Abstract
This review summarizes the long-term clinical outcomes associated with β-agonist and anticholinergic bronchodilator use in patients with chronic obstructive pulmonary disease (COPD). Pooled data from randomized placebo-controlled trials of at least three months duration were used to evaluate the risk for COPD hospitalizations, respiratory mortality, and total mortality. The results show that anticholinergic use is associated with a 30% reduction in COPD hospitalizations, a 70% reduction in respiratory mortality, and without a significant effect on total mortality. In contrast, β-agonist use had no effect on COPD hospitalizations and was associated with a two-fold increased risk for respiratory death compared with placebo. When the two bronchodilators were directly compared with each other, β-agonists were associated with a two-fold increased risk for COPD hospitalization and a five-fold increased risk for total mortality compared with anticholinergics. When β-agonists were added to either anticholinergic use or inhaled corticosteroid use alone, there was no significant improvement in any long-term clinical outcome. These results indicate that anticholinergics should be the bronchodilator of choice in COPD, while β-agonists may be associated with poorer disease control.
Long-term clinical outcomes in COPD
Chronic obstructive pulmonary disease (COPD) is characterized by partially reversible chronic airflow obstruction, caused by inflammatory reactions in the airways and lung parenchyma to inhaled toxins such as tobacco smoke (CitationCelli and MacNee 2004). The airflow obstruction is progressive over time, and is often accompanied by some degree of airway hyperreactivity, which may be partially reversible (CitationATS 1995). Acute exacerbations of COPD occur, defined loosely as an episode of increased dyspnea, cough, and sputum production (CitationMcCrory et al 2001). Exacerbations severe enough to require hospitalization are associated with 3% to 4% short-term mortality, and half of those hospitalized will be readmitted within 6 months (CitationMcCrory et al 2001). COPD is a major cause of morbidity and mortality worldwide, and the prevalence of the disease continues to rise (CitationSullivan et al 2000; CitationMichaud et al 2001).
The main therapeutic options for the management of COPD are inhaled corticosteroids and bronchodilators. Inhaled corticosteroids significantly reduce inflammatory cells in the lungs, as well as systemic inflammatory markers such as C-reactive protein, compared with placebo (CitationSin et al 2004; CitationGan et al 2005). However, there is some evidence that corticosteroids have no antiinflammatory effects in COPD patients who are still smoking (CitationVan Overveld et al 2006). The use of systemic corticosteroids in acute COPD exacerbations have been shown to improve lung function and reduce hospital stays and treatment failures (CitationWood-Baker et al 2001; CitationNiewoehner 2002; CitationSingh et al 2002). Outpatient treatment with systemic corticosteroids can improve symptoms and reduce the relapse rate (CitationAaron et al 2003). Meta-analyses have shown that long-term treatment with inhaled corticosteroids reduce the rate of COPD exacerbations by 30% and slow the progression of airway function decline (Citationvan Grunsven et al 1999; CitationAlsaeedi et al 2002; CitationSutherland et al 2003; CitationSalpeter and Buckley 2006). One meta-analysis of randomized controlled trials found a 25% reduction in all-cause mortality for inhaled corticosteroids compared with placebo (CitationSin et al 2005). However, pooled data from another meta-analysis did not show a reduction in respiratory or total mortality compared with placebo (CitationSalpeter and Buckley 2006).
The two types of bronchodilators, β-agonists and anticholinergics, are generally considered to be equivalent choices in the treatment of COPD (CitationPauwels et al 2001; CitationCelli and MacNee 2004; NCCC 2004). Many trials of these agents have concentrated on short-term physiological endpoints, such as the forced expiratory volume at 1 second (FEV1), quality of life, or symptoms. Anticholinergics have been shown to have equal or superior efficacy as β-agonists in the improvement of lung function parameters (CitationKarpel 1991; CitationRennard et al 1996; CitationMcCrory and Brown 2001; CitationSin et al 2003; CitationTashkin and Cooper 2004). However, surveys show that prescriptions for β-agonists are two times more common than anticholinergics in the UK and Europe, and ten times more common in the US (CitationRamsey 2000; CitationRudolf 2000; CitationRoche et al 2001).
This review summarizes the effect of β-agonists and anticholinergics on severe exacerbations and mortality, compared with placebo and with each other. The effects of inhaled corticosteroids on these outcomes are also evaluated. The results of two meta-analyses are presented (CitationSalpeter and Buckley 2006; CitationSalpeter, Buckley, Salpeter 2006) that pooled long-term randomized placebo-controlled trials of anticholinergic or β-agonist use and evaluated COPD hospitalizations, respiratory mortality, and total mortality. Of note, after these meta-analyses were published it was revealed that data from one of the included trials was also reported in another trial (CitationBrusasco et al 2006). We now provide the analysis with the duplicated data excluded. Information on the trials included in the meta-analysis is shown in .
Table 1 Included studies in COPD meta-analysis
Anticholinergic bronchodilators
Anticholinergic bronchodilators include the short-acting ipratropium and oxtropium (not available in the US), and the long-acting tiotropium that has been recently introduced to the market (CitationBarnes 2004). They work by inhibiting bronchoconstriction as well as mucus secretion, and have been shown in clinical trials to reduce symptoms and exacerbations, without the development of tolerance to their effects over time (CitationAshutosh and Lang 1984; CitationTashkin et al 1986; Citationvan Schayck et al 1990; CitationRennard et al 1996; CitationDonohue et al 2003; CitationBarnes 2004).
Anticholinergics compared with placebo
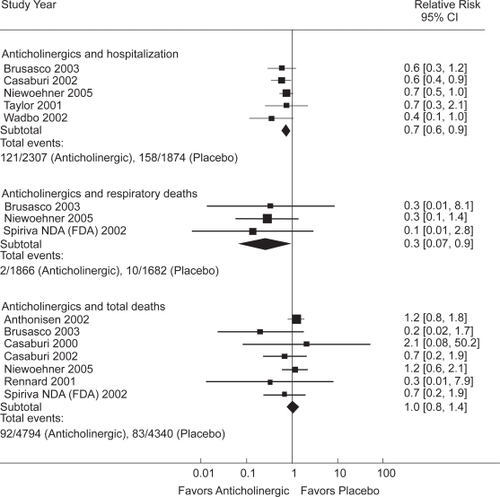
The pooled results of 9 randomized placebo-controlled trials () that ranged from three months to five years in duration (CitationSalpeter, Buckley, Salpeter 2006) showed that anticholinergics reduced the risk of COPD hospitalizations by 30% and reduced respiratory deaths by 70%, compared with placebo (). No significant effect on total mortality was seen (CitationSalpeter and Buckley 2006). It is estimated that 58% of the participants were also taking concomitant inhaled corticosteroids.
Long-acting compared with short-acting anticholinergics
When trials that compared the long-acting tiotropium with the short-acting ipratropium were pooled together, tiotropium was associated with 40% less severe exacerbations than ipratropium (CitationBarr et al 2005; CitationSalpeter and Buckley 2006). A cost-effective analysis that was funded by Boehringer Ingelheim found that the mean healthcare costs for tiotropium, including medications and hospital visits, was slightly higher than with ipratropium (CitationOostenbrink et al 2004). However the benefit of reducing hospitalizations was considered cost-effective. Tiotropium has also been shown to prevent the decline in trough FEV1 values, compared with placebo, over the course of one year (CitationCasaburi et al 2000, Citation2002; CitationFDA 2002; CitationBarr et al 2005).
β-agonist bronchodilators
β-agonist bronchodilators work by relaxing bronchial smooth muscle, and have been shown to be effective in the short-term relief of COPD symptoms (CitationSestini et al 2002). However, β-agonists have adverse cardiovascular effects and increase the risk of adverse cardiac events by over two-fold compared with placebo (CitationSalpeter et al 2004). This risk may be highest in patients with COPD and concomitant heart disease. In addition, significant tolerance to the respiratory effects of β-agonists develops with long-term use (CitationDonohue et al 2003).
Controversy has raged over the past 50 years concerning the safety of β-agonists in asthma and COPD (CitationLipworth 1992; CitationFahy and Boushey 1995; CitationTaylor et al 1996). Regular β-agonist use in reactive airway disease results in tolerance to the drug’s bronchodilator and bronchoprotective effects, and is associated with poorer disease control (CitationSears et al 1990; CitationSalpeter et al 2004). A recent meta-analysis pooled results from 19 asthma trials with 33,826 participants and found that the long-acting β-agonists salmeterol and formoterol increased asthma hospitalizations, life-threatening asthma attacks, and asthma deaths by two-fold to four-fold, compared with placebo (CitationNelson et al 2006; CitationSalpeter, Buckley, Ormiston, et al 2006). Statistically significant increases in asthma hospitalizations were seen for salmeterol and formoterol, and for children and adults. It was recently questioned whether the long-acting β-agonists should be taken off the market (CitationFDA 2005).
β-agonists compared with placebo
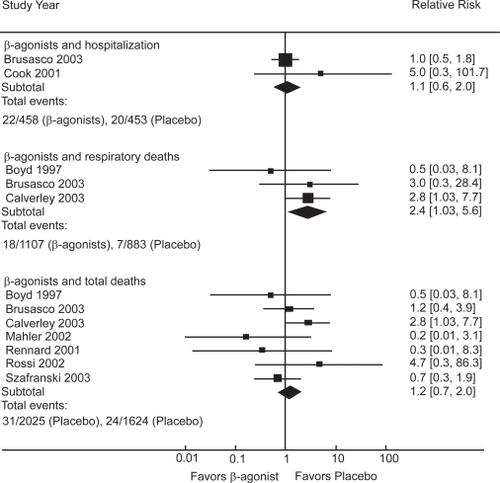
The pooled results of 9 randomized-placebo controlled trials () lasting from three to 12 months (CitationSalpeter and Buckley 2006; CitationSalpeter, Buckley, Salpeter 2006) showed that β-agonist use increased respiratory deaths by over twofold compared with placebo, without significantly affecting hospitalizations or total mortality (). It was estimated that 56% of the participants were on concomitant inhaled corticosteroids.
β-agonists compared with anticholinergics
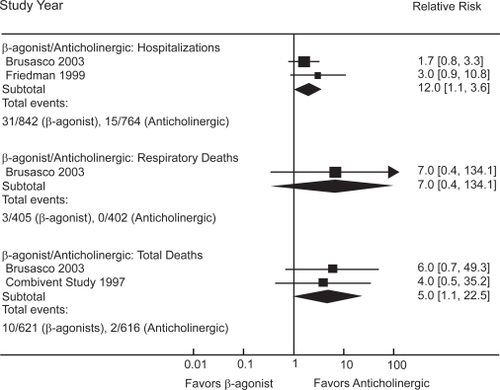
Seven trials directly compared β-agonists with anticholinergics in COPD () and reported on hospitalizations or deaths (CitationSalpeter and Buckley 2006; CitationSalpeter, Buckley, Salpeter 2006). β-agonist use was associated with a two-fold increased risk for hospitalizations and a five-fold increased risk for total mortality compared with anticholinergic use (). Four additional trials evaluated the combination of anticholinergics and β-agonists (); pooled results found that the combination was not better than anticholinergic use alone on these long-term clinical outcomes (CitationSalpeter and Buckley 2006).
β-agonists compared with inhaled corticosteroids
Only three trials () directly compared β-agonists with inhaled corticosteroids (CitationSalpeter and Buckley 2006). Pooled results found that β-agonists were associated with a two-fold increased risk for total mortality compared with corticosteroids, with marginal significance.
One meta-analysis has evaluated combined corticosteroid and long-acting β-agonist treatment compared with placebo and either modality alone (CitationNannini et al 2004). Combination treatment reduced severe COPD exacerbations by 25% compared with placebo and by 22% compared with β-agonist alone. However, the combination treatment had no significant effect on severe exacerbations compared with corticosteroid alone. Recently, an additional trial that lasted three years have been performed, but the results have not been presented (CitationGlaxoSmithKline 2006). Preliminary results show that combined treatment reduced total mortality compared with placebo, but the results compared with inhaled corticosteroids alone and β-agonist alone are not available at present.
Summary
The main therapeutic options in the treatment of COPD have been bronchodilators in combination with inhaled corticosteroid. Guidelines presently state that both bronchodilators, anticholinergics and β-agonists, are equivalent choices for use in COPD, while in practice β-agonists are prescribed 2–10 times more often than anticholinergics. This systematic review has summarized the available data on long-term clinical outcomes associated with β-agonist and anticholinergic bronchodilators in COPD. Anticholinergic inhalers reduce COPD hospitalizations by 30% and respiratory deaths by 70% compared with placebo, while β-agonists increase respiratory mortality by over twofold compared with placebo. When compared with each other, β-agonists are associated with a two-fold increased risk for COPD hospitalization and a five-fold increased risk for total mortality compared with anticholinergics. When β-agonists are added to anticholinergics or corticosteroids, there was no improvement in long-term clinical outcomes.
This meta-analysis has several limitations. Meta-analytic results can be uncertain when the numbers of events per study are small, as is the case with respiratory deaths. Another limitation is that most of the studies did not report deaths as a primary outcome, so the ascertainment of cause of death may be uncertain. It is unfortunate that there was not enough information to evaluate the protective effect of concomitant inhaled corticosteroids on the adverse effects of β-agonists. Furthermore, it was not possible to perform subgroup analysis to compare the differences in results between long-acting and short-acting β-agonists, or between the two long-acting agents, salmeterol and formoterol. Despite these limitations, this pooled analysis provides valuable information on the comparative effects of anticholinergics and β-agonists on clinical outcomes in COPD.
These results indicate that anticholinergics are superior to β-agonists in improving long-term clinical outcomes, and that guidelines should be changed so that anticholinergics are the bronchodilator of choice in COPD. Tiotropium is more effective than ipratropium for long-term clinical outcomes, but at a slightly greater cost. We provide evidence that β-agonists may actually increase respiratory mortality by over two-fold compared with placebo. More studies are needed to evaluate the long-term clinical benefit of the long-acting β-agonists, salmeterol and formoterol, in combination with inhaled corticosteroids, compared with the long-acting anticholinergic agent, tiotropium, combined with inhaled corticosteroids.
References
- AaronSDVandemheenKLHebertP2003Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary diseaseN Engl J Med34826182512826636
- AlsaeediASinDDMcAlisterFA2002The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trialsAm J Med113596512106623
- AnthonisenNRConnettJEEnrightPL2002Hospitalizations and mortality in the Lung Health StudyAm J Crit Care Med1663339
- AshutoshKLangH1984Comparison between long-term treatment of chronic bronchitic airway obstruction with ipratropium bromide and metaproterenolAnn Allergy5340166238558
- [ATP] American Thoracic Society1995Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med152(5 Pt 2)S771217582322
- BarnesPJ2004The role of anticholinergics in chronic obstructive pulmonary diseaseAm J Med117Suppl 12A24S32S15693640
- BarrRGBourbeauJCamargoCA2005Inhaled tiotropium for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev2CD00287615846642
- BoydGMoriceAHPounsfordJC1997An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD)Eur Respir J10815219150318
- BrusascoBHodderRMiravitllesM2003Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax5839940412728159
- BrusascoVHodderRMiravitllesM2006Letter to the editor. Health outcomes following treatment for 6 months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax619116396956
- CalverleyPMBoonsawatWCsekeZ2003Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J229121914680078
- CasaburiRBriggsDDJrDonohueJF2000The spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multi-center trial. The US Tiotropium Study GroupChest118129430211083677
- CasaburiRMahlerDAJonesPW2002A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J192172411866001
- CelliBRMacNeeW2004Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J239324615219010
- Combivent Inhalation Solution Study Group1997Routine nebulized ipratropium and albuterol are better than either alone in COPD. The Combivent Inhalation Solution Study GroupChest1121514219404747
- CookDGuyattGWongE2001Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary diseaseAm J Respir Crit Care Med163859011208630
- DonohueJFMenjogeSKestenS2003Tolerance to bronchodilating effects of salmeterol in COPDRespir Med9710142014509555
- FahyJVBousheyHA1995Controversies involving inhaled beta-agonists and inhaled corticosteroids in the treatment of asthmaClin Chest Med16715338565410
- [FDA] US Food and Drug Administration Advisory Committee2005Serevent, Advair, Foradil withdrawals to be considered by Advisory Committee [online]882005 URL: http://www.fdaadvisory-committee.com/FDC/AdvisoryCommittee/Committees/Pulmonary-Allergy%20Drugs/071305_betasafety/071305_BroncoP.htm
- [FDA] US Food and Drug Administration2002NDA 21–395 Spiriva (Tiotropium bromide) inhalation powder for COPD [online] Accessed on 10 August 2005. URL: http://www.fda.gov/OHRMS/DOCKETS/ac/02/briefing/3890B1_05_Clinical%20Briefing.doc
- FriedmanMSerbyCWMenjogeSS1999Pharmocoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPDChest1156354110084468
- GanWQManSFSinDD2005Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysisBMC Pulm Med5315707484
- GlaxoSmithKline2006GSK announces positive results of Seretide study in patients with chronic obstructive pulmonary disease (COPD) [online] Accessed on 18 August 2006. URL: http://www.gsk.com/ControllerServlet?appld=4&pageld=402&newsid=780
- KarpelJP1991Bronchodilator responses to anticholinergic and beta-adrenergic agents in acute and stable COPDChest9987161672634
- LipworthBJ1992Risks versus benefits of inhaled beta 2-agonists in the management of asthmaDrug Saf754701346963
- MahlerDAWirePHorstmanD2002Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16610849112379552
- McCroryDCBrownCDGelfandSE2001Management of acute exacerbations of COPD. A summary and appraisal of published evidenceChest119119020911296189
- McCroryDCBrownCD2001Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev2CD00298411406052
- MichaudCMMurrayCJBloomBR2001Burden of disease—implications for future researchJAMA285535911176854
- NanniniLCatesCJLassersonTJ2004Combined corticosteroid and long acting beta-agonist in one inhaler for chronic obstructive pulmonary diseaseCochrane Database Syst Rev3CD00379415266502
- [NCCCC] National Collaborating Centre for Chronic Conditions2004Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary careThorax59Suppl 11232
- NelsonHSWeissSTBleeckerER2006The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterolChest129152616424409
- NiewoehnerDE2002The role of systemic corticosteroids in acute exacerbation of chronic obstructive pulmonary diseaseAm J Respir Med1243814720044
- NiewoehnerDERiceKCoteC2005Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily, inhaled anticholinergic bronchodilator: a randomized trialAnn Intem Med14331726
- OostenbrinkJBRutten-van MolkenMPAlMJ2004One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary diseaseEur Respir J23241914979498
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med16312567611316667
- RamseySD2000Suboptimal medical therapy in COPD: exploring the causes and consequencesChest1172 Suppl33S7S10673472
- RennardSISerbyCWGhafouriM1996Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trialsChest11062708681667
- RennardSIAndersonWZuwallackR2001Use of a long-acting inhaled beta 2-adrenegic agonist, salmeterol xinafoate, in Patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16310879211316640
- RocheNLepageTBourcereauJ2001Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary diseaseEur Respir J18903811829094
- RossiAKristufekPLevineBE2002Comparison of the efficacy, tolerability, and safety of formoterol dry posder and oral, slow-release theophylline in the treatment of COPDChest12110586911948033
- RudolfM2000The reality of drug use in COPD: the European perspectiveChest1172 Suppl29S32S10673471
- SalpeterSRBuckleyNSOrmistonTM2006Long-acting beta-agonists increase severe asthma exacerbations and asthma-related deaths: meta-analysis of randomized controlled trialsAnn Intern Med1449041216754916
- SalpeterSRBuckleyNSSalpeterEE2006Meta-analysis: Anticholinergics, but not beta-agonists, reduce severe exacerbations and respiratory mortality in COPDJ Gen Intern Med2110111916970553
- SalpeterSRBuckleyNS2006Systematic review of clinical outcomes in chronic obstructive pulmonary disease: β-Agonist use compared with anticholinergics and inhaled corticosteroidsClin Rev Allergy Immunol312193017085795
- SalpeterSROrmistonTMSalpeterEE2004Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest12523092115189956
- SalpeterSROrmistonTMSalpeterEE2004Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthmaAnn Intern Med1408021315148067
- SearsMRTaylorDRPrintCG1990Regular inhaled beta-agonist treatment in bronchial asthmaLancet336139161978871
- SestiniPRenzoniERobinsonS2002Short-acting beta 2 agonists for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev4CD00149512519559
- SinDDLacyPYorkE2004Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med170760515229100
- SinDDMcAlisterFAManSF2003Contemporary management of chronic obstructive pulmonary disease: scientific reviewJAMA29023011214600189
- SinDDWuLAndersonJA2005Inhaled corticosteroids and mortality in chronic obstructive pulmonary diseaseThorax60992716227327
- SinghJMPaldaVAStanbrookMB2002Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic reviewArch Intern Med16225273612456224
- SullivanSDRamseySDLeeTA2000The economic burden of COPDChest1172 Suppl5S9S10673466
- SutherlandERAllmersHAyasNT2003Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysisThorax589374114586043
- SzafranskiWCukierARamirezA2003Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J21748112570112
- TashkinDPAshutoshKBleeckerER1986Comparison of the anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease. A 90-day multi-center studyAm J Med815A81902947465
- TashkinDPCooperCB2004The role of long-acting bronchodilators in the management of stable COPDChest1252495914718448
- TaylorDRSearsMCockcroftDW1996The beta-agonist controversyMed Clin N Am80719488676612
- TaylorJKotchARiceK2001Ipratropium bromide hydrofluoro-alkane inhalation aerosol is safe and effective in patients with COPDChest12012536111591569
- van GrunsvenPMvan SchayckCPDerenneJP1999Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta-analysisThorax5471410343624
- Van OverveldFJDemkowUGoreckaD2006Differences in responses upon corticosteroid therapy between smoking and non-smoking patients with COPDJ Physiol Pharmacol57Suppl 42738217072055
- van SchayckCPGraafsmaSJVischMB1990Increased bronchial hyperresponsiveness after inhaling salbutamol during 1 year is not caused by subsensitization to salbutamolJ Allergy Clin Immunol867938001977787
- WadboMLofdahlCGLarssonK2002Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled studyEur Respir J2011384612449166
- Wood-BakerRWaltersEHGibsonP2001Oral corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev2CD00128811405984