Abstract
Our understanding of the etiology, pathogenesis and consequences of acute exacerbations of chronic obstructive pulmonary disease (COPD) has increased substantially in the last decade. Several new lines of evidence demonstrate that bacterial isolation from sputum during acute exacerbation in many instances reflects a cause-effect relationship. Placebo-controlled antibiotic trials in exacerbations of COPD demonstrate significant clinical benefits of antibiotic treatment in moderate and severe episodes. However, in the multitude of antibiotic comparison trials, the choice of antibiotics does not appear to affect the clinical outcome, which can be explained by several methodological limitations of these trials. Recently, comparison trials with nontraditional end-points have shown differences among antibiotics in the treatment of exacerbations of COPD. Observational studies that have examined clinical outcome of exacerbations have repeatedly demonstrated certain clinical characteristics to be associated with treatment failure or early relapse. Optimal antibiotic selection for exacerbations has therefore incorporated quantifying the risk for a poor outcome of the exacerbation and choosing antibiotics differently for low risk and high risk patients, reserving the broader spectrum drugs for the high risk patients. Though improved outcomes in exacerbations with antibiotic choice based on such risk stratification has not yet been demonstrated in prospective controlled trials, this approach takes into account concerns of disease heterogeneity, antibiotic resistance and judicious antibiotic use in exacerbations.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and the 6th leading cause worldwide. It is estimated that by 2020, it would become the 3rd leading cause of death in the US and worldwide. Affecting 24 million people in the US, up to half of them undiagnosed, it accounts for 13.76 million office visits, 1.5 million emergency room visits and 726,000 hospitalizations annually, at an estimated direct cost of $18 billion (CitationNHLBI 2003).
COPD is a chronic disease and is mostly managed on an outpatient basis. Part of the natural history of this disease is intermittent acute episodes of increased respiratory symptoms and worse pulmonary function that may be accompanied by fever and other constitutional symptoms, which are characterized as acute exacerbations. These exacerbations are a major driver for office visits, hospitalizations and therefore cost of care in COPD. In advanced disease, they are also the most frequent cause of death in this disease (CitationBurrows and Earle 1969; CitationCalverley et al 2007). The frequency of exacerbations varies widely between patients, but is generally correlated with the severity and duration of underlying COPD.
Besides mortality, exacerbations of COPD are associated with important decrements in health-related quality of life (CitationSeemungal et al 1998; CitationSpencer et al 2001). Though the acute symptoms tend to subside over the course of 2–3 weeks, quality of life takes several months to recover. Furthermore, this recovery is delayed if exacerbations recur during this recover period (CitationSpencer and Jones 2003). Contrary to previous studies, recent data has shown that the frequency of exacerbations is associated with accelerated long term decline in lung function as measured by the forced expiratory volume in 1 second (FEV1). This has been shown in mild disease, in the Lung Health Study, where every lower respiratory tract illness was associated with an additional loss of 7 ml of FEV1 (CitationKanner et al 2001). Patients with moderate to severe COPD who experience more frequent exacerbations also experience a decline in FEV1 of 40 ml/yr in contrast to a decline of 32 ml/year among patients with infrequent exacerbations (CitationDonaldson et al 2002).
It is now clear that exacerbations are a major contributor to the morbidity, costs and mortality associated with COPD. Substantial progress has also been made in understanding their etiology and pathogenesis. In contrast, clinical studies of adequate design and quality that can help us determine the optimal management approach to exacerbations are still relatively few. Among the therapeutic options for the treatment of acute exacerbations are antibiotics. Though widely used for the treatment of exacerbations, their use is still debated and whether antibiotic choice is important is controversial (CitationHirschmann 2000; CitationMurphy et al 2000). The optimal approach to antibiotic treatment of exacerbations relies upon an accurate diagnosis, including judicious application of diagnostic tests. This needs to be followed by determining the severity of an exacerbation, the probability that it is bacterial and whether antibiotics are indicated. Once the decision is made that antibiotics are indeed indicated, then a risk stratification approach allows us to choose an appropriate antibiotic.
Diagnosis of acute exacerbation
Accurate diagnosis relies on clear definitions that are universally agreed upon and include objective measurements. Unfortunately, this is not the situation with acute exacerbations of COPD, with an imprecise and variable definition and the absence of objective measures (CitationRodriguez-Roisin 2000). There are two widely used definitions of exacerbation. The Anthonisen definition is based on the presence of one or more of three cardinal symptoms, including an increase or new onset of dyspnea, sputum production and sputum purulence (CitationAnthonisen et al 1987). In case only one cardinal symptom is present, then one or more supporting symptoms or signs are required to make the diagnosis, including an upper respiratory tract infection in the past five days, wheezing, cough, fever without an obvious source or a 20% increase in the respiratory rate or heart rate above baseline. Though simple and clinically useful, this definition is narrow in its scope and several important symptoms of exacerbation such as chest congestion, chest tightness, fatigue and sleep disturbance are not included.
A wider definition from a consensus panel defines an exacerbation as an acute sustained worsening of the patients’ condition from stable state, beyond day to day variability and which requires additional treatment (CitationRodriguez-Roisin 2000). This definition though more inclusive, is not specific with regards to the nature and duration of symptoms. Also missing in both definitions is the clinical exclusion of entities that could present in a similar manner, such as pneumonia, congestive heart failure, upper respiratory infection, noncompliance with medications etc. Older research in this field did not exclude these entities from the definition, confounding both the research findings and clinical approach to exacerbations. These clinical entities have distinct etiology, pathogenesis and treatment, and therefore should be in the differential diagnosis of an exacerbation rather than be included under the definition.
In our clinical studies, we suspect an exacerbation when a patient with COPD reports a minor increase (or new onset) of two or a major increase (or new onset) of one of the following respiratory symptoms: dyspnea, cough, sputum production, sputum tenacity, or sputum purulence (CitationSethi et al 2002). The increase in symptoms should be of at least 24 hours duration and should be of greater intensity than their normal day to day variability. Other definitions have required symptoms to be of at least 48 hrs and even 72 hrs in duration. Furthermore, as described above, clinical evaluation should exclude other clinical entities that could present in a similar manner.
The severity of an exacerbation is a complicated concept, because it is constituted by at least two factors, the severity of the underlying COPD and the acute change induced by the exacerbation itself. Therefore, a patient who has got very severe underlying COPD may have significant clinical consequences from a relatively small change from his baseline state, while a patient with mild COPD may be able to tolerate a much larger change in his symptoms and lung function.
Different notions of exacerbation severity have been used. Ideally, changes in lung function should be used to define severity of exacerbations. However, lung function is difficult to measure during exacerbations, and often the change with an exacerbation is of the same magnitude as day to day variability in these measurements. Severity has been also measured by site of care, with hospitalized exacerbations regarded as severe, outpatient exacerbations regarded as moderate and self medicated exacerbations as mild (CitationGOLD 2007). This classification is prone to error as the site of care is dependent on differences among countries and health care systems as to the threshold for admission, patient and physician preferences etc. Another suggested measure of severity of exacerbations is the intensity of treatment recommended, with treatment with bronchodilators only indicating mild exacerbations, while treatment with antibiotics and steroids in addition to bronchodilators regarded as indicating moderate or severe exacerbations. Again, this approach is beset with problems of preferences and practice approach.
One widely used determination of severity is known as the Anthonisen classification (CitationAnthonisen et al 1987). This classification relies on the number of cardinal symptoms and the presence of some supporting symptoms as shown in . Interestingly, this classification was not designed to be a classification of severity of exacerbations, but has become so over time. This determination of severity is relatively simple and does correlate with benefit with antibiotics, with such benefit seen only in Type 1 and 2 exacerbations. However, there are limitations to the Anthonisen severity classification. It has not been validated against objective measures of severity. The association with benefit with antibiotics has not been reproduced in other studies. Another limitation is the lack of gradation of severity within each symptom, such that an exacerbation with mild dyspnea and mild increase in sputum would be regarded as the same severity in this classification as one with a marked increase in both symptoms.
Table 1 Anthonisen classification of COPD exacerbations based on cardinal symptoms. Based on data from CitationAnthonisen and colleagues (1987)
It is evident that we need a better definition and objective measures of severity of exacerbations. Biomarkers represent such an objective measure and potentially could either define an exacerbation, determine its etiology or determine its severity. In a recent study by Hurst et al of 36 plasma biomarkers in 90 patients with exacerbations, none of them alone or in combination were adequate to define an exacerbation (CitationHurst et al 2006). In another study of multiple serum biomarkers in 20 hospitalized patients with exacerbation, reduction in interleukin-6 (IL-6) and interleukin-8 (IL-8) correlated with decrease in dyspnea during recovery from exacerbation, while decreases in IL-6 and tumor necrosis factor (TNFα) were proportional to recovery in FEV1 (CitationPinto-Plata et al 2007). Sputum free neutrophil elastase activity has been shown to correlate with clinical severity of an exacerbation as assessed by a clinical score based on symptoms and signs (CitationSethi et al 2000). Development of patient reported outcomes and biomarkers should provide us with a better definition and objective measures of severity of exacerbations in the future.
Pathogenesis of exacerbations
The emerging concept that an increase in airway inflammation from the baseline level characteristic of COPD is central to the pathogenesis of acute exacerbations is supported by several recent studies (CitationWhite et al 2003; CitationSethi 2004a). Measurement of airway inflammation in induced or expectorated sputum, bronchoalveolar lavage or bronchial biopsy has revealed that increased airway inflammation is indeed present in acute exacerbation and resolves with treatment. Both neutrophilic and eosinophilic inflammation has been described, with the former associated with a bacterial etiology and the latter with viral infection. Any stimulus that acutely increases airway inflammation could lead to increased bronchial tone, edema in the bronchial wall and mucus production. In an already diseased lung, these processes could worsen ventilation-perfusion mismatch and expiratory flow limitation. Corresponding clinical manifestations are dyspnea, cough, increased sputum production, tenacity and purulence along with worsening gas exchange, which are the cardinal manifestations of an exacerbation.
Increases in plasma fibrinogen, interleukin 6 (IL-6) and c-reactive protein (CRP), consistent with a heightened state of systemic inflammation have been described during exacerbations (CitationDev et al 1998; CitationWedzicha et al 2000). These and other mediators likely cause the systemic manifestations of exacerbations, including fatigue and in some instances fever.
A variety of noninfectious and infectious stimuli can induce an acute increase in airway inflammation in COPD, thereby causing the manifestations of exacerbation. Epidemiological studies have demonstrated increased respiratory symptoms and respiratory mortality among patients with COPD during periods of increased air pollution (CitationSunyer et al 1993; CitationGarcia-Aymerich et al 2000; CitationSunyer et al 2000). Indeed, environmental pollutants, both particulate matter, such as PM-10, and nonparticulate gases, such as ozone, nitrogen dioxide, sulfur dioxide, are capable of inducing inflammation in vitro and in vivo (CitationDevalia et al 1994; CitationOhtoshi et al 1998; CitationRudell et al 1999). Infectious agents, including bacteria, viruses and atypical pathogens are implicated as causes of up to 80% of acute exacerbations, and are discussed in greater detail below (CitationSethi 2000).
Microbial pathogens in COPD exacerbations
The list of potential pathogens in COPD exacerbations includes typical respiratory bacterial pathogens, respiratory viruses and atypical bacteria (). Among the typical bacteria, nontypeable Haemophilus influenzae (NTHI) is the most common and its role in COPD is the best understood (CitationEldika and Sethi 2006). Among the viruses, Rhinovirus and Respiratory Syncytial Virus (RSV) has received considerable attention in recent years (CitationSeemungal et al 2001; CitationFalsey et al 2005).
Table 2 Microbial pathogens in exacerbations of COPD
Certain shared characteristics of these pathogens provide clues to their predilection for causing infections in COPD. NTHI, Streptococcus pneumoniae, and Moraxella catarrhalis are the predominant bacterial causes of two other common respiratory mucosal infections, acute otitis media in children and acute sinusitis in children and adults. These mucosal infections have been related to anatomical abnormalities with impaired drainage of secretions, antecedent viral infections and defects in innate and adaptive immunity. All these predisposing factors likely exist in COPD. These three pathogens are all exclusively human pathogens that are transmitted among individuals. In healthy hosts, their presence is confined to the upper airway and does not cause any clinical manifestations. It is likely that acquisition of these pathogens in a patient with COPD, because of compromised lung defense, allows establishment of infection in the lower respiratory tract, with or without overt clinical manifestations.
Respiratory viruses implicated in COPD are able to cause acute tracheo-bronchial infections in healthy hosts, clinically referred to as acute bronchitis. In the setting of COPD, with diminished respiratory reserve, this acute bronchitis has more profound manifestations and serious clinical consequences.
Pathogenesis of infectious exacerbations
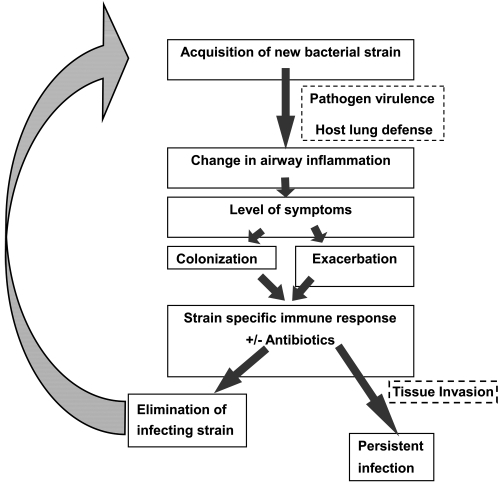
Our understanding of acute exacerbation pathogenesis, especially in relation to bacterial infection, has seen significant progress over the last few years. Both host and pathogen factors are involved in development of acute bacterial exacerbations (). Acquisition of strains of bacterial pathogens that are new to the host from the environment appears to be the primary event that puts the patient with COPD at risk for an exacerbation (CitationSethi et al 2002). Variations among strains of a species in the surface antigenic structure, as is seen with NTHI, S pneumoniae and M catarrhalis allow these newly acquired strains to escape the pre-existing host immune response that had developed following prior exposure to other strains of the same species of bacteria. These strains can therefore proliferate in the lower airways and induce acute inflammation in the airways. The virulence of the strain and as yet unidentified host factors may determine if the acute inflammatory response to the pathogen reaches the threshold to cause symptoms that present as an exacerbation (CitationChin et al 2005). In many instances, the adaptive immune response results in development of mucosal and systemic antibodies to the pathogen (CitationSethi et al 2004; CitationMurphy et al 2005). This immune response, in combination with appropriate antibiotics, is able to eliminate or control proliferation of the infecting bacteria. However, because of antigenic variability among strains of these bacterial species, the antibodies that develop to the infecting strain are usually strain-specific, and do not provide protection to the host from other antigenically distinct strains of the same species. This allows the process of recurrent bacterial infection and exacerbations in these patients.
Figure 1 Proposed model of bacterial exacerbation pathogenesis in COPD. Copyright © 2006. Reproduced with permission from Veeramachaneni SB, Sethi S. 2006. Pathogenesis of bacterial exacerbations of COPD. J Chronic Obstructive Pul Dis, 3:109–15.
The pathogenesis of acute viral exacerbations is less well understood. However, it appears to be similar to bacterial infections. A common cause of exacerbations, the rhinovirus, demonstrates considerable antigenic variation among its more than 100 serotypes, allowing for recurrent infections. The influenza virus demonstrates drift in the antigenic makeup of its major surface proteins, thereby leading to recurrent infections. In vitro, viruses can damage airway epithelium, stimulate muscarinic receptors, and induce eosinophilic influx by stimulating the secretion of RANTES (CitationJohnston et al 1998). These pro-inflammatory actions could potentially induce the pathophysiological manifestations that characterize acute exacerbation when a viral agent infects the lower airway of a patient with COPD. The pathogenesis of exacerbations with atypical bacterial infection is poorly understood.
Goals of treatment of exacerbations
Traditionally, the aims of treatment of an exacerbation are the recovery to baseline clinical status and the prevention of complications. Though these goals are undoubtedly important, several new observations question the adequacy of these goals. These include our new understanding of the importance of exacerbations in the course of COPD, the role of infection in exacerbations, the high rates of relapse with an adequate initial clinical response, and the role played by chronic infection in the pathogenesis of COPD. To draw an analogy, confining our goal in the treatment of COPD exacerbations to short term resolution of symptoms would be the equivalent of treating acute myocardial infarction with the only aim being resolution of chest pain. Several other important goals of treatment, both clinical and biological, should be considered (). For instance, clinical success in the treatment of exacerbations has been defined as resolution of symptoms to baseline or improvement of symptoms to a degree that no further treatment is required in the opinion of the treating physician. If symptoms do indeed correlate with exaggerated airway and systemic inflammation, then acceptance of clinical improvement rather than clinical resolution has important implications. Clinical improvement may reflect inadequate treatment, permitting the inflammatory process that underlies the exacerbation to persist for prolonged periods of time (CitationWhite et al 2003). Therefore, clinical resolution of symptoms to baseline may actually represent the optimal outcome.
Table 3 Proposed goals of treatment of COPD exacerbation
Other important clinical goals of treatment include delaying the next exacerbation, prevention of early relapse and more rapid resolution of symptoms (CitationAnzueto et al 1999; CitationMiravitlles et al 2001; CitationAaron et al 2003). Lengthening the inter-exacerbation interval and prevention of early relapse are being increasingly recognized as important clinical goals of treatment, because they ultimately translate to a decrease in the frequency of exacerbations, which is now a major focus of COPD treatment. Though most patients and physicians would accept faster recovery to baseline as a desirable goal of treatment, acceptance of this goal has been hampered by lack of well validated instruments to reliably measure the rate of resolution of exacerbations.
Nonclinical goals of treatment are either still in their infancy or, in the case of bacteriologic eradication, only used in clinical studies to satisfy regulatory requirements for approval of new antibiotics. If exacerbations are inflammatory events, it would be logical to have resolution of inflammation to baseline as an important goal of treatment. Similarly, exacerbations are in many instances induced by infection, therefore eradication of the offending infectious pathogen should be a goal of treatment. Practical application of these biological goals of treatment of exacerbations awaits the development of simple, rapid and reliable measurements of inflammation and infection.
Most exacerbations require a multi-pronged approach that utilizes several therapeutic modalities simultaneously, either to relieve symptoms, to treat the underlying cause or provide support till recovery occurs (CitationSethi 2002; CitationGOLD 2007). These therapies include bronchodilators, corticosteroids, antimicrobials, mucolytics, and expectorants and, in the more severe cases, oxygen supplementation and mechanical ventilation for acute respiratory failure. The subsequent discussion will focus on the role of antibiotics in the treatment of acute exacerbation.
Antibiotics in the treatment of exacerbations
The role and choice of antibiotics in the treatment of exacerbations has been a matter of controversy. Recommendations for antibiotic use among published guidelines are often inconsistent, and at times vague (CitationBach et al 2001; CitationBalter et al 2003; CitationCelli and MacNee 2004; CitationGOLD 2007). The paucity of well-designed, large randomized controlled trials in this field upon which to base solid recommendations has undoubtedly contributed to the state of affairs (CitationSethi 2004b). Recently, a few well designed placebo controlled and antibiotic comparison trials have been reported. In addition, epidemiologic studies have consistently identified certain ‘risk factors’, the presence of which in a patient with an acute exacerbation is predictive for failure of treatment or early relapse. There is increasing realization that with the heterogeneity of COPD and of exacerbations, using the same antibiotic in all episodes may not provide optimal outcome. It is likely that a proportion of treatment failures in exacerbations are related to ineffective antibiotic treatment. Therefore, patients ‘at risk’ for poor outcome are the logical candidates for aggressive initial antibiotic treatment, with the expectation that such an approach would improve overall outcomes of exacerbation. This ‘risk stratification’ approach has also been advocated for other community-acquired infections such as pneumonia and acute sinusitis (CitationMandell et al 2003; CitationSinus and Allergy Health 2004). Though improved outcomes with risk stratification has not yet been demonstrated in prospective controlled trials, such an approach takes into account concerns of disease heterogeneity, antibiotic resistance and judicious antibiotic use in exacerbations.
Placebo-controlled antibiotic trials
In contrast to the magnitude of the problem of exacerbations of COPD, resulting in significant antibiotic consumption, there are only a handful of placebo-controlled trials in this disease. Two meta-analyses of placebo-controlled trials in exacerbations have been published. In the first such analysis published in 1995, only nine trials met quality criteria and a small but significant beneficial effect of antibiotics over placebo in acute exacerbation was demonstrated (CitationSaint et al 1995). In the second analysis, 11 trials were included, and a much larger beneficial effect on mortality and prevention of clinical failure was demonstrated, especially in moderate to severe exacerbations (CitationRam et al 2006). The numbers needed to treat in severe exacerbations in hospitalized patients to prevent one death was only three patients and the number needed to treat for the prevention of one clinical failure was six patients. Diarrhea was the most frequently related adverse effect, with one episode per seven patients treated. Antibiotic treatment was beneficial in resolving sputum purulence; however benefits on lung function and gas exchange were not observed.
The different results between the two meta-analyses can be explained in large part by the addition in the later analysis of a study performed in Tunisia that was published in 2003. In this study, 93 patients with exacerbations of severe underlying COPD requiring ventilator support in an intensive care unit were randomly assigned to receive ofloxacin or placebo in a double blind manner (CitationNouira et al 2001). No systemic corticosteroids were administered. Potential respiratory bacterial pathogens were isolated in tracheo-bronchial aspirates in 61% of patients. Ofloxacin resulted in a dramatic benefit when compared to placebo, reducing mortality and the need for additional antibiotics by 17.5-fold and 28.4-fold, respectively ().
Table 4 Results of a recent placebo controlled trial in exacerbations of COPD requiring intensive care unit admission. Based on data from CitationNouira and colleagues (2001)
Another interesting study, though not included in either meta-analysis, was published in Italian by Allegra and colleagues (1991) with an additional analysis later published in English. In this trial, amoxicillin/clavunate was compared with placebo in 414 exacerbations in 369 patients with varying severity of underlying COPD (CitationAllegra et al 2001). A unique feature of this study was the measurement of primary outcome at 5 days, instead of the traditional 2–3 weeks. Clinical success (including resolution and improvement) was significantly better with the antibiotic, seen in 86.4% of patients, compared with 50.6% in the placebo arm. Greater benefit with antibiotics as compared to placebo was seen with increasing severity of underlying COPD.
Results of the meta-analyses and this study, along with those of the previous classic large placebo-controlled trial conducted by Anthonisen and colleagues show that antibiotics are beneficial in the treatment of moderate to severe exacerbations (CitationAnthonisen et al 1987; CitationAllegra et al 2001; CitationNouira et al 2001; CitationRam et al 2006). Furthermore, the benefit with antibiotics is more marked early in the course of the exacerbation, suggesting that antibiotics hasten resolution of symptoms (CitationAllegra et al 2001; CitationMiravitlles et al 2001). However, there are still important unresolved questions regarding the role of antibiotics in exacerbations. The benefit of antibiotics in mild exacerbations is unproven and warrants a placebo controlled trial. Whether antibiotics are of benefit in the treatment of exacerbations when a short course of systemic corticosteroids are co-administered has still not been studied in a large well designed trial. One would suspect that there would be additive benefits when both treatments are used over either treatment alone (CitationWood-Baker et al 2005; CitationRam et al 2006).
Antibiotic comparison trials
Though one can be quite confident that antibiotics are useful in moderate to severe exacerbations of COPD, there is considerable discussion as to antibiotic choice, especially for initial empiric therapy (CitationBach et al 2001; CitationBalter et al 2003; CitationCelli and MacNee 2004; CitationSethi and Murphy 2004; CitationGOLD 2007). As most exacerbations nowadays are treated without obtaining sputum bacteriology, this initial empiric choice often becomes the only choice made for antibiotic use in exacerbations. Results of antibiotic comparison trials should guide the recommendations for appropriate empiric antibiotics in exacerbations. However, though a large number of such trials have been conducted, in the vast majority, antibiotic choice does not appear to affect the clinical outcome. Differences in bacteriological eradication rates are seen, with an apparent dissociation between clinical and bacteriological outcomes (CitationObaji and Sethi 2001). This is contrary to expectations that antibiotics with better in vitro and in vivo antimicrobial efficacy and better pharmacodynamic and pharmacokinetic characteristics should show superiority in clinical outcomes. This paradox is likely related to several short-comings in design of these trials () (CitationSethi 2004b). Many of these deficiencies are explained by the fact that these trials are performed for regulatory approval of the drugs, therefore are designed for demonstrating noninferiority rather than differences between the two antibiotics.
Table 5 Limitations of published placebo-controlled antibiotic trials in acute exacerbations of COPD. Copyright © 2004. Reproduced with permission from Sethi S. 2004a. Bacteria in exacerbations of chronic obstructive pulmonary disease. Phenomenon or epiphenomenon? Proc Am Thorac Soc, 1:109–14
Two recent antibiotic comparison trials were designed to show differences among antibiotics and measured some unconventional but clinically relevant end-points. The GLOBE (Gemifloxacin and Long term Outcome of Bronchitis Exacerbations) trial was a prospective, double blind, randomized trial that compared a fluoroquinolone, gemifloxacin, with a macrolide, clarithromycin (CitationWilson et al 2002). End of therapy and long-term outcome assessments were made at the conventional 10–14 day and 28 day time intervals. In these assessments, in line with most antibiotic comparison trials, there was no statistically significant difference in the two arms in the clinical outcome, with clinical success rates of 85.4% and 84.6% for gemifloxacin and clarithromycin respectively. Bacteriological success, measured as eradication and presumed eradication, was significantly higher with gemifloxacin (86.7%) compared to clarithromycin (73.1%).
Patients who had a successful clinical outcome at 28 days were then offered enrollment in a follow up period for a total of 26 weeks of observation. In this time period, the primary outcomes were the rate of repeat exacerbations, hospitalizations for respiratory disease and health-related quality of life measures. Gemifloxacin was associated with a significantly lower rate of repeat exacerbations, with 71% of the gemifloxacin-treated patients remaining exacerbation free at 26 weeks in comparison to 58.5% in the clarithromycin arm. The relative risk reduction for recurrence of exacerbation was 30%. The rate of hospitalization for respiratory tract illness in the 26 weeks was also lower in the gemifloxacin-treated patients than in the clarithromycin treated patients (2.3% vs. 6.3%, p = 0.059) (CitationWilson et al 2002). Patients who had a recurrent exacerbation in the 26 week period had a lesser improvement in their health-related quality of life when compared to those who remained free of recurrence (CitationSpencer and Jones 2003). This trial clearly demonstrates the limitation of the conventional medium-term clinical outcomes to demonstrate differences among antibiotics in exacerbations. If the 26 week follow up period had not been instituted, significant differences in clinically relevant outcomes of recurrence of exacerbations and respiratory related hospitalization would have been missed.
Another recent landmark antibiotic comparison trial is the MOSAIC trial. This trial is a large study in which patients were randomized to a fluoroquinolone, moxifloxacin or to standard therapy (which could be one of the following: amoxicillin, cefuroxime or clarithromycin) (CitationWilson et al 2004). This trial had several unique design features which relate to observations made in this study. A relatively large number of patients were enrolled. In addition, patients were enrolled when stable to establish a baseline as a comparison to reliably distinguish between clinical improvement and resolution following treatment. A substantial proportion of the patients enrolled had one or more risk factors that would predispose to a poor outcome as discussed below. Patients were followed up to 9 months after randomization to provide an estimate of recurrence of exacerbation.
Interestingly, in line with usual antibiotic comparison trials, moxifloxacin and standard therapy were equivalent (88% vs 83%) when clinical success (resolution and improvement) was compared at 7–10 days after the end of therapy. However, moxifloxacin therapy was associated with a superior clinical cure rate (defined as resolution of symptoms to baseline, rather than simply improvement) than standard therapy (71% vs 63%), as well as with superior bacteriologic response (91.5% vs 81%). In addition, when other a priori unconventional end-points were examined, moxifloxacin was associated with fewer requirements for additional antibiotic therapy (8% vs 14%) and an extended time to the next exacerbation (131 versus 104 days) (CitationWilson et al 2004). A composite end-point of clinical failure, requirement of additional antibiotics and recurrence of exacerbation demonstrated a clear difference between the two arms in the study, with moxifloxacin being superior to standard therapy for up to 5 months of follow up (). Again, if the conventional outcome of clinical success would have been measured solely in this study, all the other significant differences in the two arms would have not been discovered.
Figure 2 Life-table analysis of time to the first composite event (treatment failure, and/or new exacerbation and/or any further antibiotic treatment) stratified according to the time of the last exacerbation prior to randomization. Copyright © 2004. Reprinted with permission from Wilson R, Allegra L, et al 2004. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest, 125:953–64.
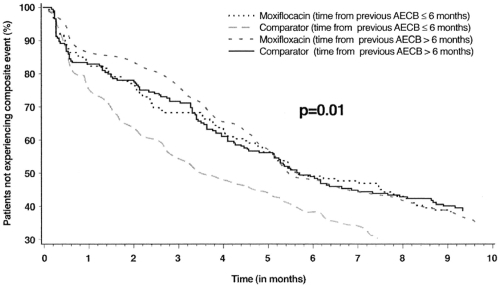
Results of the GLOBE and MOSAIC trials demonstrate that in vitro microbiologic superiority of the fluoroquinolones does translate to greater in vivo effectiveness in treating patients with acute exacerbation. Differences among antibiotics are often not perceptible with the standard regulatory end-point of clinical success at 7–14 days after the end of therapy. However, differences among antibiotics are perceptible when clinically relevant end-points such as speed of resolution, clinical cure, need for additional antimicrobials and time to next exacerbation are considered (CitationWilson et al 2002, Citation2004).
Risk stratification of patients
Based on the MOSAIC and GLOBE studies results, it would be tempting to prescribe fluoroquinolones for all moderate to severe exacerbations. However, it is clear that such a strategy, though likely to be successful in the short-term, would foster antimicrobial resistance to these valuable antibiotics in the long term. Therefore, it would be judicious to make an effort to identify those patients that are most likely to benefit from these antibiotics.
Several studies have now demonstrated that certain patient characteristics that antedate the onset of the exacerbation impact the outcome of the exacerbation (CitationBall et al 1995; CitationAdams et al 2000; CitationDewan et al 2000; CitationMiravitlles et al 2001; CitationGroenewegen et al 2003; CitationWilson et al 2006). Interestingly, several of these characteristics are relevant to outcome in more than one study. These risk factors for poor outcome should be considered in the decision regarding choice of antibiotics when treating exacerbations. Theoretically, patients at greater risk for poor outcome would have the greatest benefit from early aggressive antibiotic therapy, such as with the fluoroquinolones. These are the patients in whom the consequences of treatment with an antibiotic ineffective against the pathogen causing the exacerbation are likely to be significant, with clinical failures, hospitalizations and early recurrences likely.
Among the risk factors for poor outcome identified in various studies are increasing age, severity of underlying airway obstruction, presence of co-morbid illnesses (especially cardiac disease), a history of recurrent exacerbations, use of home oxygen, use of chronic steroids, hypercapnia and acute bronchodilator use (CitationBall et al 1995; CitationAdams et al 2000; CitationDewan et al 2000; CitationMiravitlles et al 2001; CitationGroenewegen et al 2003; CitationWilson et al 2006). Some of these risk factors are likely to be highly correlated to each other, such as home oxygen use, hypercapnia and chronic steroid use likely reflect increasing severity of underlying COPD. Acute bronchodilator use could be related to the severity of underlying COPD or reflect the wheezy phenotype of exacerbation that may be less responsive to antibiotic treatment. Many of the risk factors discussed above are continuous in severity, however, certain thresholds have been defined in studies that are clinically useful and predictive of poor outcome. These include an age of more than 65 yrs, FEV1 less than 50%, and more than 3 exacerbations in the previous 12 months.
In addition to the risk factors described above, experience in other respiratory infections tells us that recent antibiotic use, within the past 3 months, places the patient in a high risk group for harboring antibiotic resistant pathogens and therefore having a poor outcome. This has been best described for S pneumoniae among patients with community acquired pneumococcal pneumonia and recently also described for this pathogen among patients with COPD (CitationVanderkooi et al 2005; CitationSethi et al 2006). Whether such selection for antibiotic resistant strains occurs among NTHI and M catarrhalis after antibiotic exposure is not known.
Risk stratification approach to antibiotic therapy in acute exacerbation
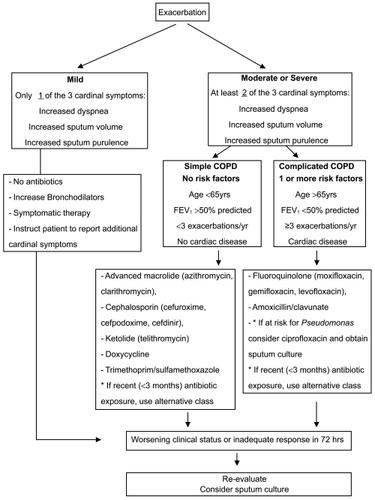
A risk stratification approach has been advocated by several experts for the initial empiric antibiotic treatment of acute exacerbation based on the risk factors discussed above and the in vitro and in vivo efficacy of antibiotics. Our current treatment algorithm which is very similar to what others have advocated is shown () (CitationBalter et al 2003; CitationSethi and Murphy 2004; CitationGOLD 2007). The initial step in the algorithm is the determination of the severity of the exacerbation. Based on the discussion above, we use the Anthonisen criteria of single cardinal symptom exacerbations defined as mild, while the presence of 2 or all 3 of the cardinal symptoms defines moderate and severe exacerbations.
Mild exacerbations are managed with symptomatic treatment and antibiotics are not prescribed unless the symptoms progress. In moderate to severe exacerbations, the important step is the differentiation of ‘uncomplicated’ (simple) patients from the ‘complicated’ patients. Uncomplicated patients do not have any of the risk factors for poor outcome. Complicated patients have one or more of the following risk factors for poor outcome: Age >65yrs, FEV1<50%, co-morbid cardiac disease, 3 or more exacerbations in the previous 12 months (CitationBalter et al 2003; CitationSethi and Murphy 2004; CitationGOLD 2007). Antibiotic choices for patients with uncomplicated COPD include an advanced macrolide (azithromycin, clarithromycin), a ketolide (telithromycin), a cephalosporin (cefuroxime, cefpodoxime or cefdinir), doxycycline or trimethoprim/sulfamethoxazole. Amoxicillin is not an appropriate choice with the considerable incidence of β-lactamase production among NTHI and M catarrhalis, two of the major pathogens of exacerbation. In complicated patients, antibiotic choices include a respiratory fluoroquinolone (moxifloxacin, gemifloxacin, levofloxacin) or amoxicillin/clavulanate.
In choosing an antibiotic, other considerations are also important. In all such patients, exposure to antibiotics within the past 3 months should be elucidated. Exposure to antibiotic is not confined to those prescribed for respiratory infections, but includes antibiotics prescribed for any indication. The antibiotic chosen should be from a different class of agents from the one prescribed within the past 3 months. For example, exposure to a macrolide in the past three months should lead to use of a cephalosporin in an uncomplicated patient. Similarly, prior use of a fluoroquinolone in a complicated patient should lead to use of amoxicillin/clavulanate.
There is a sub-group of the complicated patients who are at risk for infection by Pseudomonas aeruginosa and Enterobacteriaceae or have a documented infection by these pathogens (CitationSoler et al 1998). These patients have usually very severe underlying COPD (FEV1<35%), have developed bronchiectasis, are hospitalized (often requiring intensive care), or have been recently hospitalized or have received multiple courses of antibiotics. In such patients, empiric treatment with ciprofloxacin is appropriate. However, emerged resistance among P aeruginosa to the fluoroquinolones may compromise their efficacy. Therefore, in this sub-group of patients, a sputum (or tracheobronchial aspirate if intubated) culture should be obtained to allow adjustment of antibiotics based on the in vitro susceptibility of pathogens isolated. However, combination or parenteral antibiotic therapy for P aeruginosa in this setting has never been systematically examined and is of unproven benefit.
Patients should be re-assessed at 48–72 hrs, in which time frame clinical improvement should be apparent. In patients who fail initial empiric antimicrobial therapy, it would be appropriate to re-examine the patient to confirm the diagnosis, consider sputum studies to ascertain for resistant or difficult to treat pathogens and treat with an alternative agent with better in vitro microbiological efficacy.
Alternative approaches to antibiotic therapy in acute exacerbation
Purulence of sputum, ie, the amount of yellow and green pigmentation in sputum is related to the presence of myeloperoxidase, a product of neutrophil degranulation. As neutrophil degranulation is associated with bacterial infection, purulent sputum at exacerbation has been shown to be a marker of bacterial infection, defined by quantitative cultures of sputum as well as bronchoscopic protected specimen brush specimens (CitationStockley et al 2000; CitationSoler et al 2007). Presence of sputum purulence has been advocated as the sole determinant for antibiotic treatment of exacerbations. However, its accuracy and reproducibility as an indicator of bacterial infection is limited, and is likely to be even more so in clinical practice than in research studies (CitationStockley et al 2000; CitationSoler et al 2007). Sputum purulence is incorporated in the Anthonisen criteria, and should be used in conjunction with other symptoms and other measures of risk stratification in antibiotic treatment of exacerbations ().
Biomarkers of bacterial infection represent another approach to antibiotic use in exacerbations of COPD. The best studied biomarker of bacterial infection is serum procalcitonin level. In a recent study in patients hospitalized for exacerbations, antibiotic treatment was only prescribed if the procalcitonin level was above a certain threshold. The investigators showed no difference in outcomes in spite of reduction in antibiotic use from 72% to 40% with procalcitonin guidance (CitationStolz et al 2007). Though the findings of this study are very interesting, this approach needs to be validated in multi-center trials with varied populations (CitationMartinez and Curtis 2007). Furthermore, it needs to tested in outpatients with exacerbations, where the majority of exacerbations are treated, patients are not as closely supervised and other supportive care is less rigorous. Only a minority of patients received a fluoroquinolones in this study, which should have been the antibiotics of choice in these complicated patients, as per the risk stratification discussed above. Therefore, a reasonable speculation is that withholding antibiotics that may not be effective in the first place is likely not to have much of an impact. In this study, short term goals as well as biologic goals of bacterial eradication and inflammation reduction as discussed above were also not recorded.
Other important considerations in antibiotic prescribing are safety and tolerability of the agent, drug interactions and finally cost of treatment. Cost of the antibiotic however should not be interpreted in isolation. As shown elegantly by CitationMiravittles and colleagues (2002) exacerbations for which initial empiric treatment fails are ten times as costly as clinical successes (CitationMiravitlles et al 2002). In fact, the overall cost of care could be reduced by half with only a 33% reduction in clinical failure rates. Clinical failure rates are likely to be reduced when antibiotic choice is appropriate and logical, as discussed above.
References
- AaronSDVandemheenKLOutpatient oral prednisone after emergency treatment of chronic obstructive pulmonary diseaseN Engl J Med200334826182512826636
- AdamsSGMeloJAntibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPDChest200011713455210807821
- AllegraLBlasiFAntibiotic treatment and baseline severity of disease in acute exacerbations of chronic bronchitis: a re-evaluation of previously published data of a placebo-controlled randomized studyPulm Pharmacol Ther2001141495511273797
- AnthonisenNRManfredaJAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med19871061962043492164
- AnzuetoARizzoJAThe infection-free interval: its use in evaluating antimicrobial treatment of acute exacerbation of chronic bronchitisClin Infect Dis1999281344510451195
- BachPBBrownCManagement of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidenceAnn Intern Med20011346002011281745
- BallPHarrisJMAcute infective exacerbations of chronic bronchitisQJMed199588618
- BalterMSLa ForgeJCanadian guidelines for the management of acute exacerbations of chronic bronchitisCan Respir J200310Suppl B3B32B
- BhushanRKirkhamCAntigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalisInfect Immun1997652668759199435
- BurrowsBEarleRHCourse and prognosis of chronic obstructive lung disease. A prospective study of 200 patientsN Engl J Med19692803974045763088
- CalverleyPMAndersonJASalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med20073567758917314337
- CelliBRMacNeeWStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J2004239324615219010
- ChinCLManzelLJHaemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizersAm J Respir Crit Care Med2005172859115805181
- DevDWallaceEValue of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary diseaseRespir Med19989266479659534
- DevaliaJLRusznakCEffect of nitrogen dioxide and sulphur dioxide on airway response of mild asthmatic patients to allergen inhalationLancet19943441668717996960
- DewanNARafiqueSAcute exacerbation of COPD: factors associated with poor treatment outcomeChest20001176627110712989
- DonaldsonGCSeemungalTARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002578475212324669
- EldikaNSethiSRole of nontypeable Haemophilus influenzae in exacerbations and progression of chronic obstructive pulmonary diseaseCurr Opin Pulm Med2006121182416456381
- FalseyARHennesseyPARespiratory syncytial virus infection in elderly and high–risk adultsN Engl J Med200535217495915858184
- Garcia-AymerichJTobiasAAir pollution and mortality in a cohort of patients with chronic obstructive pulmonary disease: a time series analysisJ Epidemiol Community Health20005473410692968
- [GOLD] Global Initiative for Obstructive Lung DiseaseGlobal Initiative for Obstructive Lung Disease2007 [online]. Accessed on July 12, 2007 URL: http://www.goldcopd.com
- GroenewegenKHScholsAMMortality and mortality-related factors after hospitalization for acute exacerbation of COPDChest20031244596712907529
- HeckerlingPSThe need for chest roentgenograms in adults with acute respiratory illnessArch Intern Med1986146132143718128
- HiltkeTJSethiSSequence stability of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae in the human respiratory tractJ Infect Dis20021856273111865419
- HirschmannJVDo bacteria cause exacerbations of COPD?Chest200011819320310893379
- HurstJRDonaldsonGCUse of plasma biomarkers at exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20061748677416799074
- JohnstonSLPapiALow grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epitheliumJ Immunol19981606172819637536
- KannerRAnthonisenNRLower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health studyAm J Respir Crit Care Med20011643586411500333
- KjaergardLLLarsenFOBasophil-bound IgE and serum IgE directed against Haemophilus influenzae and Streptococcus pneumoniae in patients with chronic bronchitis during acute exacerbationsAPMIS19961046178645460
- MandellLABartlettJGUpdate of practice guidelines for the management of community-acquired pneumonia in immunocompetent adultsClin Infect Dis20033714053314614663
- MartinezFJCurtisJLProcalcitonin-guided antibiotic therapy in COPD exacerbations: closer but not quite thereChest20071311217218546
- MiravitllesMMurioCFactors associated with relapse after ambulatory treatment of acute exacerbations of chronic bronchitis. DAFNE Study GroupEur Respir J2001179283311488328
- MiravitllesMMurioCPharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPDChest200212114495512006427
- MiravitllesMRosFThe efficacy of moxifloxacin in acute exacerbations of chronic bronchitis: a Spanish physician and patient experienceInt J Clin Pract2001554374111594251
- MurphyTFBrauerALMoraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune responseAm J Respir Crit Care Med2005172195915805178
- MurphyTFSethiSThe role of bacteria in exacerbations of COPD. A constructive viewChest2000118204910893380
- NHLBI. 2003. Chronic Obstructive Pulmonary Disease Data, Fact Sheet, NIH Publication 03–5229
- NouiraSMarghliSOnce daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trialLancet20013582020511755608
- ObajiASethiSAcute exacerbations of chronic bronchitis. What role for the new fluoroquinolones?Drugs Aging20011811111232735
- OhtoshiTTakizawaHDiesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitroJ Allergy Clin Immunol19981016 Pt 1778859648705
- Pinto-PlataVMLivnatGSystemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPDChest2007131374317218554
- RamFSRodriguez-RoisinRAntibiotics for exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20062CD00440316625602
- Rodriguez-RoisinRToward a consensus definition for COPD exacerbationsChest20001175 Suppl 2398S401S10843984
- RudellBBlombergABronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particle trap filtered exhaustOccup Environ Med1999565273410492649
- SaintSBentSAntibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysisJAMA1995273957967884956
- SeemungalTHarper-OwenRRespiratory viruses symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116416182311719299
- SeemungalTARDonaldsonGCEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981571418229603117
- SethiSInfectious etiology of acute exacerbations of chronic bronchitisChest2000117380S385S10843981
- SethiSAcute exacerbations of COPD: A “multipronged” approachJ Respir Dis20022321755
- SethiSBacteria in exacerbations of chronic obstructive pulmonary disease Phenomenon or epiphenomenon?Proc Am Thorac Soc2004a11091416113422
- SethiSNew developments in the pathogenesis of acute exacerbations of chronic obstructive pulmonary diseaseCurr Opin Infect Dis2004b171131915021050
- SethiSEvansNAcquisition of a new bacterial strain and occurrence of exacerbations of chronic obstructive pulmonary diseaseN Engl J Med20023474657112181400
- SethiSMurphyTFAcute exacerbations of chronic bronchitis: new developments concerning microbiology and pathophysiology – impact on approaches to risk stratification and therapyInfect Dis Clin North Am20041886182ix15555829
- SethiSMurphyTFAntibiotic exposure in COPD and the development of penicillin and erythromycin resistance in Streptococcus pneumoniae2006ICAACSeptember 2006San Francisco CA
- SethiSMuscarellaKAirway inflammation and etiology of acute exacerbations of chronic bronchitisChest200011815576511115440
- SethiSWronaCStrain specific immune response to Haemophilus influenzae in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20041694485314597486
- Sinus and Allergy Health PAntimicrobial treatment guidelines for acute bacterial rhinosinusitis 2004Otolaryngol Head Neck Surg200413014514726904
- SolerNAgustiCBronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary diseaseThorax200762293516928715
- SolerNTorresABronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilationAm J Respir Crit Care Med199815714985059603129
- SpencerSCalverleyPMAHealth status deterioration in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001163122811208636
- SpencerSJonesPWTime course of recovery of health status following an infective exacerbation of chronic bronchitisThorax2003585899312832673
- StockleyRAO’BrienCRelationship of sputum color to nature and outpatient managment of acute exacerbations of COPDChest200011716384510858396
- StolzDChrist-CrainMAntibiotic treatment of exacerbations of COPD:a randomized controlled trial comparing procalcitonin-guidance with standard therapyChest200713191917218551
- SunyerJSaezMAir pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year studyAm J Epidemiol199313770158484361
- SunyerJSchwartzJPatients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysisAm J Epidemiol200015150610625173
- VanderkooiOGLowDEPredicting antimicrobial resistance in invasive pneumococcal infectionsClin Infect Dis20054012889715825031
- VeeramachaneniSBSethiSPathogenesis of bacterial exacerbations of COPDJ Chronic Obstructive Pul Dis2006310915
- WedzichaJASeemungalTAAcute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levelsThromb Haemost2000842101510959691
- WhiteAJGompertzSResolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitisThorax200358680512885984
- WhiteAJGompertzSChronic obstructive pulmonary disease. 6: The aetiology of exacerbations of chronic obstructive pulmonary diseaseThorax200358738012511727
- WilsonRAllegraLShort-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitisChest20041259536415006954
- WilsonRJonesPAntibiotic treatment and factors influencing short and long term outcomes of acute exacerbations of chronic bronchitisThorax2006613374216449273
- WilsonRSchentagJJA comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomesClin Ther2002246395212017408
- Wood-BakerRRGibsonPGSystemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20051CD00128815674875