Abstract
The reproducibility of exhaled breath condensate (EBC) mediators is not well documented in chronic obstructive pulmonary disease (COPD). This study assessed within assay (WA), within (WD) and between day (BD) reproducibility of EBC leukotriene B4 (LTB4) and 8-isoprostane. Three EBC samples were collected from 24 COPD patients separated by 1 h and 1 wk, to assess WD and BD reproducibility. WA reproducibility was assessed by sample analysis by enzyme immunoassay in triplicate. WA coefficient of variation for LTB4 and 8-isoprostane (18.2% and 29.2%, respectively) was lower than corresponding values for WD (47.7% and 65.3%, respectively) and BD (75.7% and 79.1%, respectively). Repeatability coefficient for 8-isoprostane and LTB4 assays were 18.6 pg/ml and 13.2 pg/ml, respectively. Group mean differences for WD and BD were small and statistically nonsignificant. Using the Bland Altman method, there were wide limits of agreement for WD (−51.6 to 47.2 for 8-isoprostane and −31.8 to 31.4 for LTB4) and BD reproducibility (−61.4 to 75.7 for 8-isoprostane and −29.3 to 38.6 for LTB4). This is the first study to fully report the variability of EBC 8-isoprostane and LTB4 in COPD. WA variability and group mean changes were small. However, we observed considerable WD and BD variability for these biomarkers.
Introduction
Biomarkers of airway inflammation and oxidative stress can be measured noninvasively using exhaled breath condensate (EBC) sampling of airway lining fluid. Leukotriene B4 (LTB4), a potent neutrophil chemoattractant, and 8-isoprostane, which is formed during oxidative stress conditions by free-radical peroxidation of arachidonic acid, are examples of biomarkers that have been measured in EBC from chronic obstructive pulmonary disease (COPD) patients. The absolute concentration of these mediators has varied greatly between studies, despite use of identical immunoassay methods. For example, mean values of LTB4 in COPD patients have ranged from 10 pg/ml (CitationBiernacki et al 2003) to 100 pg/ml (CitationMontuschi et al 2003). Similarly, mean values of 8-isoprostane in COPD patients have ranged from 9 pg/ml (CitationBiernacki et al 2003) to 47 pg/ml (CitationKostikas et al 2003). The disparity between published findings may be attributable to the small sample sizes often used, or to method variability. The reproducibility of EBC LTB4 and 8-isoprostane has not been well documented in COPD patients. This issue was highlighted as an important area for future research by the recent American Thoracic Society/European Respiratory Society (ATS/ERS) task force document on EBC methodology (CitationHovarth et al 2005). The current study was designed to investigate the variability of these mediators in COPD patients.
Methods
Twenty-four patients with COPD (16 male, mean age 65; 10 current smokers, mean pack years 40; mean % predicted forced expiratory volume in one second [FEV1] 54% standard deviation [SD] 13.5%) diagnosed according to current criteria (CitationNCCCC 2004) were recruited. Exclusion criteria were history of asthma or atopy, and respiratory tract infection within 2 weeks of sample collection. Subjects were asked to refrain from caffeine and cigarettes for 2 hours prior to each visit. Written informed consent was obtained and the local ethics committee approved the study.
Three aspects of reproducibility were studied in all subjects: 1) Within day (WD) reproducibility was assessed by the collection of 2 samples of EBC separated by 1 h. 2) Between day (BD) reproducibility was assessed by the collection of a further EBC sample 1 week later; this measurement was compared with the first collection one week earlier. 3) Within array (WA) reproducibility was assessed by analysis in triplicate of each sample. EBC was collected during tidal breathing for 10 minutes without a nose peg (EcoScreen, Jaegar, Hoechberg, Germany). Subjects were instructed to breathe normally through their mouth and to temporarily discontinue collection if they needed to swallow saliva or cough. Samples were aliquoted into separate 200 mcl tubes and frozen at −80ºC. LTB4 and 8-isoprostane were measured by enzyme immunoassays (Cayman Chemical, Ann Arbour, MI, USA). All samples were analysed in triplicate. The lower limits of detection were 13 pg/ml and 5 pg/ml for LTB4 and 8-isoprostane respectively. Samples with a concentration below the limit of the assay were assigned a level of 0 pg/ml.
Three statistical approaches were used to assess variability: 1) Coefficient of variation was used to assess WD, BD, and WA variability. 2) The repeatability coefficient was used to analyse within assay variation; this estimated the limits of the differences that can be expected to occur between 95% of repeated assays performed on the same sample. Similarly, the Bland-Altman method with limits of agreement was used to assess WD and BD variability; this estimates the differences that can be expected to occur between 95% of samples collected at different times from the same subject (CitationBland and Altman 1986). The group mean and 95% confidence intervals for the WD and BD differences were determined.
Results
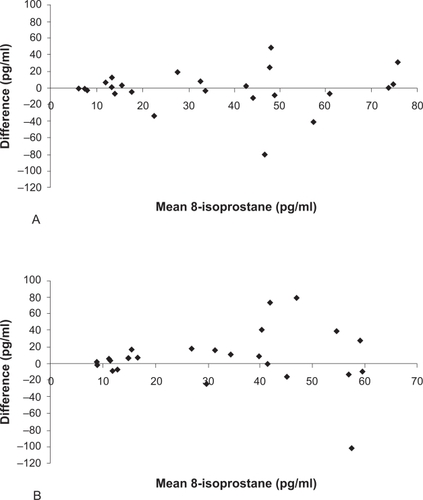
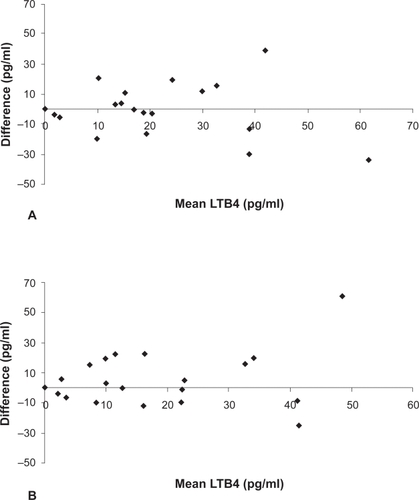
The coefficients of variation are shown in ; the WA coefficient of variation for both LTB4 and 8-isoprostane was lower than the corresponding values for WD and BD variability. The repeatability coefficient for the 8-isoprostane assay was 18.6 pg/ml and for the LTB4 assay was 13.2 pg/ml. Group mean differences for WD and BD changes were small (). In contrast, the limits of agreement for WD and BD variability were large, demonstrating that levels of LTB4 and 8-isoprostane can change markedly in some individuals even within 1 h (; , ). Limits of agreement for current smokers were in some cases wider than those for ex-smokers for both 8-isoprostane and LTB4 ().
Table 1 Coeffilcient of variation for within assay, within day, and between day variability
Table 2 Mean difference (95% CI) and limits of agreement (LA) for within and between day variability of 8-isoprostane and LTB4 in COPD
Table 3 Mean difference (limits of agreement) for within day and between day variability of 8-isoprostane and LTB4 in COPD in ex- and current smokers
Discussion
This is the first study to fully report the variability of EBC 8-isoprostane and LTB4 in COPD patients. Using the Bland Altman method, a robust technique for quantifying the potential variability during repeated sampling from the same subject, we observed considerable WD and BD variability for these biomarkers. Previous studies using these biomarkers have either failed to investigate intra-subject variability or have reported variability as being ‘minimal’. There are several reasons why the variability of EBC mediators may have been underestimated in these studies. Firstly, EBC variability has been studied in healthy subjects (CitationCsoma et al 2002). It is probable that within subject variability in patients with lung inflammation will be higher. Secondly, the use of the coefficient of variation and correlation coefficients provide statistics using arbitrary values that do not relate to the units of the measurement being studied. Thirdly, coefficient of variation, correlation coefficients and group mean statistics provide information about overall group differences. These types of analysis do not inform us of the potential for repeated samples from a single individual to vary over time. In the current study we observed no significant change in the group mean values over time. This does not mean that there was no variability; the Bland Altman method graphically shows that while some individuals have highly reproducible measurements over time, there was considerable variation in the samples from other subjects, which contributed to the wide limits of agreements. The interpretation of the limits of agreements is, for example, that repeated 8-isoprostane sampling on different days can be expected to vary from −61.4 pg/ml to 75.7 pg/ml in an individual simply due to natural variability. Using this assay as a biomarker to detect a significant biological change (greater than assay variability) in an individual, such as an exacerbation, would require a change greater than these limits of agreement.
The variability observed in this study may be explained either by (1) true changes in the composition of the airway lining fluid, (2) variability due to the sample collection methodology, or (3) the variability of the immunoassay method used to analyse the sample. The contribution of these 3 factors will now be considered: 1) We have recently shown marked WD and BD variability of EBC pH in COPD patients compared with that seen in healthy subjects, indicating that there are changes in the composition of EBC in COPD patients over time (CitationBorrill et al 2005). In the current study, there was some evidence of greater variability in current smokers compared with ex-smokers. Acute smoking was found to cause an increase in EBC 8-isoprostane after 15 minutes, but not at 5 h (CitationMontuschi et al 2000). In the current study, variation in the time since the last cigarette may have contributed to the variability observed. 2) Inconsistencies in the rate of aerosolization of airway lining fluid during sample collection may lead to increased variability. Attempts have been made to correct for this using dilution factors (CitationEffros et al 2003) and further study in this area is required. 3) The coefficient of variation showed lower within assay variability for both LTB4 and 8-isoprostane compared with within subject variation. This was confirmed by the repeatability coefficients for each assay which were lower than the limits of agreement between samples. The numerical value of the repeatability coefficient can be considered to be equivalent to the magnitude of the limits of agreement, which enables direct comparison. This indicates that the repeatability of the assay itself cannot fully explain the degree of within subject variability observed.
In a study by Citationvan Hoydonk and colleagues (2004), 8-isoprostane was undetectable in 21 of the 36 samples from healthy smokers. In the current study 8-isoprostane was detectable in all COPD samples. However, levels of LTB4 were below the limit of detection in a large number of samples, which may indicate the poor sensitivity of this assay. In a study by CitationCarpagnano and colleagues (2003) some samples had levels which were below the limit of detection of the assay. This suggests that the authors either diluted the standard below the level recommended, or that they extrapolated the standard curve below the lowest concentration of standard. These are not standard practices for immunoassays and are likely to lead to a loss of accuracy. To avoid these potential errors, we defined the lower limit of detection of the assay as the lowest concentration on the standard curve. Methods such as mass spectroscopy may offer advantages over immunoassays in terms of increased sensitivity and reduced variability (CitationCap et al 2004; CitationMontuschi et al 2004).
Overall, it is likely that the variability we have reported is multifactorial, with changes in the composition of the airway lining fluid, variability due to the sample collection methodology, and immunoassay variability and sensitivity all contributing. The high level of variability observed casts doubt on the current EBC methodology used to assess LTB4 and 8-isoprostane. Our study highlights the importance of assessing method variability. Differences between patients with disease and controls can only be properly evaluated with knowledge of the variability of the method.
References
- BiernackiWAKharitonovSABarnesPJ2003Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbation of COPDThorax58294812668789
- BlandJMAltmanDG1986Statistical methods for assessing agreement between 2 methods of clinical measurementLancet1307102868172
- BorrillZStarkeyCVestboJ2005Reproducibility of exhaled breath condensate pH in chronic obstructive pulmonary diseaseEur Respir J252697415684290
- CapPChladekJPehalF2004Gas chromatography/mass spectrometry analysis of exhaled leukotrienes in asthmatic patientsThorax594657015170025
- CarpagnanoGEKharitonovSAFoschino-BarbaroMP2003Increased inflammatory markers in the exhaled breath condensate of cigarette smokersEur Respir J215899312762340
- CsomaZSKharitonovXABalintB2002Increased leukotrienes in exhaled breath condensate in childhood asthmaAm J Respir Crit Care Med1661345912406853
- EffrosRMBillerJFossB2003A simple method for estimating respiratory solute dilution in exhaled breath condensatesAm J Respir Crit Care Med16815001514512268
- HorvathIHuntJBarnesPJATS/ERS Task Force on Exhaled Breath Condensate2005Exhaled breath condensate: methodological recommendations and unresolved questionsEur Respir J265234816135737
- KostikasKPapatheodorouGPsathakisK2003Oxidative stress in expired breath condensate in patients with COPDChest12413738014555568
- MontuschiPCollinsJVCiabattoniG2000Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokersAm J Respir Crit Care Med1621175710988150
- MontuschiPKharitonovSACiabattoniG2003Exhaled leukotrienes and prostaglandins in COPDThorax58585812832671
- MontuschiPMartelloSFelliM2004Ion trap chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensateRapid Commun Mass Spectrom1827232915499663
- [NCCCC] National Collaborating Centre for Chronic Conditions2004Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary careThorax 200459Suppl 11232
- van HoydonckPGAWuytsWAVanaudenaerdeBM2004Quantitative analysis of 8-isoprostane and hydrogen peroxide in exhaled breath condensateEur Respir J231899214979489