Abstract
A common feature of lung disorders with poor treatment options, including emphysema, is a failure to initiate a repair process of the alveolar epithelium. Several putative stem cell niches in the lung thought to be involved in lung homeostasis have been described. Apparently, under pathophysiological conditions these resident progenitor cells are unable to recover damaged alveolar epithelium, in particular in emphysema. The potential therapeutic effect of retinoic acid receptor agonists on various resident lung progenitor cells is reviewed.
Keywords:
Stem cells
Stem cells can be defined as undifferentiated cells with a long life-span, which maintain the capacity to proliferate and maintain their own progenitor population. They produce a large number of differentiated daughter cells and form new tissue after injury. They generally reside in specific anatomical localizations, termed stem niches.
Tracheal stem cells
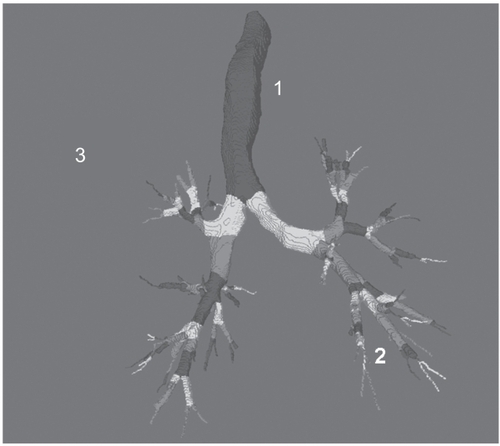
The lung can be subdivided into three regions for the classification of local stem cells (Figure ). Tracheal stem cells originate from tracheal lining epithelial cells and tracheal gland duct cells.
Figure 1 Computed tomography-derived human tracheo-bronchial tree. In the trachea (site 1) three types of cells may execute the function of stem cell, including basal cells, secretory cells and neuro-endocrine cells. In the bronchial and bronchiolar compartment (site 2) suprabasal, basal and Clara cells have similar abilities. In the bronchiolar-alveolar compartment (site 3), type II pneumocytes and bronchoalveolar stem cells may function as stem cell.
CitationDepuit and colleagues (2000) investigated cell proliferation and differentiation in a model of tracheal xenograft in rats. Denuded rat tracheae were implanted with human airway epithelial cells. Two to four weeks after implantation, the rat tracheae had repopulated with human airway epithelial cells. Cytokeratin (CK) 13 was detected in all epithelial cells whereas CK14 expression was limited to basal cells. CK18 was found in superficial cells only. High CK expression is associated with stem cell potential. After four to five weeks, the pseudostratified columnar epithelium covered the entire surface of rat epithelium and resembled fully differentiated human airway epithelium. Proliferating cells consisted of basal or epithelial cells.
Investigation of tracheal epithelium after tracheal injury in rats, induced by fluorouracil (5-FU), was found to yield cells with naked nuclei located on the basement membrane of the trachea (CitationDing et al 2004). These cells proliferate and differentiate to restore the tracheal epithelium and are thought to represent tracheal stem cells.
CitationHong and colleagues (2004a) also concluded that basal cells in the trachea are capable of restoring a fully differentiated epithelium. CitationLiu and colleagues (1994) used tracheal grafts to search for progenitor cells and their results suggest that both basal and secretory cells dedifferentiated into a highly proliferative phenotype and then redifferentiated to form mucocilliary epithelium.
Pulmonary neuroendocrine cells (PNECs) are thought to reside in niches associated with stem cells, which protect the cells from external damage. In mice, gland ducts in the trachea form such niches, as do foci near the cartilage – intercartilage junction in the distal epithelium. Due to the slow cycling of stem cells, it is necessary to damage airway epithelium to induce mitoses. Tracheal stem cells labelled with BrdU (5-bromo-2-deoxy-uridine), a base analogue of thymidine, retain the label longer than other cells as a result of their slow cycle (CitationBorthwick et al 2001). The investigators used two methods to damage the epithelium: intra-tracheal instillation of the detergent polidocanol, and SO2 inhalation. The results achieved for both methods of injury were similar. At day 21 and 95 after injury, the most densely BrdU staining cells were located at the submucosal gland ducts in the proximal trachea. Clusters were also found in the distal trachea, corresponding to the position of the cartilage- inter-cartilage junction. Tests confirmed that although there is an association between the label-retaining cells (LRCs) and PNECs, not all LRCs were in fact PNECs.
Bronchiolar stem cells
The terminal bronchiole is highly resilient; it is capable of rapid renewal of the cell population in response to epithelial injury and cell depletion.
Bronchiolar stem cells are difficult to detect, as there is not yet a satisfactory stem cell marker as there is for skin epidermis and hemato-leukopoietic tissue (CitationColler et al 2005). However, bronchiolar progenitor cells have been identified using a variety of labelling techniques in the steady state lung and in the lung after mechanical injury. It has now been established that these cells include basal cells and (nonciliated) Clara (Clara cell secretory protein (CCSP)-expressing [CE]) cells (CitationHong et al 2004b). Clara cells may be the principal progenitor for the renewal of lung tissue (Figure ). CE cells can be subdivided further according to their susceptibility to naphthalene. Naphthalene-sensitive cells are more numerous than naphthalene-resistant cells, which have stem cell properties. CE cell injury, however, is resolved by recruitment of a second type of progenitor cell, known as a GSI-B4 (Griffonia simplicifolia isolectin) reactive, cytokeratin-14-expressing basal cell.
To study the role of basal cells in bronchial epithelial regeneration, ganciclovir (GCV) or naphthalene (which specifically depletes CE cells), was administered to mice. Investigation of their bronchial epithelium after sacrifice, showed that CE cells were the preferred progenitor cell for maintenance and regeneration of the airway epithelium and that basal cells were activated in response to the depletion of CE cells.
Aurélie CitationAvril-Delplanque and colleagues (2005) tested airway epithelial cells for various cell surface antigens and found that aquaporin-3 (AQP-3) water channel expression at the cell surface is limited to basal cells. Both AQP-3+ and AQP-3– suprabasal cells are capable of restoring mucosa. However, AQP-3– cells do so much more quickly than their AQP-3+ counterparts. At four weeks, AQP-3– cells had formed a pseudo-stratified epithelium, whereas the AQP-3+ cells had formed a bilayered, poorly differentiated epithelium. The authors state that it will be important to investigate whether the AQP-3– epithelial progenitors are secretory cells, as secretory cells have, in rat models, been shown to repopulate surface epithelium in four weeks.
West and colleagues (2001) discovered that Clara cells in mice are extremely sensitive to naphthalene compared to those of rats. The extent of injury is related to the dose and method of administration. At low doses, the proximal airway is mainly affected, whereas at high doses, the terminal airways were also affected. At high doses (400 mg/kg), both Clara cells and ciliated cells were damaged in mice. In denuded areas, clusters of three to four, apparently undamaged cells were observed. The clusters appeared to be ciliated cells and were not distributed according to any anatomical area such as branch points. The investigators administered inhaled naphthalene to determine whether cells at branch points are more resistant to naphthalene injury as had been suggested. They concluded that cells at branch points are equally affected by naphthalene as cells at other anatomical locations.
Giangreco and colleagues (2002) demonstrated the existence of a CE cell which functions as a stem cell and resides in the NEB (Neuro Endocrine Body) microenvironment of the bronchiolar epithelium. These CE cells are resistant to naphthalene injury. The investigators determined in which areas of terminal bronchiolar epithelium resistant CE cells reside. The phenotype of pollutant-resistant cells was assessed by dual-color fluorescence immunostaining for the Clara cell-specific marker or the pulmonary neuroendocrine cell (PNEC)-specific marker calcitonin gene-related peptide. Clusters of resistant CE cells were found to reside adjacent to NEBs and a second population of resistant CE cells was located adjacent to the bronchoalveolar duct junction (BADJ). Further investigation using in situ hybridization and autoradiography confirmed that the second population consists of CE cells.
Naphthalene sensitivity is associated with high levels of cytochrome-450 2F2, which is responsible for the metabolism of naphthalene (CitationBuckpitt et al 1995). Buckpitt investigated naphthalene metabolic rates in airway epithelium of mice, rats and hamsters and found mice to have a relatively high rate which explains their extreme sensitivity to naphthalene.
Alveolar stem cells
An adult has on average 300,000 alveoli which are lined by three cell types (CitationBerne et al 2004). Type I epithelial cells are responsible for gas exchange and have also been found to express genes which suggest that these cells play a role in maintenance of alveolar homeostasis, tumor suppression, lung development, and remodeling and surfactant metabolism (CitationDahlin et al 2004). These cells are extremely flat and occupy 95% of the surface area of the alveolus. Due to the highly attenuated cytoplasm, these cells are difficult to isolate for study. Depletion of Type I pneumocytes by cytotoxic agents leaves the epithelial surface denuded.
Type II epithelial cells are small cuboidal cells which usually reside in the corners of the alveolus, covering roughly 2% of the alveolar surface area. Type II pneumocytes are known to produce surfactant and regenerate alveolar epithelium after injury.
In healthy lungs, type I and type II cells exist in a 1:1 ratio.
Type III pneumocytes or brush cells, were reported throughout the lung in close proximity to unmyelinated nervous fibres and are thought to function as chemoreceptors (CitationFoliguet and Grignon 1980).
It has long been established that type I pneumocytes are derived from type II pneumocytes. Evans and colleagues (1974) exposed male rats to NO2 to induce airway epithelial cell division. 3H-TdR was used to label dividing cells, most of which was taken up by type II pneumocytes. After two days, a large increase in the number of labelled type I cells had occurred, which remained stable up to day 14. These results strongly suggest that type I cells are derived from type II cells.
CitationBender Kim and colleagues (2005) isolated pulmonary stem cell populations located at the bronchoalveolar duct junction which they termed bronchoalveolar stem cells (BASCs). BASC incidence was found to increase following intranasal bleomycin administration suggesting that they play a role in the response to alveolar damage.
Side population cells
Side population (SP) cells are a rare, primitive type of stem cell, recently discovered in the lung. SP cells have previously been identified in other tissues and organs such as the bone marrow, kidney, liver, brain, heart, and small intestine. They can be identified by their capability to efflux Hoechst 33342 dye (CitationMajka et al 2005). SP cells do not have markers of differentiated cells but do express some early lineage commitment. This may be explained by the diversity of different lineages in the lung into which SP cells are capable of differentiating.
CitationSummer and colleagues (2003) found that SP cells comprise 0.03%–0.07% of total lung cells and are evenly distributed throughout the lung. All SP cells carry stem cell antigen (Sca)-1 but are lineage marker negative, confirming that they are not differentiated. SP cells are positive for breast cancer resistance protein (BCRP) 1, a protein responsible for the ATP-mediated efflux of Hoechst 33342 dye. CitationLiang and colleagues (2005) investigated the gene expression profile of CD45– and CD45+ SP cells (CD45 is a haematopoietic marker) and identified 31 and 44 highly expressed genes respectively. SP cells from mouse embryos were separated from main population (MP) cells and the cells were split into four groups: SP CD45–, SP CD45+, MP CD45–, and MP CD45+. Gene profiling data suggest that SP CD45– cells are involved in vascular development, whereas SP CD45+ cells may be progenitors for differentiated resident lung cells of myeloid lineage.
Investigators have stressed the importance of establishing a uniform protocol for the isolation of SP cells in order to ensure the comparative nature of studies. The multi-step process of isolating SP cells allows for a large inter-researcher variability. Factors include the concentration of Hoechst dye used, staining conditions and calibration of equipment.
Exogenous stem cells
The prospects of the use of exogenous stem cells to regenerate damaged and diseased tissues and organs are very exciting.
Bone marrow derived stem cells can be divided into five groups: hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), endothelial stem/progenitor cells (EPCs), SP cells, and multipotent adult progenitor cells (MAPCs). HSCs have been extensively studied and have been found responsible for the maintenance of circulating blood cell population.
Various studies have been conducted in which engraftment of bone marrow derived cells after lethal irradiation has been investigated. CitationTheise and colleagues (2002) irradiated female mice thus inducing pneumonitis and injected them with bone marrow cells from male mice. Female mice were sacrificed at various times and fluorescence in situ hybridization (FISH) was used to detect donor-derived cells with a Y chromosome in lung tissue. Immunohistochemistry for cytokeratin 7 and 8 was performed to identify pneumocytes. Y-chromosomes were found in type II pneumocytes, showing that bone marrow cells can act as alveolar stem cells.
CitationBeckett and colleagues (2005) sought to compare various mechanisms of lung injury (which consequently stimulate different inflammatory signalling cascades) and the patterns of lung engraftment they induced. Irradiated female mice were subjected to either intranasal endotoxin or inhaled NO2 one month after administration of bone marrow derived donor cells from male mice. The investigators concluded that there was no significant difference between the numbers of donor-derived type II pneumocytes in the lung in mice which had been irradiated compared to mice which had been irradiated and had received either endotoxin or NO2. The majority of donor-derived cells found in the lung were leucocytes rather than epithelial cells. Although the engraftment patterns were different for inflammatory cells, the two methods of injury did not result in varying patterns of engraftment of lung epithelial cells. The investigators state that the paucity donor-derived epithelial cells in the lung may be due to the lower dose of irradiation used.
CitationGomperts and colleagues (2006) demonstrated that early epithelial marker cytokeratin 5 (CK5) and the chemokine receptor CXCR4 are markers of epithelial progenitors in the circulation or bone marrow. The CXCR4/CXCL12 biological axis is important in mobilizing and recruiting bone marrow derived cells to the site of injury in the lung.
CitationAliotta and colleagues (2006) investigated the effect of granulocyte colony stimulating factor (G-CSF) on irradiated mice that had received donor bone marrow cells. Mice treated with G-CSF had higher levels of donor marrow – derived lung cells compared to mice whose bone marrow cells had not been mobilized by G-CSF.
Emphysema
Emphysema is one of the subtypes of COPD. The disease is defined as dilatation and destruction of lung tissue distal to the terminal bronchiole (CitationKumar and Clark 2002). It is widely accepted that the disease is caused by excessive proteolytic activity by elastases. Recent research has called for a broad interpretation of the protease-antiprotease hypothesis as many enzymes and their inhibitors, besides the initially found alpha-1-protease inhibitor, have been found to be involved in the pathogenesis of emphysema.
Macrophages release two classes of elastolytic enzymes: cysteine proteinases (cathepsin L and cathepsin S) and several matrix metalloproteinases (MMPs). MMPs are proteolytic enzymes secreted in latent form, their activation is thought to be induced by oxidants from cigarette smoke (CitationGibbs et al 1999).
Neutrophil recruitment into the lung in response to cigarette smoke is mediated by interleukin (IL) −8 secreted by alveolar epithelial cells (CitationWitherden et al 2004).
Cigarette smoke was found to induce recruitment of macrophages to alveoli in mice exposed to the smoke of nine cigarettes per day (Citationda Hora et al 2005). CitationChurg and colleagues (2004) demonstrated the role of tumor necrosis factor-α (TNF-α) in cigarette smoke-induced emphysema in mice. Mice, deficient in TNF-α receptors, show neither matrix breakdown nor airspace enlargement in response to cigarette smoke. Their lungs contain fewer neutrophils than those of their nonsmoking counterparts. Western-blotting, used to detect levels of MMPs, showed that wild-type mice that had been exposed to smoke had higher levels for each of the detected MMPs than the TNF-α receptor deficient mice. These results show that TNF-α is responsible for a significant percentage of the processes that cause airspace enlargement; TNF-α drives the production of various MMPs in mouse models.
Interestingly, the investigators discovered that the 65%–70% protection value yielded by knocking out the TNF-α receptor approximately corresponds to the protection values found in neutrophil elastase knockout mice. The detection of lower levels of MMPs in TNF-α receptor knockout mice does however suggest that these levels were induced by stimulation via an alternative pathway independently of TNF-α. CitationChurg and colleagues (2004) proposed apoptosis as a possible alternative pathway responsible for airway space enlargement.
CitationAoshiba and colleagues (2003) investigated the role of airway epithelial apoptosis in the pathogenesis of emphysema. A single intratracheal injection of active caspase-3 and Chariot, a newly developed protein transfection reagent, was administered to mice to induce alveolar wall apoptosis. Six hours later, the lungs of sacrificed mice showed focal airway enlargement and destruction of alveolar walls. Elastin was lost from alveolar walls and the lungs were more distensible than those of untreated animals.
Emerging therapy for emphysema
Retinoic acid
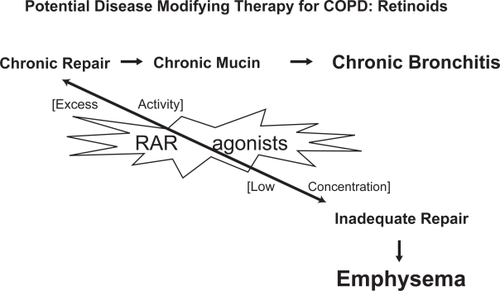
Retinoic acid is the active metabolite of vitamin A (retinol), which is acquired from the diet and has many functions in cell development, differentiation, and homeostasis. All-trans-retinoic acid (ATRA) is an analogue of vitamin A and its role in inducing alveolar regeneration has recently been under investigation due to its exciting potential as a therapy for diseases such as emphysema (Figure ).
Figure 2 Retinoid acid receptor agonists (RAR agonists) are involved in the pathogenesis of chronic bronchitis due to excess activity of type β RAR agonists involved in mucus production and in the pathogenesis of emphysema when reduced activity of type γ RAR agonists is present.
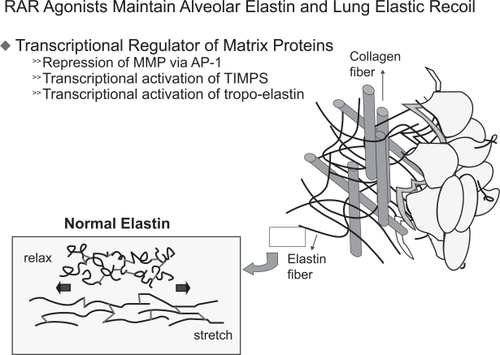
In 1987, the retinoic acid receptor was discovered (CitationChambon 1996). There are two types of retinoid receptor: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Three isoforms of the RAR are known, each of which has a different role during alveolar development (Figure ). CitationMassaro and colleagues (2003) found that RARα, one of the three RAR isoforms, is able to regulate alveolar development in mice at the age of 14–50 days. RARβ inhibits alveolar formation in the early post-natal period only. RARγ, the third isoform of RAR is necessary for formation of alveoli in mice during the first 28 days after birth. Additional experiments showed that RARγ drives tropoelastin gene expression (Figure ; CitationMcGowan et al 2000) and that RARγ agonists promote alveolar repair in animals with experimentally-induced emphysema (CitationMassaro et al 1997).
Figure 3 Type γ retinoid acid receptor is required for alveolar septation and branching morphogenesis. In this system, plasma retinol and retinol binding protein may have an important regulatory role in COPD.
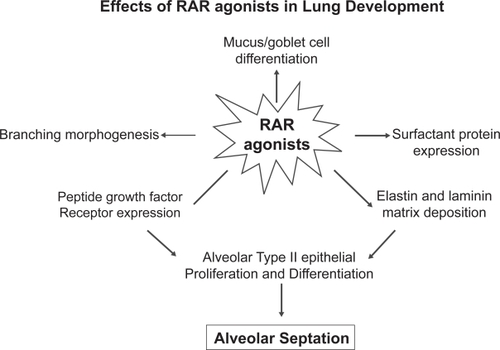
Figure 4 RARγ drives tropo-elastin gene expression. Tropo-elastin is the precursor of elastin, the later has a critical function in the elastic recoil of the lung. Destruction of elastin content in the lung will result in emphysema.
CitationSnyder and colleagues (2005) proposed that RARβ, plays an important role in neo natal alveolar growth. Mice lacking the RARβ gene were found to have impaired neonatal lung function. The area for gas exchange was smaller than that of their wild-type counterparts.
Experiments conducted by CitationCardoso and colleagues (1995) have shown that retinoids influence the developmental pattern of lung tissue in vitro.
CitationFujita and colleagues (2004) treated mice with daily intraperitoneal ATRA injections for 12 days, following induction of emphysema with porcine pancreatic elastase. Histopathological analysis revealed no significant difference between mice treated with ATRA and controls.
CitationHind and Maden (2004) demonstrated that mice treated with dexamethason, which disrupts alveolar development, followed by retinoic acid (RA) administration had a more complex alveolar structure and more alveoli than control mice that had not received RA following dexamethason. Lungs of mice treated with both dexamethason and RA resembled those of mice who had received neither dexamethason or RA.
However, experiments conducted with rat emphysema models showed that ATRA induced alveolar regeneration. CitationTepper and colleagues (2000) administered two doses of porcine elastase intratracheally to male rats. Nine weeks later, the rats were treated with daily intraperitoneal ATRA injections for 2 weeks. Elastase-induced airway obstruction worsened progressively in rats treated with elastase only, whereas rats treated with elastase followed by ATRA showed no progression of airway obstruction. CT scans showed that ATRA caused a restoration of lung density, which was not significant, whereas rats treated with elastase only displayed a reduced density. There were, however, small but significant changes in lung volume after two weeks of intraperitoneal RA infusions.
CitationIshizawa and colleagues (2004) investigated the effect of G-CSF and ATRA on emphysema induced by elastase in mice. Combination of G-CSF and ATRA had an additive effect, indicating that mobilization of BMCs may play an important role in lung regeneration after injury.
CitationNakajoh and colleagues (2003) investigated whether retinoic acid protects epithelial cells from elastase-induced damage and apoptosis. Three cell types were exposed to elastase and their viability was monitored following exposure to various amounts of elastase. Retinoic acid without prior elastase treatment caused no change in the viability of the lung epithelial cells. Retinoic acid administered to cells damaged by elastase improved the viability of each of the three cells types. The investigators also found that pre-treatment with retinoic acid, followed by elastase, improves cell viability significantly.
CitationBelloni and colleagues (2000) performed immunohistochemistry on alveolar tissue. Within 24 hours of ATRA treatment, they found a threefold increase in apoptotic cells in rats to which elastase had previously been administered compared with rats that had not received ATRA. These findings suggest that ATRA induces apoptosis which is a normal part of the wound-healing process in which specific cell populations are eliminated without inducing an inflammatory response.
CitationNabeyrat and colleagues (2000) found evidence that RA stimulates proliferation of type II alveolar cells. Their results show that RA acts on regulatory proteins in the cell cycle leading them to suggest that RA is capable of causing cells which have ceased proliferating to resume growth.
A double blind, placebo-controlled trial, conducted by CitationMao and colleagues (2002) in which 20 patients with moderate to severe emphysema participated, showed that ATRA is well-tolerated by humans. Besides their usual therapy, patients received twice-daily doses of 25 mg/m2 ATRA for four days a week for twelve weeks. Side effects included dry skin and cracked lips, headache, hyperlipidemia, mild pruritis, mild transaminase elevations, mild muscle and bone pain/soreness, and a sensation of clogged ears. The study did not show any reversal of emphysema.
Due to the side effects caused by long-term use of oral retinoid acid, CitationBrooks and colleagues (2000) proposed the use of aerosolized ATRA as a safer alternative. Tests using rats revealed that intra-tracheal administration of ATRA resulted in a long half-life in the lung and low systemic levels, proving that aerosolized ATRA is a feasible therapy.
The development of selective RARgamma agonists was taken forward by a pharmaceutical company and resulted in a large proof-of-concept study currently conducted in 20 hospitals world-wide with the aim to proof that agonists of RARgamma are able to induce lung alveolar growth and improved gas exchange in patients with emphysema.
Conclusion
Despite significant advances over the past decades, much remains unknown about the processes involved in lung epithelial repair. As our knowledge of lung physiology improves, new therapies will emerge for destructive diseases such as emphysema.
Research into pulmonary stem cells has yielded valuable information concerning the origins and functions of stem cells at various anatomical locations, such as the trachea, bronchus and alveolus. It is thought that stem cells may hold the key to new treatments, possibly even a cure for emphysema.
Currently, further research and trials are being carried out to explore the use of retinoid acid receptor agonists, discussed in the second part of this review, as a potential therapy for emphysema. Hopefully these studies will yield useful data concerning the molecular signaling pathways involved in alveolar epithelial regeneration.
References
- AliottaJMKeanePPasseroM2006Bone marrow production of lung cells: The impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotypeExp Hematol342304116459191
- AoshibaKYokohoriNNagaiA2003Alveolar wall apoptosis causes lung destruction and emphysematous changesAm J Respir Cell Mol Biol285556212707011
- Avril-DelplanqueACasalICastillonN2005Aquaporin-3 Expression in Human Fetal Airway Epithelial Progenitor CellsStem Cells23992100116043462
- BeckettTLoiRPrenovitzR2005Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrowMol Ther12680616027045
- BelloniPNGarvinLMaoCP2000Effects of all-trans-retinoic acid in promoting alveolar repairChest117Suppl 1235S41S10843926
- Bender KimCFJacksonELWoolfendenAE2005Identification of bronchioalveolar stem cells in normal lung and lung cancerCell1218233515960971
- BerneRMLevyMNKoeppenBM2004Physiology5th ed453
- BorthwickDWShahbazianMKrantzQT2001Evidence for stem-cell niches in the tracheal epitheliumAm J Respir Cell Mol Biol246627011415930
- BrooksADTongWBenedettiF2000Inhaled aerosolization of all-trans-retinoic acid for targeted pulmonary deliveryCancer Chemother Pharmacol463131811052629
- BuckpittAChangAMWeirA1995Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamstersMol Pharmacol4774817838135
- CardosoWVWilliamsMCMitsialisSA1995Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitroAm J Respir Cell Mol Biol12464767742011
- Chambon1996A decade of molecular biology of retinoic acid receptorsFASEB J10940548801176
- ChurgAWangRDTaiH2004Tumor necrosis factor-α drives 70% of cigarette smoke–induced emphysema in the mouseAm J Respir Crit Care Med170492815184206
- CollerHAKhrapkoKHerrero-JimenezP2005Clustering of mutant mitochondrial DNA copies suggests stem cells are common in human bronchial epitheliumMutat Res5782567116009384
- DahlinKMagerEMAllenL2004Identification of genes differentially expressed in rat alveolar type I cellsAm J Respir Cell Mol Biol313091615205179
- DingQJiaXSZhouY2004Study of tracheal regeneration after injury induced by 5-fluorouracil in ratsZhonghua Bing Li Xue Za Zhi33143515132852
- DupuitFGaillardDHinnraskyJ2000Differentiated and functional human airway epithelium regeneration in tracheal xenograftsAm J Physiol Lung Cell Mol Physiol278L1657610645904
- FoliguetBGrignonG1980Type III pneumocyte. The alveolar brush-border cell in rat lung. Study by transmission electron microscopy (author’s transl)Poumon Coeur36149537465487
- FujitaMYeQOuchiH2004Retinoic acid fails to reverse emphysema in adult mouse modelsThorax592243014985558
- GibbsDFWarnerRLWeissSJ1999Characterization of matrix metalloproteinases produced by rat alveolar macrophagesAm J Respir Cell Mol Biol2011364410340932
- GompertsBNBelperioJARaoPN2006Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injuryJ Immunol17619162716424223
- HindMMadenM2004Retinoic acid induces alveolar regeneration in the adult mouse lungEur Respir J2320714738226
- HongKUReynoldsSDWatkinsS2004aIn vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulationsAm J Physiol Lung Cell Mol Physiol286L643912871857
- HongKUReynoldsSDWatkinsS2004bBasal cells are a multipotent progenitor capable of renewing the bronchial epitheliumAm J Pathol1645778814742263
- da HoraKValencaSSPortoLC2005Immunohistochemical study of tumor necrosis factor-alpha, matrix metalloproteinase-12, and tissue inhibitor of metalloproteinase-2 on alveolar macrophages of BALB/c mice exposed to short-term cigarette smokeExp Lung Res317597016368650
- IshizawaKKuboHYamadaM2004Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysemaFEBS Lett5562495214706858
- KumarPClarkM2002Clinical Medicine; Respiratory Disease863
- LiangSXSummerRSunXFineA2005Gene expression profiling and localization of Hoechst-effluxing CD45– and CD45+ cells in the embryonic mouse lungPhysiol Genomics231728116076931
- LiuJYNettesheimPRandellSH1994Growth and differentiation of tracheal epithelial progenitor cellsAm J Physiol266L2963078166299
- MassaroGDMassaroD1997Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in ratsNat Med367579176496
- MassaroGDMassaroDChambonP2003Retinoic acid receptor-alpha regulates pulmonary alveolus formation in mice after, but not during, perinatal periodAm J Physiol Lung Cell Mol Physiol284L431312533315
- MajkaSMBeutzMAHagenM2005Identification of novel resident pulmonary stem cells: form and function of the lung side populationStem Cells2310738115987674
- MaoJTGoldinJGDermandJ2002A pilot study of All-trans-Retinoic Acid for the treatment of human emphysemaAm J Respir Crit Care Med1657182311874821
- McGowanSJacksonSKJenkins-MooreM2000Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbersAm J Respir Cell Mol Biol23162710919981
- MichaelJEvansLCabralJ1974Transformation of alveolar Type 2 cells to Type 1 cells following exposure to NO2Expir Mol Pathol22142150
- NabeyratECorroyerSEpaudR2000Retinoic acid-induced proliferation of lung alveolar epithelial cells is linked to p21CIP1 downregulationAm J Physiol Lung Cell Mol Physiol278L42L5010645889
- NakajohMFukushimaTSuzukiT2003Retinoic acid inhibits elastase-induced injury in human lung epithelial cell linesAm J Respir Cell Mol Biol2829630412594055
- SnyderJMJenkins-MooreMJacksonSK2005Alveolarization in retinoic acid receptor-beta-deficient micePediatr Res573849115635054
- SummerRKottonDNSunX2003Side population cells and Bcrp1 expression in lungAm J Physiol Lung Cell Mol Physiol285L9710412626330
- TepperJPfeifferJAldrichM2000Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat?Chest117242S4S10843928
- TheiseNDHenegariuOGroveJ2002Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrowExp Hematol301333812423687
- WitherdenIRvanden BonEJGoldstrawP2004Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastaseAm J Respir Cell Mol Biol30500915033639