Abstract
Bacteria are isolated in more than 50% of exacerbations of chronic bronchitis (CB) and chronic obstructive pulmonary disease (COPD). The most prevalent respiratory pathogens include Gram-positive (Streptococcus pneumoniae) and Gram-negative (Haemophilus influenzae, Moraxella catarrhalis) microorganims. Moxifloxacin is a fourth-generation fluoroquinolone that has been shown to be effective against respiratory pathogens, including atypicals and those resistant to most common antibiotics. The bioavailability and half-life of moxifloxacin provides potent bactericidal effects at a dose of 400 mg once daily. Among the fluoroquinolones, the ratio of the area under the concentration-time curve (AUC) to minimal inhibitory concentration of moxifloxacin is the highest against S. pneumoniae. Moxifloxacin has demonstrated better eradication in exacerbations of CB and COPD compared with standard therapy, in particular, with macrolides. Patients treated with moxifloxacin showed a prolonged time to the next exacerbation and observational studies suggest that moxifloxacin induces a faster release of symptoms of exacerbation. Some guidelines recommend the use of moxifloxacin as first-line therapy in bacterial exacerbations in patients with moderate to severe COPD and in patients with mild COPD with risk factors. The current article reviews the use of moxifloxacin in bacterial exacerbations of CB and COPD.
Introduction
After cardiovascular disease, respiratory diseases rank second in terms of mortality, incidence, prevalence, and costs. The European prevalence of chronic obstructive pulmonary disease (COPD) ranges from <2% in France and the UK, to 9% in Spain (CitationSobradillo et al 2000), and >10% in Germany and Italy (CitationLoddenkemper 2003). Moreover, a European-wide increase in the prevalence and mortality of COPD and other smoking-related diseases, particularly in women, is projected for the coming decades (CitationPeto et al 1992). Chronic bronchitis (CB), a component of COPD, usually associated with frequent exacerbations, is even more prevalent; as an example, 4.1% of the French population aged 25+ years develop this disease (CitationHuchon et al 2002). Nonetheless, the prevalence of CB differs between countries. In a recent study of young European adults, the prevalence of CB in subjects aged 20–44 years ranged from 0.7% in the UK to 9.7% in Spain (CitationDe Marco et al 2004), with the prevalence being directly associated with smoking prevalence (CitationCerveri et al 2001).
Due to the high prevalence and chronic course of these conditions, COPD and CB represent a great burden to society. The mean annual direct medical costs of a COPD patient are an estimated €1800, with 42% of the costs being due to hospitalisations, most because of exacerbations (CitationMiravitlles et al 2003a). Each exacerbation treated in the community has a mean cost of €175, but costs are higher for complicated exacerbations requiring hospital admission (CitationMiravitlles et al 2002).
In addition, COPD and CB are debilitating conditions affecting fundamental aspects of everyday life including normal physical exertion, work, and social and family activities (CitationMurray and Lopez 1997). Chronic lung disease thus has substantial effects on health-related quality of life (HRQoL), with increasing CB severity and factors such as frequency and nature of exacerbations being associated with increasing symptoms and deteriorating HRQoL (CitationSeemungal et al 1998; CitationMiravitlles et al 2004a). HRQoL has been found to be significantly adversely affected during exacerbations with increased anxiety about breathlessness and the coughing up of phlegm in public (CitationNicolson and Anderson 2000).
Bacterial are isolated in 50%–75% of exacerbations (CitationMiravitlles 2002a; CitationPapi et al 2006). Because of the difficulties in establishing a bacteriological diagnosis of respiratory tract infections (RTIs) in ambulatory clinical practice, it is common practice to commence antibiotic treatment empirically. In an analysis of initial antibiotic treatment in Europe for all infections, including RTIs, 85% of these therapies were started empirically (CitationHalls 1993). In view of the limits of the spectrum of activity of some antibiotics, as well as the increasing prevalence of bacterial resistance to older generation agents, multiple antibiotics are administered in some cases of RTIs or when resistant or polymicrobial infection is suspected (CitationSmith et al 1999). There is a continued need to find antibiotics that can be used in empiric treatment regimens so that the patient responds rapidly and the risk of development of resistance is limited.
Moxifloxacin hydrochloride (Avelox®, Bayer AG) is a fluoroquinolone that has been shown to be effective against Moraxella catarrhalis, Haemophilus influenza, and multi-drug resistant pneumococcal strains (CitationBlondeau 1999; CitationSaravolatz et al 2001; CitationMiravitlles 2005). Moxifloxacin is strongly targeted to alveolar tissue (CitationSoman 1999 et al; CitationMiravitlles 2000), and has shown rapid initial killing and eradication rates for pneumococcal bacteria (CitationLister and Sanders 2001).
This paper will focus on recent advances in the study of antimicrobial treatment of exacerbations of CB and COPD with moxifloxacin. In this respect, new trials have documented improved outcomes related to time to resolution of symptoms and extended time to the next exacerbation. These new outcomes derive from the better understanding of the natural history of CB and COPD and their exacerbations.
Pharmacology
Mechanism of action
Moxifloxacin is a forth generation fluoroquinolone with bactericidal activity as a result of its interference with topoisomerase II (DNA gyrase) and IV (CitationScheld 2003). Topoisomerases are essential enzymes which play a crucial part in the replication, transcription, and repair of bacterial DNA. Topoisomerase IV is also known to influence bacterial chromosome division. Kinetic investigations have demonstrated that moxifloxacin exhibits a concentration-dependent killing rate; minimum bactericidal concentrations (MBC) were found to be within the range of the minimum inhibitory concentrations (MIC) (CitationStass 1999; CitationKrasemann et al 2001).
Antimicrobial activity
Moxifloxacin demonstrates excellent in vitro activity (as assessed by MIC90 concentrations of 0.06 to 0.25 mg/L) against all the predominant respiratory pathogens including Gram-positive (Streptococcus pneumoniae), Gram-negative (H. influenzae, M. catarrhalis), anaerobic (peptostreptococci or Prevotella spp.), and atypical strains (C. pneumoniae, M. pneumoniae). Against both beta-lactamase positive and negative M. catarrhalis and H. influenzae, MIC90 values to moxifloxacin were ≤0.06 μg/mL. Similarly, moxifloxacin MIC90 values ranged from 0.06 to 0.25 μg/mL against clinical isolates of S. pneumoniae regardless of penicillin susceptibility (CitationWoodcock 1997; CitationBlondeau 1999) (Table ).
Table 1 Minimum inhibitory concentrations (MIC) of moxifloxacin and other quinolones against common respiratory pathogens
Pharmacokinetics
Following oral administration moxifloxacin is rapidly and almost completely absorbed, with an absolute bioavailability of approximately 91% (CitationStass et al 2001). Its penetration into pulmonary tissues is excellent and concentrates in alveolar macrophages (Table ). Following a 400 mg oral dose, peak concentrations of 3.1 mg/L are reached within 0.5–4 hours post administration. Peak and trough plasma concentrations at steady-state (400 mg once daily) are 3.2 and 0.6 mg/L, respectively. Moxifloxacin is rapidly distributed to extra-vascular spaces; and an area under the concentration-time curve (AUC) of 35 mg·h L−1 is observed after a dose of 400 mg. Moxifloxacin is mainly bound to serum albumin. In vitro and ex vivo experiments have shown a protein binding of approximately 40%–42%, independently of the concentration of the drug (CitationSoman et al 1999; CitationSullivan et al 1999).
Table 2 Tissue concentrations by site 10 hours after oral administration of 400 mg of moxifloxacin
Moxifloxacin is eliminated from plasma with a mean terminal half-life of approximately 12 hours (CitationStass 1999). After a 400 mg dose, recovery of parent compound and metabolites of moxifloxacin from urine and feces totals approximately 96% (Table ).
Table 3 Main pharmacokinetic/pharmacodynamic parameters of moxifloxacin after an oral dose of 400 mg
The pharmacokinetic and pharmacodynamic parameters for antibacterial agents can be integrated into the ratio of the AUC to MIC90, or area under the inhibition curve (AUIC), ie, the AUC/MIC normalized for 24 hours (CitationSchentag et al 1996).
Agents with a low Cmax, short t1/2 and low in vitro activity against a specific pathogen (viz: higher MIC90 values) have a lower AUIC than those with high Cmax, prolonged t1/2 and high in vitro activity. Quinolones with AUIC values above 85–125 and Cmax to MIC ratios of 8–10 have been associated with better clinical and bacteriological cure rates compared with agents with lower AUIC values (CitationForrest et al 1993). High AUIC values indicate rapid eradication of pathogens and a reduced likelihood of resistance development, as pathogens are killed before they have time to mutate (CitationSchentag et al 1996).
Compared with other fluoroquinolones, moxifloxacin has the highest AUIC ratio against S. pneumoniae (192–400 35 mg·h L−1) (CitationWise 1999) (Table ). Despite achieving good tissue penetration, levofloxacin is only moderately active against S. pneumoniae, whereas gatifloxacin is more bactericidal but only achieves modest tissue levels. Levofloxacin does not have the chemical structure of moxifloxacin to fight resistance, eg, a methoxy moiety at position C-8 – a substitution that has been shown to select for mutants much less frequently than a hydrogen moiety (CitationWise 1999). In addition, moxifloxacin has a 7-azabicyclo side chain that makes it more difficult to efflux the antibiotic out of the bacterial cell (CitationWise 1999).
The approved dose and regimen of oral moxifloxacin for the indication of exacerbations of CB is: 400 mg orally once daily for 5–7 days.
Efficacy of moxifloxacin in exacerbations of chronic bronchitis and COPD
Registration clinical trials
The clinical program for moxifloxacin included four studies comparing the efficacy and safety of moxifloxacin (400 mg once daily, 5 days) with either clarithromycin (500 mg twice daily, 7–10 days) or cefuroxime-axetil (500 mg twice daily, 10 days) in 2381 patients with exacerbations of CB (CitationMiravitlles 2005).
The clinical and bacteriological success rates 7–14 days post-treatment were comparable between the three treatment groups. However, there was a trend towards improved bacteriological success after 5 days of moxifloxacin therapy compared with 7–10 days of clarithromycin treatment. The three treatment regimens were also similar with respect to eradication of S. pneumoniae and M. catarrhalis. In contrast, moxifloxacin showed improved eradication rates against H. influenzae (129/132, 97%) as compared with clarithromycin (62/86, 72%) (CitationNiederman et al 2006). Taken together, the studies of the clinical program demonstrated that treatment with moxifloxacin 400 mg once daily for 5 days achieves a clinical response rate of 89% and a bacteriological response rate of 87% (CitationMiravitlles 2005).
Clinical studies of antimicrobials in exacerbations of CB such as those performed in the original clinical program have been limited by factors such as inadequate information on patient condition prior to the exacerbation and lack of long-term follow-up, as well as a lack of prospective control for steroid use, which can positively affect the outcome of the episode (CitationMiravitlles and Torres 2004a), and for prognostic factors that can have a negative impact (CitationDewan et al 2000; CitationMiravitlles 2001a) (Table ). After the registration trials, new studies have been designed to evaluate the clinical efficacy of moxifloxacin in different patient populations with exacerbations of chronic bronchial disease. In parallel, new outcomes have been analyzed in this new generation of clinical trials.
Table 4 Risk factors for relapse after ambulatory treatment of exacerbations of chronic bronchitis and COPD
Clinical efficacy of moxifloxacin: short-term outcomes
The MOSAIC study (CitationWilson et al 2004, Citation2006) was a multicenter, multinational, randomized study of two parallel treatment arms including outpatients aged >45 years with stable CB, history of smoking of ≥20 packs/year, ≥2 documented exacerbations in the previous year, and FEV1<85% of predicted value. Patients with a severe exacerbation of CB within 12 months of enrolment were randomized to receive either oral moxifloxacin 400 mg once daily for 5 days or one of the comparators: amoxicillin 500 mg tid for 7 days, clarithromycin 500 mg bid for 7 days or cefuroxime-axetil 250 mg bid for 7 days. Patients were assessed at screening, end of treatment and 7–10 days after treatment, and were contacted by phone monthly until a new exacerbation occurred or up to a maximum of 9 months. Clinical cure was defined as a return to pre-exacerbation status, and clinical success as cure and improvement combined, overall and by strata of steroid use and prognostic factors. Other efficacy measures were needed for further antimicrobials, time to next exacerbation, and bacteriological treatment success. Of 1935 enrolled patients, 733 (37.9%) had severe (Anthonisen type I) exacerbation within 12 months of screening and were randomized; 730 receiving either moxifloxacin (n = 354) or comparator (n = 376). Clinical success was seen in 83.0%–87.6% of patients across treatment arms and populations, with statistical equivalence in all populations except a significant difference in favor of moxifloxacin patients not receiving steroids. However, clinical cure rates were significantly higher with moxifloxacin (70.9%) compared with the comparators (62.8%) (95% confidence interval (CI) of the difference = 0.3%–15.6%); the clinical cure rate was also significantly greater in the moxifloxacin arm when analyzed by prognostic factors of age, airway obstruction, number of exacerbations of CB in the previous year, duration of CB and cardiopulmonary comorbidity (CitationWilson et al 2004).
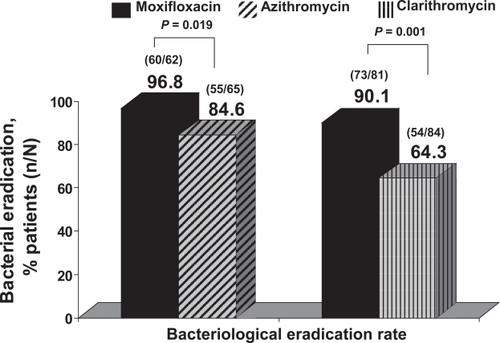
Bacterial eradication with moxifloxacin in bacteriologically evaluable patients was significantly higher with moxifloxacin compared with the control antibiotics (91.5% vs 81%; 95% CI = 0.4%–22%) (CitationWilson et al 2004). Bacterial eradication with moxifloxacin has been demonstrated to be superior compared with macrolides. In a pooled analysis of moxifloxacin phase III trials compared with macrolide agents in eradication of H. influenzae, the results showed superiority of moxifloxacin against both azithromycin and clarithromycin. Eradication of H. influenzae with moxifloxacin was 93% (133/143) compared with 73.2% (109/149) with macrolides, p = 0.001. Considering both macrolides separately, eradication rates were 96.8% with moxifloxacin vs 84.6% with azithromycin (p = 0.019) and 90.1% with moxifloxacin vs 64.3% with clarithromycin (p = 0.001) (CitationNiederman et al 2006) (Figure ).
Figure 1 Eradication rates for H. influenzae in exacerbations of CB treated with moxifloxacin or macrolides. Derived from CitationNiederman et al (2006).
The results of the MOSAIC trial also showed that up to 14.8% of patients treated with a comparator antibiotic required a second prescription of antibiotics to control symptoms of exacerbation, compared with only 8.8% of patients randomized to receive moxifloxacin (p = 0.03) (CitationWilson et al 2004).
Although initially designed to demonstrate non-inferiority of moxifloxacin over standard therapy, the MOSAIC study was the first clinical trial to demonstrate the superiority of an antibitic, moxifloxacin, over the comparators in clinical cure and bacteriological eradication rates.
A recent publication has provided a systematic analysis and meta-analysis of published clinical trials with moxifloxacin in the treatment of exacerbations of CB up to June 2005 (CitationMiravitlles et al 2007a) with the objective to determine the superiority of moxifloxacin over standard therapy in this indication. A total of 45 studies were identified, but only 9 fulfilled the inclusion criteria in the meta-analysis. Of them, 5 were randomized and double-blind and 4 were randomized and open labeled.
All studies concluded that the efficacy of treatment with moxifloxacin was at least as good as treatment with the comparator. One of the studies concluded that eradication and cure rates were superior with moxifloxacin versus comparators, as highlighted above (CitationWilson et al 2004).
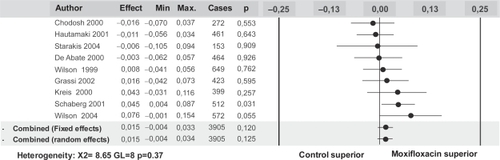
The global analysis included 3905 randomized patients and showed an aggregated superior clinical success rate of moxifloxacin of 1.5% (95% CI = −0.4%–3.4%) which did not reach statistical significance, but showed a trend towards a better clinical outcome with moxifloxacin (Figure ). It is of note that these results were obtained from a combined analysis of studies individually designed to show equivalence between both treatment arms and without enough statistical power to demonstrate superiority.
Figure 2 Results of the meta-analysis of clinical trials with moxifloxacin in exacerbations of CB and COPD. Data obtained from 9 studies including 3,905 patients. Reproduced with permission from CitationMiravitlles M, Molina J, Brosa M. 2007a. Clinical efficacy of moxifloxacin in the treatment of exacerbations of chronic bronchitis: a systematic review and meta-analysis. Arch Bronconeumol, 43:22–28. Copyright © 2007 Elsevier.
Long-term outcomes: prevention of exacerbations with moxifloxacin
The most important unmet need in the treatment of exacerbations of COPD is the prevention of recurrence (CitationMiravitlles et al 2007b). One of the characteristics of the MOSAIC trial was the follow-up period of up to 9 months after recovery from the exacerbation. The hypothesis was that a better eradication rate with moxifloxacin would translate into a prolonged time to the next exacerbation. In 405 patients with new exacerbations during the follow-up period, the mean time to event was 10 days longer with moxifloxacin treatment (127.6 ± 68.1 days) than comparators (116.7 ± 68.8), with more patients receiving the comparator having new exacerbations within 60 days of treatment (51/208, 24.5%) than moxifloxacin (31/197, 15.7%). The log rank test showed a significant difference in favor of moxifloxacin for up to 5 months of follow-up (p = 0.03) (CitationWilson et al 2004).
In a secondary analysis, the authors attempted to identify the variables independently and significantly associated with a prolonged time to the next exacerbation. Treatment with moxifloxacin was associated with a prolonged time to recurrence (hazard ratio for recurrence with moxifloxacin versus comparators 0.82; 95% CI = 0.68–0.98) (CitationWilson et al 2006).
This effect of moxifloxacin on time to relapse has not been observed with other fluoroquinolones. In a recent study, levofloxacin was compared with clarithromycin in a group of 434 patients with exacerbations of CB. After a 1-year follow-up, no differences in time free from exacerbation were observed between patients treated with levofloxacin (mean of 100.5 days) or clarithromycin (95 days), p = 0.32 (CitationLode et al 2004). These results may be the consequence of a lower antibacterial activity of levofloxacin compared to moxifloxacin or more likely to the inclusion of a milder patient population in the latter study, making clinical differences against macrolides less likely (CitationMiravitlles and Torres 2004b).
The rationale for the prevention of exacerbations with moxifloxacin derives from a combination of at least three different factors: a) an immunomodulatory effect of the drug (CitationDalhoff and Shalit 2003); b) the rapid and more complete eradication prevents epithelial damage and restores the local defence mechanisms of the bronchial mucosa (CitationDe Benedetto and Sevieri 2006); c) as a consequence of the better eradication, fewer viable pathogens remain in the bronchial tissue after antimicrobial treatment, requiring longer for the bacterial population to increase sufficiently to induce a new exacerbation (CitationChodosh 2005). This last hypothesis is also known as the “fall and rise” hypothesis of bacterial exacerbation of CB (CitationMiravitlles 2002b). In clinical trials with antibiotics, a prolonged time to the next exacerbation has been observed in patients who eradicate the bronchial pathogen after an exacerbation compared with those who cured the exacerbation but without effective eradication (CitationChodosh et al 1998). According to the “fall and rise” hypothesis, patients who effectively eradicate bacteria would need a longer time to achieve the threshold of bacterial counts compared with patients who cured the exacerbation but in whom bacteria still persisted after antibiotic treatment (CitationMiravitlles 2002b). This hypothesis would also explain why patients with acute exacerbations may be clinically cured even without eradication of the bacterial pathogen (CitationWilson et al 1999). This is not proof that this particular bacterium is not the cause of the exacerbation, but does demonstrate that the antibiotic only needs to reduce bacterial counts to below the threshold to eliminate symptoms. Nevertheless, if eradication also occurs, the time for a number of bacteria above the threshold to be reached will be longer (Figure ).
Figure 3 The “fall and rise” hypothesis of bacterial exacerbations of COPD.
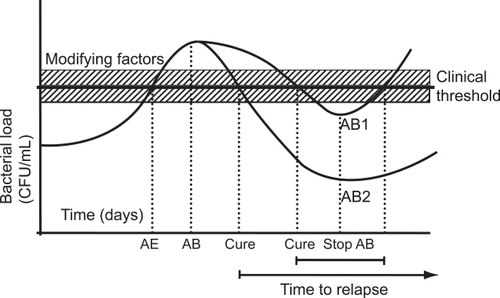
The quantitative or “fall and rise” hypothesis may explain the basic mechanism of bacterial exacerbations in patients with chronic bronchial colonisation and frequent exacerbations. In this group of patients, the change in the serotype of the colonising bacteria may act as a trigger that initiates profileration of microorganisms in some cases. The “fall and rise” can also explain relapses when bacteria have not been eradicated after antibiotic treatment of the exacerbation. In contrast, the change of serotype of infective bacteria may be crucial in patients who do not suffer frequent exacerbations, ie, less than two in a year. It is difficult to explain the appearance of an exacerbation more than four months after a preceding episode based only on bacterial growth in the airways without any precipitating factor, such as change in serotype (CitationSethi et al 2002). New evidence is required on the complex relationship between microorganisms and the host, particularly considering that important therapeutic implications may be derived from these findings. In fact, if the new exacerbation is caused by the regrowth of the same bacteria that remained unkilled, the logical approach to treatment would be to use a different antibiotic and rotation of antibiotics should be recommended to prevent bacteriological failure and the development of resistance. In contrast, if new exacerbations are caused by the acquisition of new strains, the same antibiotic can be used in repeated exacerbations without concern about increased exposure to the same antibiotic.
Speed of recovery after antibiotic treatment of an exacerbation
Fast recovery of the symptoms of an exacerbation is highly demanded by patients (CitationMiravitlles et al 2007b). Antibiotics that exhibit faster bacterial killing in vitro should provide faster relief of symptoms in patients with exacerbations of CB and COPD.
Observational studies and cross-sectional analyses in patients with CB and COPD have suggested that patients recover from symptoms of exacerbations more rapìdly with moxifloxacin than with other commonly used treatments (CitationKreis et al 2000; CitationMiravitlles et al 2003b, Citation2004b).
One large, multicenter comparative study using a 2-year protocol (IMPAC study) showed a significant reduction in the time to recovery from exacerbations of moderate to severe COPD (CitationMiravitlles et al 2003b). In 441 patients with COPD, all with a FEV1 <50% predicted (mean FEV1 = 36% predicted), the investigators treated 614 exacerbations with moxifloxacin (exacerbations = 111) and 503 with either amoxicillin/clavulanate, cefuroxime, or clarithromycin. Moxifloxacin was available in the second study year only. In year 2, exacerbations treated with moxifloxacin resulted in complete recovery from symptoms in a mean of 4.6 days, compared with 5.8 days for the other treatments administered (p = 0.02). In the longitudinal analysis of 27 patients treated with moxifloxacin who had received other treatments in year 1, the mean time to recovery from exacerbations was significantly reduced from a mean of 6.8–3.7 days (p = 0.02). These results were confirmed in another study, the EFEMAP study (Estudio FarmacoEconómico de Moxifloxacino en las Agudizaciones de la EPOC), with mild to moderate COPD patients in primary care centers (CitationMiravitlles et al 2004b). A total of 1456 patients with COPD (mean FEV1 = 52% predicted) received either moxifloxacin (n = 575), amoxicillin/clavulate (n = 460), or clarithromycin (n = 421). Clinical success was observed in 97.2%, 93.1%, and 94.4% of the cases, respectively, without significant differences among the groups. However, symptoms of purulence and the volume of expectoration resolved a mean of one day earlier with moxifloxacin compared with the other groups (CitationMiravitlles et al 2004b). In a secondary analysis of the EFEMAP study, the factors associated significantly and independently with a slow recovery (more than 5 days) were the use of long-term oxygen therapy (odds ratio (OR) = 1.97; 95% CI = 1.35–2.85); the increased use of rescue medication with short-acting beta-2 agonists (OR = 1.51; 95% CI = 1.17–1.92); and the use of amoxicillin/clavulanate or clarithromycin compared with moxifloxacin (OR = 2.94; 95% CI = 2.22–3.84 and OR = 2.43; 95% CI = 1.17–3.22, respectively) (CitationMiravitlles et al 2005). Although all studies consistently indicate a faster resolution of symptoms of exacerbations with moxifloxacin compared to other frequently used antibiotics, these results must be interpreted with caution because they derive from observational studies and, as such, are subjected to possible sources of biases.
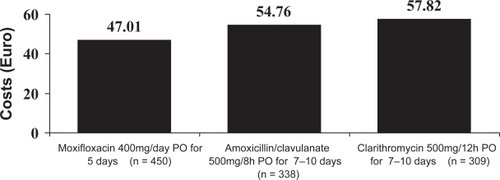
A economic analysis derived from the results of the EFEMAP study has shown that exacerbations treated with moxifloxacin generated a direct medical cost similar to those treated with amoxicillin/clavulanate (€111.46; 95% CI =€73.4–149.5 compared with €109.45; 95% CI = €68.2–150.7, respectively); and lower, although not significantly, than those treated with clarithromycin (€138.95; 95% CI = €89.4–188.5). These differences were mainly due to the higher costs associated with relapse in the group treated with clarithromycin (CitationLlor et al 2004) (Figure ).
Figure 4 Mean cost per patient incurred by treatment failures of exacerbations of CB and COPD in each group. Derived from CitationLlor et al (2004).
Clinical efficacy of moxifloxacin in patients belonging to risk groups
Failure rates after ambulatory treatment derive from clinical trials on antibiotics in CB and range from 7% to 12%. Nevertheless, these results cannot be extrapolated to everyday practice, since patients included in clinical trials consist of CB patients and include subjects with ages ranging from 18 to 90 years, a significant proportion of never smokers, and individuals without ventilatory impairment (not COPD) (CitationMiravitlles and Torres 2004b). However, more recently, some studies have addressed treatment failure in observational “real-life” studies and showed a failure rate ranging from 12% to 26% (CitationDewan et al 2000; CitationAdams et al 2000; CitationMiravitlles 2000b; CitationMiravitlles et al 2001a; CitationMacfarlane et al 2001). Identification of risk factors for failure may permit the implementation of more aggressive broad spectrum treatment and closer follow-up. In a further step, risk factors associated with relapse should be incorporated into management guidelines to aid in identifying at-risk patients. Among the risk factors, severity of the underlying pulmonary disease is probably the most important. A summary of the main risk factors for relapse is presented in Table .
Some of the studies included in the meta-analysis of clinical trials evaluated the efficacy of moxifloxacin in patients belonging to different risk groups. In a study comparing moxifloxacin and amoxicillin-clavulanate (CitationSchaberg et al 2001), individuals older than 60 years showed a significantly better success rate with moxifloxacin (p = 0.048) and a tendency towards a better outcome with moxifloxacin in patients with cardiopulmonary comorbidity (p = 0.054). The MOSAIC study has also demonstrated the superiority of moxifloxacin over the comparators in elderly patients and those with a higher number of previous exacerbations (CitationWilson 2006). Table presents the results of moxifloxacin and comparators in patients belonging to different risk groups.
Table 5 Clinical success rates with moxifloxacin and comparators in patients belonging to risk groups in clinical trials of exacerbations of chronic bronchitis and COPD
The main studies of moxifloxacin for the treatment of exacerbations of CB and COPD are summarized in Table .
Table 6 A summary of clinical trials with moxifloxacin (400 mg once daily) in E-CB and E-COPD
Safety
Adverse events
Moxifloxacin has shown a highly favorable safety and tolerability profile in clinical trials as well as in the clinical setting. In a meta-analysis of data from clinical trials and post-marketing surveillance comprising over 25 million patient treatments, the most frequent adverse events reported in clinical trials were nausea (7.1%), diarrhea (5.2%), and dizziness (2.6%) following the administration of a dosage of 400 mg, once daily (CitationBall et al 2004). In all cases the frequency of side-effects was not statistically different to that of the comparators. There was no evidence that moxifloxacin caused disturbances in glucose metabolism in patients with or without diabetes mellitus, and there was no evidence of an increased risk of adverse cardiovascular events (CitationBall 2004).
Conclusions
The prevalence of chronic bronchial diseases caused by tobacco smoking, namely CB and COPD is great in developed and developing countries. Exacerbations constitute the mean cause for medical consultation of patients with these diseases and bacterial infection is involved in more than half of the cases. Most of the microorganisms involved in pathogenesis of exacerbations of CB and COPD have developed diverse degrees of resistance to the traditional antibiotics used as first-line treatment. Development of resistance is caused by the increased volume of prescription, the inadequate indication of the antibiotic and/or inadequate administration or treatment compliance by the patient. Patients with acute (viral) bronchitis and those with exacerbations without changes in the volume or characteristics of sputum should not be treated with antibiotics. When bacterial infection is suspected, there is a growing tendency to treat exacerbations more aggressively with shorter courses of antimicrobials to help reduce antibiotic resistance (CitationPerez-Gorricho and Ripoll 2003). This is possible with the new generation fluoroquinolones such as moxifloxacin. Moxifloxacin is a fourth generation fluoroquinolone that has an excellent in vitro activity against the most common respiratory pathogens and its rapid bactericidal action allows short-course 5-day therapy for exacerbations of CB and COPD. In addition, its long half-life allows administration in single daily doses. The once-daily, short-course administration guarantees better patient compliance and makes the development of resistance less likely.
Moxifloxacin is 4- to 10-fold more active than levofloxacin against S. pneumoniae. A greater intrinsic activity is linked with faster eradication and reduced susceptibility of the development of resistance. In fact, some reports have described the development of resistance of S. pneumoniae during treatment with levofloxacin (CitationDavidson et al 2002), but no reports have shown the same phenomenon in patients treated with more active fluoroquinolones such as moxifloxacin or gatifloxacin. Therefore, these findings support the concept of “use the best first”. The most potent agent of the class should be used as first line therapy to avoid the development of resistance to the entire class of antimicrobials, particularly in moderate to severe cases or those with risk factors for relapse or poor compliance.
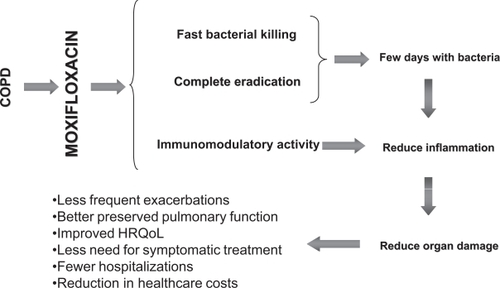
Moxifloxacin has demonstrated better eradication in exacerbations of CB and COPD compared to standard therapy, in particular compared to macrolides. The complete eradication of bacteria present in the airways is important for the prevention of resistance development (CitationStratton 2003) and for clinical outcome. The persistence of bacteria after antibiotic treatment of an exacerbation has been associated with increased inflammation, poorer evolution of the baseline disease and early recurrence of exacerbation. Fast and complete eradication of bacteria may induce a “virtuous circle” preserving and/or restoring the integrity of the bronchial mucosa and making it more resistant to further bacterial colonization and the development of exacerbations. In addition, the reduction in the frequency of exacerbations will contribute to preserve pulmonary function and health status (CitationDe Benedetto and Sevieri 2006) (Figure ). In the MOSAIC trial, patients treated with moxifloxacin showed a significantly better eradication, together with an extended time to the next exacerbation compared with the standard treatments.
Figure 5 The “virtuous circle” of antibiotic treatment of exacerbations of CB and COPD. Derived from CitationDe Benedetto and Sevieri (2006).
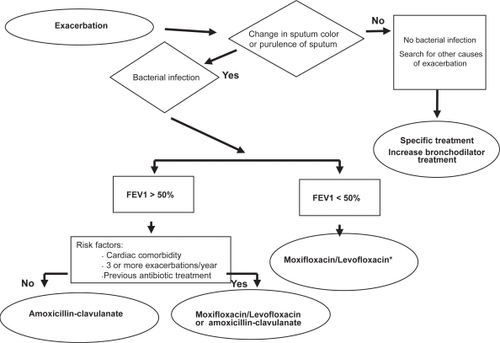
The results obtained in registration trials of patients with exacerbations of CB and COPD, together with the new trials performed after launching moxifloxacin have had an impact on the development of guidelines of antibacterial treatment of exacerbations. The Canadian guidelines indicate fluoroquinolones as the first-line treatment for patients with exacerbated CB with risk factors (FEV1 <50% predicted, more than 4 exacerbations/year, cardiac disease, use of home oxygen, chronic steroid use or antibiotic use in the past 3 months) and second-line therapy in uncomplicated CB (CitationBalter et al 2003). The Latin American guidelines recommend moxifloxacin as first-line therapy in exacerbated COPD patients with FEV1 between 35% to 50%, those with an FEV1 <35% without risk factors for Pseudomonas infection and those with FEV1 >50% with risk factors (CitationALAT Work Group 2004). Recent guidelines published in Spain by the Spanish Society of Pneumologists and Thoracic Surgeons and the Spanish Society of Geriatrics recommend moxifloxacin as first-line therapy in patients with suspected bacterial exacerbation of COPD with an FEV1 <50% predicted and without risk factors for Pseudomonas infection and in patients with FEV1 >50% with risk factors (CitationMiravitlles and Martín Graczyk 2006) (Figure ).
Figure 6 Algorithm of antibiotic treatment of ambulatory patients with exacerbations of COPD from the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and the Spanish Society of Geriatry and Gerontology (SEEG). Derived from CitationMiravitlles and Martín Graczyk (2006).
The appropriate use of moxifloxacin in patients with exacerbated chronic bronchial disease and clear criteria of bacterial infection may improve the short- and long-term clinical outcome. Restriction of the use of fluoroquinolones, such as moxifloxacin, in patients with acute bronchitis, suspected viral infections or benign infections that can be successfully treated with older antibiotics will help to preserve the antibacterial activity of the class. The use of the most active fluoroquinolone first (“use the best first”) may also contribute to preserve the antibacterial activity of all quinolones and allow its use in patients who really benefit from the better outcome obtained with moxifloxacin. Physicians need to be familiar with criteria for suspecting bacterial infection in exacerbated patients and identify risk factors for relapse in order to indicate the use of moxifloxacin according to local and international guidelines.
References
- AdamsSGMeloJLutherM2000A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPDChest11713455210807821
- ALAT Work Group2004Update to the Latin American Thoracic Society (ALAT) recommendations on infectious exacerbation of COPDArch Bronconeumol403152515225518
- BallPStahlmannRKubinR2004Safety profile of oral and intravenous Moxifloxacin: Cumulative data from clinical trials and postmarketing studiesClin Ther269405015336463
- BalterMSLa ForgeJLowDE2003Canadian guidelines for the management of acute exacerbations of chronic bronchitisCan Respir J10Suppl B3B32B
- BlondeauJ1999A review of the comparative in vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolonesJ Antimocrob Chemother43111
- CerveriIAccordiniSVerlatoG2001Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adultsEur Respir J18859211510810
- ChodoshSDeabateCAHaverstockD2000Short-course moxifloxacin therapy for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study GroupRespir Med94182710714475
- ChodoshSSchreursASiamiG1998The efficacy of oral ciprofloxacin vs. clarithromycin for the treatment of acute bacterial exacerbations of chronic bronchitisClin Infect Dis2773089798025
- ChodoshS2005Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitisChest1272231615947342
- DavidsonRCavalcantiRBruntonJl2002Resistance to levofloxacin and failure of treatment of pneumococcal penumoniaN Engl J Med3467475011882730
- DalhoffAShalitI2003Immunomodulaotry effects of quinolonesLancet Infect Dis33597112781508
- DeabateCAMathewCPWarnerJH2000The safety and efficacy of short course (5-day) moxifloxacin vs. azithromycin in the treatment of patients with acute exacerbation of chronic bronchitisRespir Med9410293711127487
- De BenedettoFSevieriG2006Chronic obstructive pulmonary disease (COPD) exacerbation and inflammation of the respiratory tract: clinical implication, prognostic consequences, and therapeutic strategiesMultidisciplinary Respiratory Medicine13648
- De MarcoRAccordiniSCerveriI2004An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stagesThorax59120514760151
- DewanNARafiqueSKanwarB2000Acute exacerbation of COPD. Factors associated with poor outcomeChest1176627110712989
- EllerJEdeASchabergT1998Infective exacerbations of chronic bronchitis. Relation between bacteriologic etiology and lung functionChest113154289631791
- ForrestANixDEBallowCH1993Pharmacodynamics of intravenous ciprofloxacin in seriously ill patientsAntimicrob Agents Chemother371073818517694
- GrassiCCasaliLCurtiE2002Efficacy and safety of short course (5-day) moxifloxacin vs 7-day ceftriaxone in the treatment of acute exacerbations of chronic bronchitis (AECB)J Chemother1459760812583552
- HallsGA1993The management of infections and antibiotic therapy: a European surveyJ Antimicrob Chemother3198510008360135
- HautamakiDBruyaTKureishiA2001Short-course (5-day) moxifloxacin versus 7-day levofloxacin therapy for treatment of acute exacerbations of chronic bronchitisToday’s Therapeutic Trends1911736
- HuchonGJVergnenègreANeukirchF2002Chronic bronchitis among French adults: high prevalence and underdiagnosisEur Respir J208061212412668
- KrasemannCMeyerJTillotsonG2001Evaluation of the clinical microbiology profile of moxifloxacinClin Infect Dis32Suppl 1S516311249830
- KreisSRHerreraNGolzarN2000A comparison of moxifloxacin and azithromycin in the treatment of acute exacerbations of chronic bronchitisJ Clin Outcomes Manag7337
- LandenHMöllerMTillotsonGS2001Clinical experience in Germany of treating community-acquired respiratory infections with the new 8-methoxyfluoroquinolone, moxifloxacinJ Int Med Res29516011393349
- ListerPDSandersCC2001Pharmacodynamics of moxifloxacin, levofloxacin and sparfloxacin against Streptococcus pneumoniaeJ Antimicrob Chemother478111811389113
- LlorCNaberanKCotsJM2004Economic evaluation of the antibiotic treatment of exacerbations of chronic bronchitis and COPD in Primary care centersInt J Clin Pract589374415587773
- LoddenkemperR2003The European Lung White BookEuropean Respiratory Society
- LodeHEllerJLinnhoffA2004Levofloxacin versus clarithromycin in COPD exacerbation: focus on infection-free intervalEur Respir J249475315572537
- LorenzJBuschWThate-WaschkeI-M2001Moxifloxacin in acute exacerbations of chronic bronchitis:clinical evaluation and assessment by patientsJ Int Med Res29617311393350
- MacfarlaneJHolmesWGardP2001Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the communityThorax561091411209098
- MiravitllesM2000Moxifloxacin: an antibiotic designed for use in the communityEur Respir Rev101619
- MiravitllesM2002aEpidemiology of chronic obstructive pulmonary disease exacerbationsClin Pulm Med9191197
- MiravitllesM2002bExacerbations of chronic obstructive pulmonary disease: when are bacteria important?Eur Respir J20Suppl 369s19s
- MiravitllesM2005Moxifloxacin in respiratory tract infectionsExpert Opin Pharmacother628329315757424
- MiravitllesMAnzuetoALegnaniD2007bPatient’s perception of exacerbations of COPD – the PERCEIVE studyRespir Med1014536016938447
- MiravitllesMEspinosaCFernández-LasoE1999Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPDChest11640610424501
- MiravitllesMFerrerMPontA2004afor the IMPAC study group:Exacerbations impair quality of life in patients with chronic obstructive pulmonary disease. A two-year follow-up studyThorax593879515115864
- MiravitllesMGuerreroTMayordomoC2000Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysisRespiration6749550111070451
- MiravitllesMLlorCNaberanK2004bThe effect of various anti-microbial regimens on the clinical course of exacerbations of chronic bronchitis and chronic obstructive pulmonary disease in Primary CareClin Drug Invest246372
- MiravitllesMLlorCNaberanK2005Variables associated with recovery from acute exacerbations of chronic bronchitis and chronic obstructive pulmonary diseaseRespir Med999556515950136
- MiravitllesMMartín GraczykA2006Tratamiento del paciente con EPOC agudizada. Guía de buena práctica clínica en Geriatría “Enfermedad Pulmonar Obstructiva Crónica”. Normativa conjunta SEPAR-SEGG (Sociedad Española de Geriatría y Gerontología)Ed Elsevier Farma7588
- MiravitllesMMolinaJBrosaM2007aClinical efficacy of moxifloxacin in the treatment of exacerbations of chronic bronchitis: a systematic review and meta-analysisArch Bronconeumol431621
- MiravitllesMMurioCGuerreroT2001aFactors associated with relapse after ambulatory treatment of acute exacerbations of chronic bronchitis. A prospective multicenter study in the communityEur Respir J179283311488328
- MiravitllesMMurioCGuerreroT2002Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPDChest12114495512006427
- MiravitllesMMurioCGuerreroT2003aCosts of chronic bronchitis and COPD. A one year follow-up studyChest1237849112628879
- MiravitllesMRosFCobosA2001bThe efficacy of moxifloxacin in acute exacerbations of chronic bronchitis: a Spanish physician and patient experienceInt J Clin Pract554374411594251
- MiravitllesMTorresA2004aNo more equivalence trials for antibiotics in exacerbations of COPD, pleaseChest1258111315006934
- MiravitllesMTorresA2004bAntibiotics in exacerbations of COPD: lessons from the pastEur Respir J24896715572528
- MiravitllesMZalacainRMurioC2003bSpeed of recovery from acute exacerbations of COPD after treatment with antimicrobials: Results of a two-year studyClin Drug Invest2343950
- MonsóEGarcía-AymerichJSolerN2003Bacterial infection in exacerbated COPD with changes in sputum characteristicsEpidemiol Infect13179980412948381
- MurrayCJLLopezAD1997Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease studyLancet34914985049167458
- NicolsonPAndersonP2000The patient’s burden: physical and psychological effects of acute exacerbations of chronic bronchitisJ Antimicrob Chemother45253210719009
- NiedermanMSAnzuetoASethiS2006Eradication of H. influenzae in AECB: A pooled analysis of moxifloxacin phase III trials compared with macrolide agentsRespir Med10017819016531032
- PapiABellettatoCMBraccioniF2006Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med17311142116484677
- Perez-GorrichoBRipollMFor the PACE Study Group2003Does short-course antibiotic therapy better meet patient expectationsInt J Antimicrob Agents21222812636982
- PetoRLopezADBorehamJ1992Mortality from tobacco in developed countries: indirect estimation from vital statisticsLancet3391268781349675
- PrescottELangePVestboJ1995Chronic mucus hypersecretion in COPD and death from pulmonary infectionEur Respir J8133387489800
- SaravolatzLManzorOCheckC2001Antimicrobial activity of moxifloxacin, gatifloxacin and six fluoroquinolones against Streptococcus pneumoniaeJ Antimicrob Chemother47875711389122
- SchabergTBallinIHuchonG2001A multinational, multicentre, non-blinded, randomized study of moxifloxacin oral tablets compared with amoxicillin-clavulanate oral tablets in the treatment of acute exacerbation of chronic bronchitisJ Int Med Res293142811675905
- ScheldWM2003Maintaining fluoroquinolone efficacy:review of influencing factorsEmerg Infect Dis91912533274
- SchentagJNixDEForrestA1996AUIC – the universal parameter within the constraint of a reasonable dosing intervalAnn Pharmacother30102488876867
- SeemungalTADonaldsonGCPaulEA1998Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1571418229603117
- SethiSEvansNGrantBJ2002New strains of bacteria and exacerbations of chronic obstructive pulmonary diseaseN Engl J Med3474657112181400
- SmithJARedmanPWoodheadMA1999Antibiotic use in patients admitted with acute exacerbations of chronic obstructive pulmonary diseaseEur Respir J13835810362049
- Sobradillo PenaVMiravitllesMGabrielR2000Geographical variations in prevalence and underdiagnosis of COPD. Results of the IBERPOC multicentre epidemiological studyChest118981911035667
- SomanAHoneybourneDAndrewsJ1999Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopyJ Antimicrob Chemother44835810590288
- StarakisIGogosCABassarisH2004Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitisInt J Antimicrob Agents231293715013037
- StassHKubitzaD1999Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in manJ Antimicrob Chemother43Suppl B839010382880
- StassHKubitzaDSchühlyU2001Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone, after repeated oral administrationClin Pharmacokinet40Suppl 11911352436
- StrattonCW2003Dead bugs don’t mutate: susceptibility issues in the emergence of bacterial resistanceEmerg Infect Dis9101612533275
- SullivanJTWoodruffMLettieriJ1999Pharmacokinetics of a once-daily oral dose of moxifloxacin (BAY 12-8039), a new enantiomerically pure 8-methoxyquinoloneAntimicrob Agents Chemother432793710543767
- Urueta-RobledoJArizaHJardimJR2006Moxifloxacin versus levofloxacin against acute exacerbations of chronic bronchitis: the Latin American cohortRespir Med10015041116504492
- WilsonRKubinRBallinI1999Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitisJ Antimicrob Chemother445011310588312
- WilsonRAllegraLHuchonG2004Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitisChest1259536415006954
- WilsonRJonesPSchabergT2006Antibiotic treatment and factors influencing short and long term outcomes of acute exacerbations of chronic bronchitisThorax613374216449273
- WiseR1999A review of the clinical pharmacology of moxifloxacin, a new 8-methoxyquinolone and its potential relation to therapeutic efficacyClin Drug Invest1736587
- WoodcockJMAndrewsJMBoswellFJ1997In vitro activity of BAY 12-8039, a new fluoroquinoloneAntimicrob Agents Chemother4110168980763