIRS and the reliability and validity of the measurement have been previously described in detail (CitationMancini et al 1994; CitationAustin et al 2005). Briefly, the NIRS probe was placed on the right vastus lateralis muscle approximately 14–20 cm from the knee joint. The measurement site for each individual was marked with a permanent marker, to locate the same placement of the probe for both exercise tests. Before each exercise test, the NIRS probe was calibrated to known wavelengths equivalent to a StO2 measurement of 36 ± 2% and 85 ± 2%, according to the manufacturer’s protocol and specifications. The NIRS unit was interfaced with a computer and %StO2 data were averaged over the final 20 sec of each minute of collection.
Arterial oxygen saturation was continuously estimated (%SpO2), during rest, exercise, and recovery periods with a pulse oximeter (SensorMedics, StatTrak™, Yorba Linda, CA) and attached to the subject by a finger probe. The pulse oximeter was electronically integrated to the SensorMedics, Vmax 229™ Metabolic Cart System. The %SpO2 data were averaged over the final 20 sec of each minute of collection.
Pulmonary function testing
During the second visit and prior to exercise, participants completed a pulmonary function test (SensorMedics, Vmax 229™ Metabolic Cart System, Yorba Linda, CA) using standard spirometry procedures (CitationATS 1995) for the determination of forced vital capacity (FVC), and forced expiratory volume in one second (FEV1). Before each pulmonary function test, the flow-volume sensor was calibrated with a 3-L calibration syringe. Reference equations from CitationMorris and colleagues (1971) were used to derive the subject’s predicted spirometric values.
Statistical analysis
Independent t-tests were utilized to compare differences between groups for measures of the participant physical characteristics, and for the measures of workload during GXT and constant load exercise. A repeated measures analyses of variance (ANOVA; group × time) was utilized to compare differences between groups and across time for the measures of VO2, HR, %StO2, %SaO2, and dyspnea during the GXT, and the constant load exercise test. When interactions or main effects were determined to be significantly different, independent t-testing was used to determine which individual means were significantly different. Simple linear regression analysis was utilized to examine possible relationships between %StO2 and VO2, %SaO2, and measures of pulmonary function (FEV1, FVC, and %FEV1/FVC), during GXT and constant load exercise. A statistical significance of p ≤ 0.05 was used for all analyses.
Results
Physical characteristics of participants
presents the descriptive characteristics of the participants. Both groups were closely matched in gender, age, height, weight, and body mass index (BMI). Persons with COPD had significantly lower FEV1, percent predicted FEV1, and %FEV1/FVC values than control participants. Persons with COPD were classified as having moderate to severe COPD, based on percent predicted values () (CitationATS 1986).
Table 1 Physical characteristics of participants (N = 16)
Graded exercise testing
Significantly greater workloads were attained for control participants, compared with persons with COPD, at peak GXT (73.8 ± 36.2W vs 36.9 ± 11.9W). presents data from the GXT. A significant interaction between groups and time was observed for VO2 and HR. Control participants compared with persons with COPD, had significantly greater peak VO2 values (12.3 ± 3.5 ml O2*kg−1*min−1 vs 7.9 ± 2.9 ml O2*kg−1*min−1) and peak HR values (122 ± 11 beats*min−1 vs 110 ± 7 beats*min−1). No significant differences between groups were found for measures of dyspnea.
Table 2 Graded exercise test (GXT) (N = 16)
No significant differences in %StO2 were observed between groups (control vs COPD), at rest, peak exercise, or during recovery from exercise. A significant interaction between groups and time was observed for %SaO2. Significantly higher %SaO2 values for control participants, compared with persons with COPD, were found at rest (96.8 ± 1.0% vs 93.5 ± 1.7%) and during min 1 of recovery (96.0 ± 1.3% vs 92.8 ± 2.4%) from exercise.
Constant Load Exercise testing
The eight control participants completed 6 min of constant load exercise. Only six COPD participants completed 6 min of constant load exercise. Seven COPD participants completed 5 min of constant load exercise and all eight COPD participants completed 4 min of constant load exercise. Significantly greater workloads were attained for control participants, compared with persons with COPD, during constant load exercise (36.9 ± 18.1W vs 18.1 ± 6.5W).
presents data from the constant load exercise test. A significant interaction between groups and time was observed for VO2 and HR. Control participants, compared with persons with COPD, had significantly greater VO2 values at min 4 of constant load exercise (9.3 ± 3.6ml O2*kg−1*min−1 vs 6.1 ± 1.7 ml O2*kg−1*min−1). Control participants, compared with persons with COPD, had significantly lower HR values at rest (67 ± 11 beats*min−1 vs 86 ± 8 beats*min−1), and during recovery from exercise at min 1 (77 ± 13 beats*min−1 vs 93 ± 12 beats*min−1) and min 5 (72 ± 12 beats*min−1 vs 89 ± 11 beats*min−1). No significant differences between groups were found for measures of dyspnea.
Table 3 Constant load exercise
No significant differences in %StO2 were observed between groups (control vs COPD), at rest, constant load exercise, or during recovery from exercise. A significant interaction between groups and time was observed for %SaO2. Significantly higher %SaO2 values for control participants, compared with persons with COPD, were found at rest (96.3 ± 1.3% vs 93.6 ± 1.5%), at min 4 of constant load exercise (95.8 ± 2.2% vs 92.9 ± 2.2%), and during recovery from exercise at min 1 (95.9 ± 2.0% vs 93.4 ± 2.6%) and min 5 (96.8 ± 0.7% vs 94.9 ± 1.0%).
Relationships between %StO2 and VO2, %SaO2 and measures of pulmonary function
shows correlation coefficients (r values) between %StO2 and VO2, %SaO2 and pulmonary function measures (FEV1, FVC, and %FEV1/FVC) during incremental and constant load exercise, in all subjects, at various time points. The only significant correlations observed were between %StO2 and VO2, at rest, during both GXT (r = 0.60) and constant load exercise (r = 0.62).
Table 4 Correlation coefficients (r values) between peripheral muscle oxygenation (%StO2) and oxygen uptake (VO2), arterial oxygenation (%SaO2) and pulmonary function measures (forced expiratory volume in one second [FEV1], forced vital capacity [FVC], and %FEV1/FVC) during incremental (GXT) and constant load exercise, in all subjects (N = 16)
Responses of %StO2 during exercise
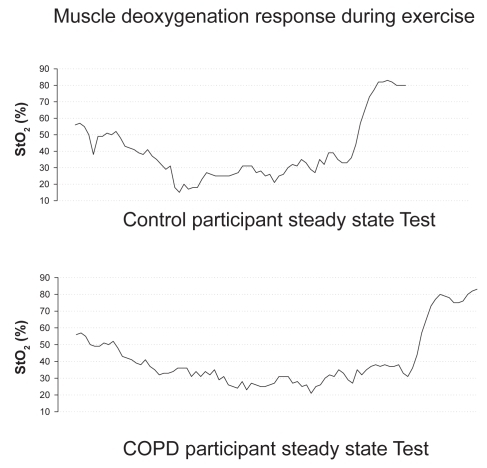
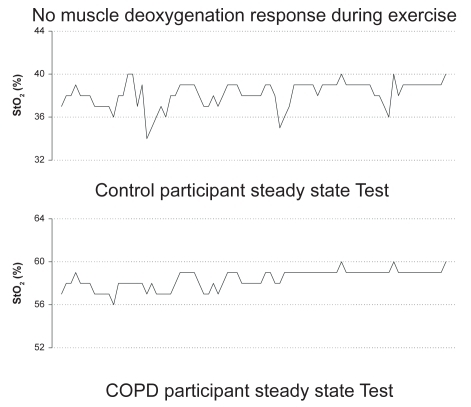
Differences in the exercise and post-exercise %StO2 measures that were demonstrated in both COPD and control participants during the GXT and CON tests can be found in and . In most participants (5 COPD, 5 control), no significant change in %StO2 was found from rest to peak exercise load during the incremental exercise test or during the constant load exercise test (). A significant change in %StO2 was based on the known standard error of the equipment during cycle exercise and placing a 95% confidence interval around the resting %StO2 value; thus participants had to achieve a greater than 8% change in StO2 values. In contrast, six participants (3 COPD, 3 control) demonstrated a steady decline in %StO2 (). The post exercise %StO2 response was characterized by a rapid re-oxygenation in these 6 participants (3 COPD, 3 controls). However, the other participants (5 COPD, 5 controls) demonstrated almost no change or an increase above resting values in %StO2 following the incremental and constant load exercise tests.
Discussion
Prior research examining the causes of exercise intolerance in persons with COPD attributed exercise cessation primarily to dyspnea and limitations in ventilation and gas exchange that limit O2 delivery to the exercising muscle (CitationMaltais et al 1998; CitationATS and ERS 1999; CitationRichardson et al 2004). However, it has been demonstrated that patients with similar FEV1 values have a wide range of exercise capacity (CitationJones and Killian 1991) and that cardiorespiratory fitness is partially dependent on peripheral muscle function (CitationMaltais et al 1998). Previous findings have also suggested that peripheral muscle weakness in persons with COPD is a result of functional changes that have lead to skeletal muscle dysfunction (CitationATS and ERS 1999). However, these same observed changes are also found in deconditioned muscle and suggest that disuse rather than pathological dysfunction is what causes changes in muscle structure and function (CitationBooth and Gollnick 1983; Larson and Ansved 1985).
In the present study we attempted to match our participants for age, gender, BMI, and activity levels. Nonetheless, control participants had greater lung function and higher exercise capacities than the COPD participants as was evidenced by the pulmonary function data, VO2 data, exercise workloads, and the inability of 2 COPD participants to complete 6 min of constant load exercise. Cessation of exercise for the two participants that completed only 4 min and 5 min of exercise, respectively, at their constant workload, was because of muscular fatigue. However, it is interesting to note that during both exercise tests, that there were no significant differences in dyspnea ratings between COPD and control participants. Since the Borg rating scale used in this study is a validated assessment tool for breathlessness (CitationKillian 1985), we believe the results reflect that the exercise intensities were relatively low. Thus, our findings are in agreement with previous studies (CitationMaltais et al 1998; CitationATS and ERS 1999; CitationRichardson et al 2004) suggesting that exercise intolerance in COPD may be primarily attributed to impaired oxygen delivery associated with COPD pathology causing limitations in ventilation and gas exchange. Furthermore, in the present study we observed that control participants and persons with COPD had similar levels of %StO2 during exercise. This suggests that COPD pathology does not cause muscle dysfunction in terms of impaired muscle oxygenation in persons with moderate COPD.
The findings of our study are in agreement with CitationRichardson and colleagues (2004) who employed direct measurements of oxygen utilization and blood flow in evaluating metabolic capacity in persons with COPD. Despite the finding of a greater percentage of Type II fibers, structure in terms of muscle fiber cross sectional area, capillary density and mitochondrial volume were not different between COPD and controls. Thus, Richardson concluded that limitations in exercise capacity were a result of mechanical inefficiency (ie, work performed per unit of oxygen consumed) due to an increase in Type II muscle fibers, and that persons with COPD do not demonstrate a diminishment in muscle O2 utilization due to dysfunction. While we did not perform assessment of muscle type and structure in the present study, the nonsignificant differences observed between the groups in measures of %StO2 provide evidence to support the theory that muscle disuse leading to inefficiency rather than muscle dysfunction may further be reducing exercise capacity in persons with COPD and sedentary, healthy adults.
Others have also reported that muscle O2 delivery is maintained in those with COPD, however impaired O2 utilization was reported and muscle dysfunction suggested (CitationMaltais et al 1998; CitationSimon et al 2001). Maltais and colleagues have shown that peripheral O2 delivery is preserved in persons with COPD and that the significantly greater skeletal muscle acidosis observed in these individuals at peak O2 uptake was potentially a cause for the early cessation of exercise; thus reflecting an altered metabolic function of the skeletal muscle and indicating dysfunction.
Several other explanations for the reported disparity between supply of muscle oxygen and utilization have been proposed; however all have concluded that this indicates muscle dysfunction rather than detraining. Saey and colleagues have suggested that exercise tolerance in COPD during constant load cycling is dependent on contractile leg fatigue, and that differences in fatigue existed between individuals. When participants were divided based on fatigability during the exercise test, it was found that despite improving FEV1 through the use of a bronchodilator, that peripheral muscle fatigue limited exercise tolerance in those who demonstrated early fatigue. Unfortunately a control group was not utilized for comparison purposes.
In a study examining limitations in lower limb oxygen consumption of persons with COPD, CitationSimon and colleagues (2001) reported that exercise capacity was significantly greater in participants who did not demonstrate a plateau in leg oxygen consumption, than in those participants who did plateau in leg oxygen consumption despite a continued increase in total body oxygen uptake. This was attributed to a redistribution of cardiac output O2 from the lower extremities to pulmonary musculature in the plateau group. The rationale provided for redistribution was in part attributed to a limitation in O2 extraction by peripheral exercising muscle. The results of CitationSimon et al (2001) and Saey et al (2001) indicate that despite ventilatory limitation, some individuals with COPD are additionally limited during whole body cycling exercise by the ability of the peripheral musculature to utilize oxygen.
An additional study by CitationOkamoto and colleagues (2003) which also utilized NIRS to assess peripheral skeletal muscle oxygen utilization, reported a significantly greater time for re-oxygenation following submaximal exercise in persons with COPD when compared with controls. It was additionally found in persons with COPD that a significant positive relationship existed between the time VO2 remained elevated during exercise and duration needed for complete re-oxygenation of peripheral skeletal muscle. This relationship was not found in the present study. In addition, VO2 and %StO2 were not significantly different between the groups at any time point of recovery during the GXT or constant load exercise test; however, %SaO2 was significantly lower in COPD than controls during recovery. The discrepancy between our study and that of CitationOkamoto and colleagues (2003) might be attributed to differences in the control groups that were utilized for comparison purposes.
Several differences in the exercise and post-exercise %StO2 measures were observed ( and ) for the COPD and control participants of the present study. Some subjects demonstrated a desaturation response during both types of exercise. As noted above, we believe that the exercise workloads in this study were likely of relatively low intensity, as indicated by similarities in dyspnea ratings between groups. Thus, it is possible that the intensity was not high enough to stimulate a desaturation response. However, this same phenomena (lack of tissue desaturation) has been observed in well trained triathletes, cyclists, and runners during high intensity exercise (ie, exercise tests to lactate threshold and maximal tests to exhaustion) (CitationSnyder and Parmenter 2002; CitationSnyder et al 2003; CitationAustin et al 2005). Thus, we believe that the workload intensities were sufficient for the study purpose, and this lack of tissue desaturation response, in some individuals, does occur in healthy subjects, athletes, and persons with COPD at various exercise intensities. What other possibilities might explain this lack of tissue desaturation response during exercise? Citationvan-Beekvelt and colleagues (2001) have suggested that NIRS measurements may be affected by adipose tissue thickness. These researchers reported muscle oxygen consumption, as measured by NIRS, is negatively correlated (r = −0.70, p < 0.01) to adipose tissue thickness, indicating that adipose tissue is a confounding factor in obtaining valid NIRS measurements. In the present study we did not measure skinfold thickness, thus it is possible that % StO2 readings were skewed because of an excess in adipose tissue at the measurement site. However, we found that when we compared BMI values with the %StO2 response, those with normal BMIs (18.5–24.9) had just as frequent a variation in the %StO2 response as did those with overweight or obese BMIs (≥ 25.0) (CitationExpert Panel 1998). CitationAustin and colleagues (2005) have also reported the same type of varied response for %StO2 in well trained athletes who did not appear to have large amounts of body adiposity at the measurement sites; thus, something in addition to the thickness of adipose tissue appears to influence the results obtained by NIRS and warrants further investigation.
Impaired muscle oxygen utilization has also been reported in persons with insulin resistance, hypertension, cardiovascular disease (CVD), diabetes, and peripheral arterial disease (PAD) (CitationReusch 2002; CitationBauer et al 2004). Prior literature has reported that COPD causes vasoconstriction in the pulmonary vasculature, resulting in pulmonary hypertension (CitationHida et al 2002). Additionally, in COPD there is evidence of an impaired endothelial cell release of the vasodilator and nitric oxide in the pulmonary vasculature due to atherosclerosis (CitationHida et al 2002). This is also reported for persons with cardiovascular disease and PAD in the coronary and peripheral vasculature due to atherosclerosis (CitationReusch 2002). While none of the participants had known PAD, CVD, or diabetes, it may be hypothesized that individuals demonstrating impaired muscle oxygen use and without the post-exercise vasodilatory response may additionally be suffering from a lack in vasodilatory capacity due to impairments of endothelial cell release of nitric oxide.
Limitations of the present study
The major limitation of the present study is the small sample size which may have prevented us from determining any significant differences in %StO2. We had a large and varied response in %StO2 within the COPD and control groups, which limits the power of the data. This may be a result of the varied activity levels of the COPD and control participants, and should be further examined through a larger population of exercising and nonexercising persons for both of these populations. Additionally, several of our participants were taking medications for both hypertension and hyperlipidemia, which may contribute to decreases in nitric oxide, and thus may have further led to the variance in %StO2 responses that were seen in the present study. Further work is needed to assess the impact of such medications on peripheral muscle oxygenation, along with the effects which could be solely attributable to hypertension and hyperlipidemia. Future research that examines the role of peripheral oxygenation in persons with COPD should include the assessment of blood flow, hemoglobin concentrations, and muscle fiber type to further elucidate what may cause such a varied response in %StO2 values.
Summary
Even though we attempted to match our participants for age, gender, BMI, and activity levels, our results indicate that the control participants had higher exercise capacities and greater lung function than the COPD participants as was evidenced by the VO2 data, exercise workloads, and the inability of 2 COPD participants to complete 6 min of constant load exercise. Control participants and persons with COPD had similar levels of %StO2 during exercise in our study. This suggests that COPD pathology does not cause muscle dysfunction in terms of impaired muscle oxygenation in persons with moderate COPD. It is concluded that peripheral skeletal muscle oxygenation is not compromised in individuals with moderate COPD during submaximal aerobic exercise, and that limitations in exercise capacity are most likely a result of muscle disuse and poor lung function.
References
- [ATS] American Thoracic Society1986Evaluation of impairment/disability secondary to respiratory disordersAm Rev Respir Dis133120593509148
- [ATS] American Thoracic Society1995Standardization of spirometry: 1994 updateAm J Respir Crit Care Med1521107367663792
- [ATS and ERS] American Thoracic Society and European Respiratory Society1999Skeletal muscle dysfunction in chronic obstructive pulmonary disease: a statement of the American Thoracic Society and European Respiratory SocietyAm J Respir Crit Care Med159S1S4010194189
- AustinKGDaigleKCowmanJ2005Reliability of near-infrared spectroscopy for determination of muscle oxygen saturation during exerciseRes Q Exerc Sport76440916739682
- BauerTBrassEHiattW2004Impaired muscle oxygen use at onset of exercise in peripheral arterial diseaseJ Vasc Surg404889315337878
- BoothFWGollnickPD1983Effects of disuse on the structure and function of skeletal muscleMed Sci Sport Exerc1541520
- CasaburiRPatessioJIoliF1991Reduction in exercise lactic acidosis and ventilation as a result of exercise training in obstructive lung diseaseAm Rev Respir Dis1439181986689
- Expert Panel1998Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adultsArch Intern Med1581855679759681
- HidaWTunYKikuchiY2002Pulmonary hypertension in patients with chronic obstructive pulmonary disease: recent advances in pathophysiology and managementRespirology731311896895
- JonesNLKillianKJChermiackNS1991Limitation of exercise in chronic airway obstructionChronic obstructive pulmonary disease1st edPhiladelphiaWB Saunders196206
- JonesNL1988Clinical exercise testing3rd edPhiladelphiaWB Saunders77
- KillianKJ1985The objective measurement of breathlessnessChest88885905
- LarssonLAnsvedT1985Effects of long term physical training and detraining on enzyme histochemical and functional skeletal muscle characteristics in manMuscle Nerve8714222932641
- MaltaisFJobinJSullivanMJ1998Metabolic and hemodynamic responses of lower limb during exercise in patients with COPDJ App Physiol84157380
- ManciniDBolingerLLiH1994Validation of near-infrared spectroscopy in humansJ App Physiol7727407
- MorrisJFKoskiAJohnsonLC1971Spirometric standards for healthy nonsmoking adultsAm Rev Respir Dis10357675540840
- OkamotoTKanazawaHHirataK2003Evaluation of oxygen uptake kinetics and oxygen kinetics of peripheral skeletal muscle during recovery from exercise in patients with chronic obstructive pulmonary diseaseClin Physiol Funct Imaging232576212950322
- ReuschJ2002Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndromeAm J Cardiol90suppl19G26G12088773
- RichardsonRLeekBGavinT2004Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak Vo2 with small muscle mass exerciseAm J Respir Crit Care Med169899614500263
- SaeyDDeblgareRLeBlancP2003Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1684253012714348
- SalaERocaJMarradesR1999Effect of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med15917263410351910
- SimonMLeBlancPJobinJ2001Limitation of lower limb VO2 during cycling exercise in COPD patientsJ App Physiol90101319
- SnyderACDormanJCParmenterMA2003Muscle oxygen saturation measurements track changes due to training in ironman triathletesMed Sci Sport Exerc35Suppl 1S37
- SnyderACParmenterMA2002Use of muscle oxygen saturation in determining maximal steady state exercise intensityMed Sci Sport Exerc34Suppl 1S78
- vanBeekveltMBorghuisMvanEngelenB2001Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscleClin Sci10121811410110