Abstract
Influenza is a major respiratory pathogen, which exerts a huge human and economic toll on society. Influenza is a vaccine preventable disease, however, the vaccine strains must be annually updated due to the continuous antigenic changes in the virus. Inactivated influenza vaccines have been used for over 50 years and have an excellent safety record. Annual vaccination is therefore recommended for all individuals with serious medical conditions, like COPD, and protects the vaccinee against influenza illness and also against hospitalization and death. In COPD patients, influenza infection can lead to exacerbations resulting in reduced quality of life, hospitalization and death in the most severe cases. Although there is only limited literature on the use of influenza vaccination solely in COPD patients, there is clearly enough evidence to recommend annual vaccination in this group. This review will focus on influenza virus and prophylaxis with inactivated influenza vaccines in COPD patients and other “at risk” groups to reduce morbidity, save lives, and reduce health care costs.
Introduction
Influenza virus is one of the leading causes of viral disease resulting in widespread morbidity, a large number of lives lost and substantial economic loss. During annual influenza outbreaks, an estimated 5%–15% of the world’s population is infected causing an estimated one million deaths every year (CitationWHO 2002; CitationYewdell and Garcia-Sastre 2002). Moreover, we are currently facing a potential new pandemic that could cause unprecedented levels of global morbidity and mortality, particularly in the developing countries but also in the industrialized world. Today’s inactivated influenza vaccines provide satisfactory protection against seasonal influenza in healthy adults (CitationBeyer et al 2002; CitationWHO 2005) and annual vaccination is cost effective (CitationNichol et al 1995). However, there is still room for improvement in current vaccines, especially in the elderly “at risk” groups where vaccine efficacy is not optimal. Two classes of antiviral agents are also licensed for therapeutic and prophylactic use against influenza, complementing but not replacing, the use of influenza vaccines (Reviewed in CitationOxford et al 2003).
In man, influenza virus is transmitted via droplets expelled upon coughing and sneezing and normally infects the epithelial cells of the upper respiratory tract (CitationNicholson 1998). The incubation period is usually 2–3 days, but can be as long as 7 days. The patient is generally contagious during the febrile phase, but cases of viral spread have been observed prior to the onset of symptoms (CitationNicholson 1998). The duration of illness is usually one week and is normally accompanied by high fever, headache, myalgia, sore throat and rhinitis. People with low levels of viral shedding often have fewer clinical symptoms or are asymptomatic (CitationMurphy et al 1973; CitationMurphy and Webster 1996).
Healthy people usually recover within one week of illness without requiring any medical intervention. In the very young, the elderly and people with underlying medical problems (eg, chronic obstructive pulmonary disease (COPD), diabetes, cancer, heart disease) influenza poses a serious risk, and infection may lead to hospitalization and in some cases death (CitationNguyen-Van-Tam 1998). Frequently, the cause of hospitalization or death is viral pneumonia or secondary bacterial pneumonia. Substantial levels of community morbidity occur during an influenza outbreak, resulting in a significant strain on the health care system. The economic burden of influenza on society can also be significant. In the USA alone, a conservative estimate predicted an annual loss of 12–17 billion dollars (CitationWilliams et al 1988; CitationWHO 2005).
Respiratory tract infections such as influenza often lead to exacerbation of disease in COPD patients. Influenza infection in such patients requires increased medical intervention and serious complications can result in hospitalization and death (CitationYap et al 2004). Each exacerbation in COPD patients leads to a progressive irreversible decrease in lung function, the frequency and severity of the exacerbations increases as the disease worsens (CitationNiewoehner 2006). The patient’s quality of life therefore deteriorates with an increased need for hospitalization. Importantly, influenza is a vaccine preventable disease and this review will focus on influenza virus and prophylaxis with inactivated influenza vaccines in “at risk” groups, herein COPD patients.
The influenza virus and its life cycle
Influenza belongs to the family of Orthomyxoviridae (CitationFauquet et al 2004). There are three types of influenza, A, B and C, which are classified on the basis of antigenic differences in the internal proteins (nucleoprotein (NP) and matrix (M1) protein). Influenza A and B viruses are important human pathogens, whereas influenza C infection results only in a mild respiratory infection in man and will not be discussed further in this review. The influenza A genus is further subdivided based on the antigenic properties of its surface glycoproteins, the haemagglutinin (HA or H) and the neuraminidase (NA or N). Currently, there are 16 HA and 9 NA subtypes recognized by the WHO (CitationWHO 2005) and of these the H3N2 and H1N1 subtypes are circulating widely in man today.
Influenza virus has a negative sense, segmented, single stranded (ss) RNA genome. The genome of influenza A virus has 8 segments, each coding for one or two proteins, in all a total of 11 proteins (Table ). Each segment is encapsulated by the NP to form a ribonucleoprotein complex (RNP). Bound to each RNP, is the viral RNA polymerase complex, consisting of the three viral gene products (PB1, PB2, PA) (Figure ). Three viral proteins are found in the viral envelope of influenza A virus; the HA, NA and the ion channel protein M2. The M1 protein lines the viral envelope in close proximity to the RNP and is hypothesized to interact with the cytoplasmic tails of the surface glycoproteins (CitationLamb and Krug 1996). The virion is pleomorphic in structure, the main form is a spherical particle (80–120 nm in diameter), but also filamentous and bean-like structures are found.
Table 1 Influenza A proteins
Figure 1 Schematic figure of influenza virus. On the surface of the virus there are three viral proteins, haemagglutinin (HA), neuraminidase (NA) and the matrix 2 protein (M2). Underlining the viral envelope is the matrix 1 protein (M1), the nucleoprotein (NP) encapsidates the genome segments with one complex of the polymerase attached (PB1, PB2 and PA). The non-structural protein 2 (NS2) is also contained in the virion in low numbers.
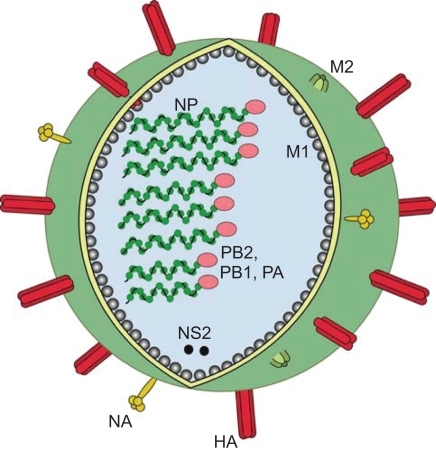
The HA and NA are the major antigenic proteins of the virus. Most antibodies produced are directed against these proteins and they will be briefly discussed here. The HA is a trimeric protein, composed of three identical monomers, which must be post-translationally cleaved by cellular proteases in order to be functional (CitationColman 1994). The distal tip of HA contains the receptor binding sites and the transmembrane stalk attaches the HA to the viral envelope (Figure ). NA is a tetramer with a mushroom shape and contains the viral enzyme (neuraminidase), which is responsible for release of newly assembled virus from the cell. The antiviral drugs Oseltamivir and Zanamivir bind to the enzymatic site of NA, reducing or hindering the release of new virus.
Influenza virus replicates in the epithelial cells lining the respiratory tract. The enzymatic activity of NA helps the virus in navigating through the mucus layer and upon cell contact allows the HA to attach to the sialic acid containing host cell receptor. The virus particle is then engulfed and taken up into the cell in a vesicle (endosome) in a process called receptor-mediated endocytosis (CitationLamb and Krug 1996). The low pH initiates a conformational change in the HA molecule facilitating a fusion with the endosomal membrane. At the same time the M2 ion channel protein lowers the pH inside the virion, so that the RNPs become disassociated from M1, allowing the RNPs to enter the cytosol. The M2 protein is the target for the influenza antiviral drugs Amantadine and imantadine (CitationOxford et al 2003).
The RNPs migrate to the nucleus and the viral polymerase complex transcribes and replicates the viral RNA to form new viral RNA and mRNA. The NP and the M1 are translated in the cytosol, before they migrate to the nucleus to take part in the RNP assembly and transport. Viral proteins, possibly NP, may regulate the switch between transcription and replication (CitationPortela and Digard 2002). New viral genomes are encapsidated by NP and migrate to the cytosol. The HA, NA and the M2 proteins are translated on the endoplasmatic reticulum and transported to the cell membrane. M1 and RNPs interact with the cytoplasmic tails of HA and NA at the cell membrane. The M1 protein further interacts with cellular proteins and is involved in determining both the virion shape and size, as well as being involved in the viral budding process (CitationLamb and Krug 1996; CitationHui et al 2003). The virus is then released by budding from the cell surface membrane, which is facilitated by the NA.
Influenza ecology
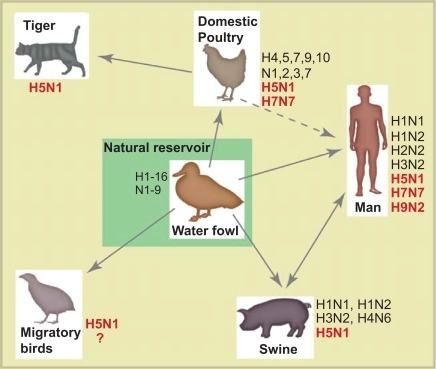
Influenza A viruses infect a wide range of species such as birds, seals, horses, man and pigs (CitationMurphy and Webster 1996). In contrast, influenza B viruses are mainly found in man. The natural reservoir for influenza A viruses is birds, with waterfowl being the most important host and will, for the foreseeable future, continue to be a threat to human health (CitationWebster et al 1992; CitationMurphy and Webster 1996). The virus may spread from this reservoir to other species including man (Figure ). Normally, avian influenza is a mild or asymptomatic intestinal infection in birds and the virus may be secreted in high titers through the cloacae for a period up to 30 days (CitationHinshaw et al 1980). The practice in many parts of the world of keeping free-ranging poultry close to the family dwelling, allowing both exchange of avian influenza with wild birds as well as facilitating zoonoses, is considered to be unsafe (reviewed in CitationWebster and Bean 1998).
Figure 2 A simplified overview of the ecology of influenza A virus. The subtypes that have been detected in each species are shown and the subtypes marked in bold are the highly pathogenic avian influenza subtypes that have caused illness in humans. The main reservoir of influenza A viruses is waterfowl, which may carry highly pathogenic subtypes of influenza without visible illness. The H5N1 has also been detected in domestic cats, however, little is known about their role in H5N1 epidemiology. Currently, we do not have enough knowledge about the importance of migratory birds in the spread of influenza virus (hence the question mark). Interested readers can consult the reviews by Webster et al 1992 or Murphy et al 1996 for further information.
The receptor binding specificity of the surface glycoprotein HA is an important determinant of host range. Only one amino acid substitution in HA (226Leu→Gln) may change the receptor specificity of HA (CitationEisen et al 1997), shifting from human (SAα-(2, 6)Gal) to avian (SAα-(2, 3)Gal) receptor preference. Humans also express a low level of the avian receptor (SAα-(2, 3)Gal) in the lower respiratory tract, which may explain why avian influenza virus only rarely infects man (CitationShinya et al 2006). In addition to the HA gene, the pathogenicity of influenza is also determined by a number of gene segments including the polymerase complex, the NS1 and the NA (CitationGoto and Kawaoka 1998; CitationBasler et al 2001; CitationTaubenberger et al 2005).
Influenza epidemiology
The annual influenza outbreaks usually start during the winter months in temperate climates. In contrast, in the tropics and subtropics influenza virus may be isolated throughout the year. The number of suspected influenza cases, designated influenza like illness (ILI), is a frequently used as a measure of epidemic activity (CitationFleming et al 2000; CitationStephenson and Zambon 2002). However, there is more than one case definition of ILI used. The WHO defines ILI as a sudden onset of fever (>38 °C), cough or sore throat in the absence of other diagnoses (CitationWHO 1999), whereas others have used a sudden onset of fever, cough and fatigue (CitationThursky et al 2003), or fever as well as least two of the following symptoms: headache, cough, sore throat and myalgia (CitationBoivin et al 2000). Generally, influenza A H3N2 normally results in the most serious illness, B viruses intermediate and influenza A H1N1 cases present with the mildest manifestations (CitationMonto et al 1985). Influenza A viruses are normally responsible for four-fold more hospitalizations than influenza B viruses (CitationMurphy and Webster 1996).
Influenza related deaths are frequently under-reported because influenza often exacerbates underlying disease, which may be recorded as the primary cause of death (CitationNicholson 1998). The number of influenza related deaths is therefore often monitored as the number of excess deaths compared to a period without (known) influenza activity. Influenza mortality usually occurs in the “at risk” groups; people with underlying chronic medical conditions like COPD, diabetes, cancer and heart disease as well as in the elderly. Influenza related deaths worldwide are estimated to be about 1 million people each year (CitationWHO 2002; CitationYewdell and Garcia-Sastre 2002) and in the USA about 60%–70% of deaths occur in people over 65 years old (CitationPerrotta et al 1985). However, the true number of influenza related deaths worldwide is difficult to estimate, since most epidemiological data are derived from industrialized countries in the temperate zone (CitationWHO 2005).
Antigenic drift of influenza
Influenza is an RNA virus and as such has a high frequency of copy errors during replication due to a lack of proof-reading by the polymerase. This results in substitutions in the genome at a rate that is many-fold higher than that found for DNA viruses (CitationDrake et al 1998).
The accumulation of mutations, particularly in the HA, may lead to changes in the antigenic signature of the virus allowing the virus to escape herd immunity and cause new outbreaks, a process called antigenic drift (CitationMurphy and Webster 1996). Influenza A viruses mutate more frequently than influenza B viruses and hence influenza B is more antigenically stable (CitationYamashita et al 1988). The slower evolution of influenza B viruses may be attributed to a longer co-evolution in man and host specific adaptations (CitationWebster et al 1992). The risk of reassorting with avian subtypes makes influenza A virus a particularly dangerous infectious agent for man.
Antigenic shift of influenza
During the 20th century there were three pandemics, namely in 1918, 1957 and 1968 (CitationWHO 2005). The term pandemic is only used when an antigenic shift occurs and the novel influenza A subtype infects humans causing global widespread outbreaks resulting in substantial morbidity and mortality. The 1918 pandemic had the highest mortality rates of the 20th century pandemics, causing approximately 40 million deaths worldwide, with an unprecedented number of deaths in young adults (review in CitationReid et al 2001).
Antigenic shift may occur after reassortment of viral genome segments from two different influenza A subtypes. When two viruses co-infect the same cell and exchange segments, a novel reassorted virus with new combinations of HA and NA surface glycoproteins may be generated. When a virus with a novel HA (and NA) spreads efficiently in man, the virus may cause a pandemic (CitationWebster et al 1992). These novel influenza A viruses cross the species barrier from the large avian reservoir and can strike at unpredictable intervals. Pigs, having receptors for both the human and avian influenza viruses, and are thought to play a role as a “mixing vessel” producing new reassortments of influenza A virus (see Figure ). The pandemics of 1957 and 1968 were the result of a reassortment between avian and human influenza viruses, possibly in pigs (CitationWebster et al 1992).
An antigenic shift can also occur after direct transfer of an avian virus into man. The H1N1 virus responsible for the 1918 pandemic was not a reassortant, but was transmitted to man in toto from an avian source (CitationTaubenberger et al 2005). The zoonotic cases of avian H5N1 in Hong Kong in 1997 and the ongoing zoonosis of H5N1 demonstrate that avian viruses can directly infect man and do not necessarily require a “mixing vessel” (CitationSubbarao et al 1998; CitationChotpitayasunondh et al 2005). In fact, the genetic reassortment between avian and human subtypes could well take place in man. However, to date there has not been an adaptation of this novel H5N1 subtype to allow a sustained human-to-human spread.
Vaccines and immunity
The two main types of influenza vaccine are inactivated virus, which is by far the most commonly used, and live virus vaccines. Inactivated vaccines are normally administered parenterally. In contrast, live virus vaccines are administered intranasally with an attenuated virus to produce a limited upper respiratory tract infection without causing any overt clinical illness. However, live influenza vaccines are not licensed outside Russia and the USA. In the USA, live vaccines are so far only recommended for healthy subjects 5–49 years old, thus excluding their use in COPD patients (CitationHarper et al 2005).
Current inactivated influenza vaccines are trivalent containing strains from two influenza A subtypes (H1N1 and H3N2) and one influenza B variant. The vaccine is normally administered during October and November in the northern hemisphere. A single-dose regime is mostly used, but for preschool children two doses are recommended at one-months intervals. The vaccine strains need to be epidemiologically relevant, and the WHO updates their strain recommendation on an annual basis for each hemisphere. The selected strains are either reassorted with a high-growth laboratory strain or adapted to give better yields in embryonated eggs. The reassorted strain expresses the desired HA and NA of the field virus, while maintaing the high growth potential in eggs (CitationKilbourne 1969). Embryonated hens’ eggs are the most commonly used vaccine substrate. However, some vaccine manufacturers are now licensing their cell culture based vaccines. This will give the vaccine industry greater flexibility and allow scaling up their production if a sudden surge in demand should occur.
Formulation of inactivated vaccines
There are three main formulations of inactivated vaccines (extensively reviewed in CitationFurminger 1998). Whole virus vaccine is inactivated by chemical agents (eg, formaldehyde or β-propiolactone) in a procedure that does not destroy the viral envelope (CitationGoldstein and Tauraso 1970). This type of vaccine was widely utilized until the end of the 1970s, but its use was largely discontinued due to a somewhat higher frequency of side reactions (CitationBarry et al 1976; CitationBoyer et al 1977; CitationGross et al 1977; CitationHehme et al 2002, Citation2004). A split virus vaccine is produced using chemical agents (eg, ether or tributyl phosphate) to disrupt the viral envelope (CitationDavenport et al 1960; CitationBarry et al 1976). Immunization with split virus vaccine produces fewer side reactions (ie, has a lower reactogenicity), but has also a somewhat reduced immunogenicity compared to whole virus vaccine (CitationBarry et al 1976; CitationGross et al 1977). A third vaccine formulation, the subunit type, consists of highly purified surface antigens, HA and NA, and is the least reactogenic influenza vaccine on the market today (CitationPotter et al 1975). All inactivated vaccines are administered parenterally, either intramuscularly or deep subcutaneously. However, more recent studies using highly purified virus have found that split and whole virus vaccine induce similar levels of side reactions (CitationHehme et al 2002; CitationHehme et al 2004).
The normal adult human dose is standardized to a concentration of 15 μg HA per strain, and thus the trivalent vaccine contains a total of 45 μg of HA. Current influenza vaccines are not adjuvanted, the use of adjuvants may be necessary for pandemic vaccines based on avian influenza viruses where the vaccine recipient would be immunologically naïve (CitationStephenson et al 2004).
Immunity after vaccination
Humans have generally experienced a number of influenza infections, which prime the immune system and create an immunological memory (CitationSmith 1977; CitationPalladino et al 1995; CitationTamura and Kurata 2004). Upon vaccination with inactivated vaccine, reactivation of immunological memory results in production of mainly IgG as well as some IgA antibodies (CitationBrokstad et al 1995; CitationBrokstad et al 2002; CitationCouch 2003; CitationGuthrie et al 2004). In contrast, after a natural infection the immune response will elicit more IgA antibodies and a cellular T-cell response (CitationBeyer et al 2002). If there is no pre-existing immunity (for example in children), two doses of vaccine are recommended given at least one month apart to achieve a satisfactory immune response (CitationHarper et al 2005). It is likely that a two dose regime will be required for a pandemic vaccine containing a novel influenza subtype, in addition to the use of an adjuvanted formulation (CitationStephenson et al 2003; CitationHehme et al 2004).
The systemic response after vaccination is rapid in healthy subjects with the antibody secreting cell response peaking after one week, while the serum antibody continues to increase up to 2–3 weeks after vaccination (CitationCox et al 1994). The concentration of influenza-specific serum antibody then wanes over time, but remains elevated at least 8 months post vaccination (CitationClark et al 1983). The importance of serum antibody is shown by the fact that the higher the serum antibody level, the less likely the person is to experience clinical illness upon subsequent infection (CitationHobson et al 1972; CitationKendal et al 1982). Passively derived serum IgG probably leaks through the infected epithelial cell layer and neutralizes the virus and thus prevents binding to the cellular receptor (CitationTamura and Kurata 2004). IgG diffuses more readily across the alveolar wall in the lower respiratory tract than across the epithelial cells in the upper airways (CitationMurphy 2005), which makes IgG antibodies particularly important in avoiding the most serious complications of infection.
COPD patients have a damaged epithelial cell layer and it has been shown in experimental models for asthma and chronic bronchitis, a lasting change in the airway epithelium and smooth muscle behavior after viral infection (CitationHoltzman et al 2005). These authors have hypothesized a viral cause of both acute and chronic manifestations of asthma and chronic bronchitis. Studies in healthy individuals and animal models have shown apoptosis of cells in the epithelial cell layer and the resulting inflammation also attract effector cells to the site of infection causing further cell death (CitationBrydon et al 2005). This could have consequences also for the immune response in the damaged lungs of COPD sufferers, but no study to date has adequately addressed this issue. A thorough review of the aetiology of exacerbations in COPD sufferers has recently been published (CitationSapey and Stockley 2006).
Immunity after influenza infection
Initially, influenza virus replicates in the epithelial cells of the respiratory tract and this is also an important site for the immune response (CitationTamura and Kurata 2004). IgA is actively transported in its secretory form, S-IgA, across the epithelial surfaces of the respiratory tract and neutralizes virus by binding to its surface proteins. Once the infection is established, a cytotoxic response is induced, often against the internal viral proteins, and is involved in viral clearance and recovery from infection (CitationSambhara et al 2001; CitationTakada et al 2003). The internal influenza proteins are more conserved than HA and NA, thus memory T-cells may be more cross-reactive against drifted viruses and possibly across influenza A subtypes.
Infection-induced immunity is thought to be long-lived, but will more or less become redundant due to the continuous antigenic changes of the virus. A special case was demonstrated when the H1N1 virus re-appeared in 1977, which had circulated 20 years earlier (CitationDowdle 1999). Before the first H1N1 wave, antibodies were only detected in people that were in their childhood in the 1950s and not in older or younger individuals (CitationHaaheim 2003). The long-lived immune response to influenza virus experienced in early childhood is often referred to as ‘Antigenic Sin’ (CitationFrancis et al 1953). During the first wave, people under the age of 20 were almost exclusively infected (CitationPotter 1998) and they also had a marked lower immune response to inactivated vaccines containing H1N1, suggesting that they did not have any immunological memory against this virus (CitationSmith 1977). The 1977 virus was antigenically very similar to a 1950 isolate and was possibly inadvertently released into the human population (CitationPalese 2004).
Target groups for vaccination
The WHO has issued a priority list of groups, which should be annually vaccinated (Listed in full in Table (CitationWHO 2005)). These groups are: (1) Residents of institutions for the elderly or the disabled; (2) Elderly non-institutionalized individuals with certain chronic medical conditions; (3) Other individuals in the community with certain chronic medical conditions; (4) Individuals who are above a nationally defined age limit; and (5) Other groups defined on the basis of national data such as those with frequent contact with high-risk persons, health care workers, pregnant women and children age 6–23 months old. The WHO has to date not issued any recommendations on the use of live influenza vaccines (CitationWHO 2005).
Table 2 Target groups for vaccination (CitationWHO 2005)
Since influenza strains are prone to drift antigenically from one influenza season to the next, annual vaccination is recommended. Repeated influenza vaccination does not compromise the immune response, and should therefore not be used as an argument against annual vaccination (CitationKeitel et al 1997; CitationBeyer et al 1999).
Safety of inactivated influenza vaccines
More than 300 million doses of influenza vaccines are administered each year and the vaccine has an excellent safety record (CitationBeyer et al 2002). Local side reactions usually occur in 15%–20% of the vaccinees, most commonly pain and redness at the injection site (CitationWHO 2005). Transient and mild systemic reactions can also occur and include low-grade fever and headache, especially in children, but generally do not interfere with daily activities (CitationWiselka 1998).
Most people can be vaccinated without any complications. However, children younger than 6 months and people with known allergies to egg protein are advised against vaccination, whereas persons with an acute febrile illness should just postpone their vaccination until they have recovered (CitationWiselka 1998; CitationWHO 2005). However, a mild febrile illness is not considered a contraindication to vaccination (CitationHarper et al 2005).
Influenza vaccination is recommended for a number of groups with underlying medical conditions and there is considerable information on the vaccine’s safety in these patients. A good safety record has been observed using inactivated influenza vaccines in patients with COPD (CitationHowells and Tyler 1961; CitationPoole et al 2000; CitationWongsurakiat et al 2004) In adults with asthma there is a very low incidence of clinically significant adverse reactions, for example, out of 40 million vaccine doses administered to such patients, only 5 cases of asthma exacerbations were reported over a ten-year period (CitationPalache and van der Velden 1992). In asthmatic patients on oral steroid therapy, no serious local or systemic side reactions were reported after vaccination with an inactivated influenza vaccine (CitationPark et al 1996).
Influenza vaccination will of course only protect against illness caused by influenza virus and not by other acute respiratory infections as shown in a recent study where the incidences of respiratory illnesses over a year was not affected by influenza vaccination (CitationWongsurakiat et al 2004). This is to be expected as, at least in the temperate zone, influenza normally is only epidemic for a few months each year. About 30% of exacerbations of COPD are caused by viral pathogens, and of these less than half are caused by influenza (CitationJohnson and Stevenson 2002; CitationCameron et al 2006). Even so, influenza vaccination clearly reduced the frequency of exacerbations in COPD cohorts (CitationPoole et al 2000). Some COPD patients occasionally report a worsening of their condition immediately following vaccination. However, in a recent study no increased usage of corticosteroids was found after vaccination (CitationTata et al 2003). People with asthma and COPD are considered to be one of the groups that benefit most from influenza vaccination, consequently they are included in the “at risk” groups by the WHO (CitationWHO 2000, Citation2005).
Vaccine efficacy: measurement and contributing factors
The protection elicited by influenza vaccine is notoriously difficult to quantify. The term vaccine efficacy refers to well-controlled experiments with a placebo control group (CitationHannoun et al 2004) and sometimes involves young study subjects with a better than average health. Vaccine effectiveness is used to describe the protective effect of the vaccine when used as a part of a public health scheme and can be considerably lower than the efficacy. Both vaccine effectiveness and efficacy are highly dependent upon the specific outcome being measured, the degree of antigenic match between epidemic virus in circulation and the vaccine strain, as well as the severity of the epidemic. However, the vaccine efficacy, especially when measured by serological correlates of protection, may be an underestimate of the true effect of vaccination, partly because little data is available on the number of vaccinees who undergo a sub-clinical influenza infection (CitationPalache 1997).
Vaccine efficacy is commonly measured using surrogate correlates of protection. The serum antibody response is often tested by the haemagglutination inhibition (HI) assay (CitationHobson et al 1972; CitationKendal et al 1982). An HI titer ≥40 indicates a 50% protective level against influenza (CitationHobson et al 1972; CitationKendal et al 1982). The level of seroprotection, ie, the percentage of vaccinees that have an HI titer after vaccination of at least 40, was found in a meta-study of mainly young health subjects under 65, to be 80%–90% (Table ) (CitationBeyer et al 2002). Vaccination with inactivated influenza vaccine has been shown to prevent laboratory confirmed influenza in 70%–90% of healthy adults (CitationRuben et al 1973; CitationWilde et al 1999; CitationBridges et al 2000; CitationBeyer et al 2002; CitationKawai et al 2003; CitationHarper et al 2005; CitationWHO 2005). The vaccine efficacy is reduced if a more general clinical outcome is measured, for instance one study showed only a 25% reduction in upper respiratory tract illness and a 43% reduction in absenteeism from work (CitationNichol et al 1995).
Table 3 The efficacy of influenza vaccine in different population groups from representative studies
A significant factor when evaluating the efficacy of the vaccine is the antigenic match between the vaccine strains and the circulating influenza viruses in the community. Normally, the antigenic difference between them is trivial, but occasionally there is a mismatch. Due to the poorer immune response in the elderly, this may be more important in this group than in healthy adults (Citationde Jong et al 2000). In healthy subjects, however, the vaccine is effective (49%–53%) in preventing illness also in years with a sub-optimal match between the vaccine and circulating influenza strains (CitationPyhälä et al 2001; CitationRitzwoller et al 2005). The level of circulating influenza in the community also complicates the calculation of the effectiveness of the vaccine. In years of high influenza activity and widespread outbreaks, the vaccine will show a higher efficacy (CitationJefferson et al 2005) and better cost/benefit ratio (CitationNichol et al 2005; CitationTurner et al 2005).
Efficacy and effectiveness in “at risk” populations
The immune response elicited after influenza vaccination in the elderly is poorer than in younger subjects (Table ) (CitationPalache et al 1993), which is important for COPD patients as they are often at an advanced age (CitationVilkman et al 1996). This is reflected in the influenza vaccine licensing criteria in the EU, which have less stringent requirements for the vaccine’s immunogenicity in subjects over 60 years of age (CitationCHMP 1997). In older patients (>65), a lower number of subjects elicits an increase in serum antibody after vaccination than in healthy adults, and the seroprotection rate was generally 40%–70% (CitationGross et al 1987; CitationMcElhaney et al 1993; CitationPalache et al 1993; CitationGovaert et al 1994; CitationMcElhaney et al 2005). Similar findings were observed in a trial with COPD patients with 45%–87% achieving a protective HI titer (CitationWongsurakiat et al 2004). However, institutionalized elderly and infirm subjects often have a particularly poor response after vaccination (CitationGross et al 1989). For ethical reasons few case-control studies have been undertaken investigating the efficacy of inactivated vaccines in the “at risk” groups.
A much used marker for measuring vaccine efficacy is the prevention of ILI. Some ILI cases are not due to influenza virus, but other respiratory pathogens and the vaccine efficacy in the elderly and probably also COPD sufferers against ILI is relatively low (35%) (CitationVu et al 2002). The positive predictive value of ILI actually being due to influenza is 23%–60%, and is very dependent on the case definition (CitationThursky et al 2003). The study conducted by Wongsurakiat et al showed a significant reduction (66%) in the number of ILI cases between vaccinated and unvaccinated COPD patients, despite a small patient group size (CitationWongsurakiat et al 2004). Using a more specific influenza diagnosis, namely laboratory confirmed influenza, the vaccine had a 58% efficacy in healthy elderly subjects (CitationGovaert et al 1994). This is similar to the reduction of laboratory confirmed influenza observed in adults 16–64 years old (CitationKawai et al 2003).
During the influenza season there is an increase of hospitalization of COPD patients, clearly demonstrating the impact influenza illness can have on this group of patients (CitationYap et al 2004). Influenza vaccination, however, only partly protects this group against hospitalization for pneumonia and influenza (P&I) as there are several causes not related to influenza for P&I. Several studies in the elderly population, found the reduction in hospitalization for P&I to be significant after influenza vaccination (33%–52%) (CitationGross et al 1995; CitationNichol et al 1996; CitationVu et al 2002). There is an rapid deterioration in the quality of life with increasing number of COPD exacerbations (CitationNiewoehner 2006) and influenza vaccine has been shown to have a 75%–80% effectiveness in reduction of acute respiratory illnesses, independently of the severity of COPD (mild, moderate and severe) (CitationHowells and Tyler 1961; CitationWongsurakiat et al 2004).
Older people may not fully recover after an influenza infection and thus one significant consequence of hospitalization may be permanent disability (CitationMcElhaney 2005). Influenza vaccination reduced the hospitalization rate by 52% in elderly patients with chronic lung disease (CitationNichol et al 1999). An additional strategy for protecting these residents is therefore vaccination of the nursing-home staff caring for them (CitationPotter et al 1997). Vaccination of family members and other close contacts will therefore indirectly protect the “at risk” groups (CitationPiedra et al 2005) and is a policy that is advocated by the WHO (CitationWHO 2005).
The efficacy of inactivated influenza vaccines in preventing influenza related deaths is 50%–75% in elderly “at risk” groups (CitationFleming et al 1995; CitationGross et al 1995; CitationNichol et al 1996; CitationJefferson et al 2005). This has been verified by trials in non-institutionalized elderly (CitationVu et al 2002) and in elderly with pulmonary or heart diseases (CitationNichol 1999). A large recent study involving over 100,000 people in Taiwan investigating the impact of influenza vaccination on mortality, also demonstrated a 45% reduction in mortality of COPD patients following influenza vaccination and the vaccination was strongly correlated with prevention of death from lung disease in general (CitationWang et al 2007).
The assumption that influenza vaccination also appears to protect the vaccinee from all causes of death and not only influenza related deaths, is controversial (CitationSimonsen et al 2005) and underlines the difficulty in determining the vaccines effectiveness in large cohort studies. The efficacy of influenza vaccines to prevent serious influenza-related complications in the elderly, can be increased by using both influenza and pneumococcal vaccines and results in a reduction of the number of hospitalizations. Both vaccines are safe and can be co-administered without impairing the antibody response to either vaccine (CitationCDC 1997).
Future challenges in the use of influenza vaccines
One of the most critical challenges is to increase the coverage rate of current influenza vaccines among the “at risk” groups, and many developed countries have adopted a policy to increase vaccine uptake. The World Health Assembly has set a goal of annual immunization of at least 75% of people over 65 years of age by 2010 (CitationWHO 2005). In recent years, some countries have had vaccine supply problems, which can only be rectified by increasing vaccine production and encouraging more manufacturers to produce influenza vaccine. This will benefit not only COPD sufferers, but also the general public, as the industry’s production capacity will be better placed to meet the considerable demand for vaccine when a new pandemic strikes (CitationWood 2001). Most influenza vaccine manufacturers use embryonated hens’ eggs as the vaccine substrate. Quality-assured eggs cannot be delivered at short notice, as the manufacturers need to plan their production a year in advance. The use of cell culture systems that are more easily scaled up provides more flexibility to accommodate the increasing demand for influenza vaccines and improves pandemic preparedness.
Currently used inactivated influenza vaccines are safe, but the immunogenicity, especially in elderly, is suboptimal. There are a number of different approaches being employed to improve the vaccine efficacy. One option is to return to the use of the more immunogenic whole virus vaccine formulation, which in recent trials has also been shown to have an acceptable reactogenicity profile (CitationHehme et al 2002) or alternatively, to use a virosomal influenza vaccine, which has the viral surface antigens in a reconstituted viral envelope (CitationHuckriede et al 2005). The vaccine immunogenicity can also be increased by adjuvanting the vaccine. This raises new safety issues, especially in patients on medication. However, one of these adjuvants, MF59, has been shown not to cause more side reactions in COPD patients on steroid therapy than healthy subjects (Citationde Roux et al 2005). Several trials have investigated the combined effect of both live and inactivated influenza vaccination, this is however, not considered as a practical routine procedure (CitationHarper et al 2005). To date, no beneficial effect of vaccination with both live and inactivated influenza vaccines has been found in older patients with COPD (CitationGorse et al 2003; CitationGorse et al 2004).
Conclusion
Influenza remains today an important cause of morbidity and mortality, especially in groups with underlying medical conditions like COPD. Inactivated influenza vaccines have been used for many years with hundreds of millions of doses administered and have an excellent safety record in all patients groups. There have been relatively few studies based solely on COPD patients, but nonetheless the conclusion is that there is enough evidence to recommend annual vaccination in this group (CitationPoole et al 2000; CitationBaydur 2004; CitationWongsurakiat et al 2004). Even if the efficacy of current influenza vaccine is not optimal, there is no doubt that its use in COPD sufferers will continue to reduce morbidity, save lives, and reduce health care costs.
References
- BarryDWMaynerREStatonE1976Comparative trial of influenza vaccines. I. Immunogenicity of whole virus and split product vaccines in manAm J Epidemiol1043446947145
- BaslerCFReidAHDybingJK2001Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genesProc Natl Acad Sci USA9827465111226311
- BaydurA2004Influenza vaccination in vulnerable populationsChest1251971215189905
- BeyerWEde BruijnIAPalacheAM1999Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studiesArch Intern Med15918289927102
- BeyerWEPalacheAMde JongJC2002Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysisVaccine2013405311818152
- BoivinGHardyITellierG2000Predicting influenza infections during epidemics with use of a clinical case definitionClin Infect Dis311166911073747
- BoyerKMCherryJDWelliverRC1977IgM and IgG antibody responses after immunization of children with inactivated monovalent (A/New Jersey/76) and bivalent (A/New Jersey/76-A/Victoria/75) influenza virus vaccinesJ Infect Dis136SupplS66571342632
- BridgesCBThompsonWWMeltzerMI2000Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trialJama28416556311015795
- BrokstadKACoxRJOlofssonJ1995Parenteral influenza vaccination induces a rapid systemic and local immune responseJ Infect Dis1711982037798664
- BrokstadKAErikssonJCCoxRJ2002Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosaJ Infect Dis1858788411920311
- BrydonEWMorrisSJSweetC2005Role of apoptosis and cytokines in influenza virus morbidityFEMS Microbiol Rev298375016102605
- CameronRJde WitDWelshTN2006Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilationIntensive Care Med321022916791664
- CDC1997Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep46RR–8124
- CHMP (1997). Committee for Proprietary Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines. CPMP/BWP/214/96, Circular No. 96-0666:1–22.
- ChotpitayasunondhTUngchusakKHanshaoworakulW2005Human disease from influenza A (H5N1), Thailand, 2004Emerg Infect Dis11201915752436
- ClarkAPotterCWJenningsR1983A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunityJ Hyg(Lond)90361706863910
- ColmanPM1994Influenza virus neuraminidase: structure, antibodies, and inhibitorsProtein Sci31687967849585
- CouchRB2003An overview of serum antibody responses to influenza virus antigensDev Biol(Basel)115253015088772
- CoxRJBrokstadKAZuckermanMA1994An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccinationVaccine1299397975853
- DavenportFMRottRSchaeferW1960Physical and biological properties of influenza virus components obtained after ether treatmentJ Exp Med1127658213719952
- de JongJCBeyerWEPalacheAM2000Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderlyJ Med Virol6194910745239
- de RouxAMarxABurkhardtO2005Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patientsVaccine
- DowdleWR1999Influenza A virus recycling revisitedBull World Health Organ77820810593030
- DrakeJWCharlesworthBCharlesworthD1998Rates of spontaneous mutationGenetics1481667869560386
- EisenMBSabesanSSkehelJJ1997Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallographyVirology23219319185585
- FauquetCMMayoMAManiloffJ2004Eight report of the International Committee on Taxonomy of VirusesSan Diego, Wien, New YorkAcademic Press, Elsevier
- FlemingDMWatsonJMNicholasS1995Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989–90 using a general practice databaseEpidemiol Infect11558198557090
- FlemingDMZambonMBarteldsAI2000Population estimates of persons presenting to general practitioners with influenza-like illness, 1987–96: a study of the demography of influenza-like illness in sentinel practice networks in England and Wales, and in The NetherlandsEpidemiol Infect1242455310813150
- FrancisTJrDavenportFMHennessyAV1953A serological recapitulation of human infection with different strains of influenza virusTrans Assoc Am Physicians66231913136267
- FurmingerIGS1998Vaccine productionNicholsonKGWebsterRGHayAJTextbook of InfluenzaOxfordBlackwell Science32432
- GoldsteinMATaurasoNM1970Effect of formalin, beta-propiolactone, merthiolate, and ultraviolet light upon influenza virus infectivity chicken cell agglutination, hemagglutination, and antigenicityAppl Microbiol1929045437304
- GorseGJO’ConnorTZNewmanFK2004Immunity to influenza in older adults with chronic obstructive pulmonary diseaseJ Infect Dis190111915195238
- GorseGJO’ConnorTZYoungSL2003Efficacy trial of live, cold-adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative studyVaccine2121334412706704
- GotoHKawaokaY1998A novel mechanism for the acquisition of virulence by a human influenza A virusProc Natl Acad Sci USA951022489707628
- GovaertTMSprengerMJDinantGJ1994Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trialVaccine12118597839722
- GovaertTMThijsCTMasurelN1994The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trialJama272166157966893
- GrossPAEnnisFAGaerlanPF1977A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in childrenJ Infect Dis13662332335000
- GrossPAHermogenesAWSacksHS1995The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literatureAnn Intern Med123518277661497
- GrossPAQuinnanGVJrWekslerME1989Relation of chronic disease and immune response to influenza vaccine in the elderlyVaccine730382815966
- GrossPAWekslerMEQuinnanGVJr1987Immunization of elderly people with two doses of influenza vaccineJ Clin Microbiol25176353654947
- GuthrieTHobbsCGDavenportV2004Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adultsJ Infect Dis19019273515529256
- HannounCMegasFPiercyJ2004Immunogenicity and protective efficacy of influenza vaccinationVirus Res103133815163501
- HarperSAFukudaKUyekiTM2005Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep54RR–814016086456
- HehmeNEngelmannHKuenzelW2004Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic useVirus Res1031637115163505
- HehmeNEngelmannHKunzelW2002Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccinesMed Microbiol Immunol(Berl)191203812458361
- HinshawVSBeanWJWebsterRG1980Genetic reassortment of influenza A viruses in the intestinal tract of ducksVirology10241296245516
- HobsonDCurryRLBeareAS1972The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B virusesJ Hyg (Lond)70767774509641
- HoltzmanMJTynerJWKimEY2005Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary diseaseProc Am Thorac Soc21324016113481
- HowellsCHTylerLE1961Prophylactic use of influenza vaccine in patients with chronic bronchitis. A pilot trialLancet214283214449407
- HuckriedeABungenerLStegmannT2005The virosome concept for influenza vaccinesVaccine23Suppl 1S263816026906
- HuiEKBarmanSYangTY2003Basic residues of the helix six domain of influenza virus M1 involved in nuclear translocation of M1 can be replaced by PTAP and YPDL late assembly domain motifsJ Virol7770789212768027
- HaaheimLR2003Original antigenic sin. A confounding issue?Dev Biol (Basel)115495315088775
- JeffersonTRivettiDRivettiA2005Efficacy and effectiveness of influenza vaccines in elderly people: a systematic reviewLancet36611657416198765
- JohnsonMKStevensonRD2002Management of an acute exacerbation of copd: are we ignoring the evidence?Thorax57Suppl 2II15II2312364706
- KawaiNIkematsuHIwakiN2003A prospective, Internet-based study of the effectiveness and safety of influenza vaccination in the 2001–2002 influenza seasonVaccine2145071314575760
- KeitelWACateTRCouchRB1997Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year periodVaccine151114229269055
- KendalAPPereiraMSSkehelJ1982Concepts and procedures for laboratory-based influenza surveillancePublication no. B17–35.Centers for Disease Control Atlanta, Ga
- KilbourneED1969Future influenza vaccines and the use of genetic recombinantsBull World Health Organ4164355309489
- LambRAKrugRM1996Ortomyxoviridae: the viruses and their replicationFieldsBNKnipeDMHowleyPMFields VirologyPhiadelphiaLippincott-Raven Publishers3rd edition135395
- McElhaneyJE2005The unmet need in the elderly: designing new influenza vaccines for older adultsVaccine23Suppl 1S102515908062
- McElhaneyJEHootonJWHootonN2005Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adultsVaccine23329430015837235
- McElhaneyJEMeneillyGSLecheltKE1993Antibody response to whole-virus and split-virus influenza vaccines in successful ageingVaccine111055608212827
- MontoASKoopmanJSLonginiIMJr1985Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981Am J Epidemiol121811224014174
- MurphyBR2005Mucosal immunity to virusesMesteckyJLammMEMcGheeJRMucosal ImmunologyLondonElsevier Academic Press799813
- MurphyBRChalhubEGNusinoffSR1973Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in manJ Infect Dis128479874743544
- MurphyBRWebsterRG1996OrthomyxovirusesFieldsBNKnipeDMHowleyPMFields VirologyPhiladelphiaLippincott-Raven Publishers3rd edition1397445
- Nguyen-Van-TamJS1998Epidemiology of influenzaNicholsonKGWebsterRGHayAJTextbook of InfluenzaOxfordBlackwell Science181206
- NicholKL1999Complications of influenza and benefits of vaccinationVaccine17Suppl 1S475210471180
- NicholKLBakenLNelsonA1999Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung diseaseAnn Intern Med13039740310068413
- NicholKLLindAMargolisKL1995The effectiveness of vaccination against influenza in healthy, working adultsN Engl J Med33314889937666874
- NicholKLMargolisKLWouremnaJ1996Effectiveness of influenza vaccine in the elderlyGerontology4227498940650
- NicholKLNordinJMulloolyJ2005Influence of clinical outcome and outcome period definitions on estimates of absolute clinical and economic benefits of influenza vaccination in community dwelling elderly personsVaccine
- NicholsonKG1998Human influenzaNicholsonKGWebsterRGHayAJTextbook of InfluenzaOxfordBlackwell Science21964
- NiewoehnerDE2006The impact of severe exacerbations on quality of life and the clinical course of chronic obstructive pulmonary diseaseAm J Med11910 Suppl 1384516996898
- OxfordJSBossuytSBalasingamS2003Treatment of epidemic and pandemic influenza with neuraminidase and M2 proton channel inhibitorsClin Microbiol Infect911412691538
- PalacheAM1997Influenza vaccines. A reappraisal of their useDrugs54841569421692
- PalacheAMBeyerWESprengerMJ1993Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteersVaccine11398427034
- PalacheAMvan der VeldenJW1992Influenza vaccination in asthmaLancet3397411347604
- PaleseP2004Influenza: old and new threatsNat Med1012 SupplS82715577936
- PalladinoGMozdzanowskaKWashkoG1995Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID miceJ Virol692075817884853
- ParkCLFrankALSullivanM1996Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapyPediatrics98(2 Pt 1)1962008692617
- PerrottaDMDeckerMGlezenWP1985Acute respiratory disease hospitalizations as a measure of impact of epidemic influenzaAm J Epidemiol122468764025296
- PiedraPAGaglaniMJKozinetzCA2005Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in childrenVaccine231540815694506
- PoolePChackoEWood-BakerR2000Influenza vaccine for patients with chronic obstructive pulmonary diseaseThe cochrane database of systematic reviews (3).
- PortelaADigardP2002The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replicationJ Gen Virol83(Pt 4)7233411907320
- PotterCW1998Chronicle of Influenza PandemicsNicholsonKGWebsterRGHayAJTextbook of InfluenzaOxfordBlackwell Science318
- PotterCWJenningsRMcLarenC1975A new surface-antigen-adsorbed influenza virus vaccine. II. Studies in a volunteer groupJ Hyg(Lond)75353621059705
- PotterJStottDJRobertsMA1997Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patientsJ Infect Dis175168985189
- PyhäläRHaanpaaMKleemolaM2001Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic matchVaccine1932536011312022
- ReidAHTaubenbergerJKFanningTG2001The 1918 Spanish influenza: integrating history and biologyMicrobes Infect381711226857
- RitzwollerDPBridgesCBShetterlyS2005Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 dosesPediatrics116153915995046
- RubenFLAkersLWStanleyED1973Protection with split and whole virus vaccines against influenzaArch Intern Med132568714200477
- SambharaSKurichhAMirandaR2001Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage functionCell Immunol2111435311591118
- SapeyEStockleyRA2006COPD exacerbations. 2: aetiologyThorax61250816517585
- ShinyaKEbinaMYamadaS2006Avian flu: influenza virus receptors in the human airwayNature440435616554799
- SimonsenLViboudCTaylorR2005Influenza vaccination in elderly peopleLancet366208616360785
- SmithJWG1977Antibody responses and reactogenicity of graded doses of inactivated influenza A/New Jersey/76 whole-virus vaccine in humansJ Infect Dis136SupplS47583342621
- StephensonINicholsonKGColegateA2003Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human populationVaccine2116879312639491
- StephensonINicholsonKGGluckR2003Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trialLancet36219596614683655
- StephensonINicholsonKGWoodJM2004Confronting the avian influenza threat: vaccine development for a potential pandemicLancet Infect Dis449950915288823
- StephensonIZambonM2002The epidemiology of influenzaOccup Med (Lond)52241712181371
- SubbaraoKKlimovAKatzJ1998Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illnessScience27939369430591
- TakadaAMatsushitaSNinomiyaA2003Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in miceVaccine213212812804850
- TamuraSKurataT2004Defense mechanisms against influenza virus infection in the respiratory tract mucosaJpn J Infect Dis572364715623947
- TataLJWestJHarrisonT2003Does influenza vaccination increase consultations, corticosteroid prescriptions, or exacerbations in subjects with asthma or chronic obstructive pulmonary disease?Thorax58835914514932
- TaubenbergerJKReidAHLourensRM2005Characterization of the 1918 influenza virus polymerase genesNature4378899316208372
- ThurskyKCordovaSPSmithD2003Working towards a simple case definition for influenza surveillanceJ Clin Virol27170912829039
- TurnerDAWailooAJCooperNJ2005The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of ageVaccine
- VilkmanSKeistinenTTuuponenT1996Age distribution of patients treated in hospital for chronic obstructive pulmonary diseaseAge Ageing25109128670537
- VuTFarishSJenkinsM2002A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the communityVaccine201831611906772
- WangCSWangSTLaiCT2007Impact of influenza vaccination on major cause-specific mortalityVaccine25119620317097773
- WebsterRGBeanWJ1998Evolution and ecology of influenza viruses: interspecies transmissionNicholsonKGWebsterRGHayAJTextbook of influenzaOxfordBlackwell Science10919
- WebsterRGBeanWJGormanOT1992Evolution and ecology of influenza A virusesMicrobiol Rev56152791579108
- WHO1999WHO recommended surveillance standardsGenevaWHO/CDS/CSR/ISR/99.2, WHO1116
- WHO2000Influenza vaccines. Recommendations for the use of inactivated influenza vaccines and other preventive measuresWkly Epidemiol Rec7528188
- WHO2002Draft WHO guidelines on the use of vaccines and antivirals during influenza pandemicsWkly Epidemiol Rec7739440412498024
- WHO 2005. Influenza pandemic preparedness and response. Geneva, Executive Board rapport - EB115/44.
- WHO2005Influenza vaccines -WHO position paperWkly Epidemiol Rec802798716171031
- WildeJAMcMillanJASerwintJ1999Effectiveness of influenza vaccine in health care professionals: a randomized trialJama2819081310078487
- WilliamsWWHicksonMAKaneMA1988Immunization policies and vaccine coverage among adults. The risk for missed opportunitiesAnn Intern Med108616252964806
- WiselkaMJ1998Vaccine safetyNicholsonKGWebsterRGHayAJTextbook of InfluenzaOxfordBlackwell Science34657
- WongsurakiatPMaranetraKNGulprasutdilogP2004Adverse effects associated with influenza vaccination in patients with COPD: a randomized controlled studyRespirology9550615612969
- WongsurakiatPMaranetraKNWasiC2004Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled studyChest12520112015189916
- WoodJM2001Developing vaccines against pandemic influenzaPhilos Trans R Soc Lond B Biol Sci35619536011779397
- YamashitaMKrystalMFitchWM1988Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C virusesVirology163112223267218
- YapFHHoPLLamKF2004Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong KongJ Med Virol736172315221909
- YewdellJGarcia-SastreA2002Influenza virus still surprisesCurr Opin Microbiol54141812160862