Abstract
Objective
This pilot study concerns the evaluation of the acute cytokine response to exercise and changes in this throughout a 7 week pulmonary rehabilitation programme.
Methods
17 (10 male, 7 female) stable COPD patients, mean (SD) age 69 (8) yrs, mean FEV1, 51.3 (17.3) % predicted entered into 7 weeks of rehabilitation. The acute cytokine response (ACR) was measured from serum cytokine levels; Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) taken pre, post and 1 hour post-maximal incremental shuttle walking test (ISWT). The ACR to maximal exercise was determined before rehabilitation (T0) and post rehabilitation (T7). The ACR (pre/post test) to iso-distance exercise (based on initial ISWT distance) was determined throughout the rehabilitation period at 2 (T2), 4 (T4) weeks and at the end (T7).
Results
12 patients completed the study. Maximal ISWT distance significantly increased after rehabilitation. There was no significant change in baseline cytokine level throughout; or in pre/post-exercise cytokine levels prior to, during or following rehabilitation.
Conclusions
There was no significant inflammatory response associated with maximal exercise before or after training. Cytokine responses to a fixed bout of exercise did not alter markedly throughout. Clinical PR is unlikely to exacerbate systemic inflammation in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by fixed airflow obstruction and systemic inflammatory complications. Serum inflammatory markers are elevated in patients with COPD compared with healthy subjects (CitationGan et al 2004) and catabolic and anabolic imbalances may have deleterious effects on peripheral muscle (CitationDebigare et al 2001). There is increasing evidence to support the premise that inflammation may contribute to impairment in health status and exercise tolerance in patients with COPD (CitationBroekhuizen et al 2006). Exercise as part of pulmonary rehabilitation is a proven beneficial therapy for COPD, improving symptoms and functional outcomes (CitationLacasse et al 2002). In healthy individuals strenuous exercise has been shown to provoke an inflammatory response with predominant production of anti-inflammatory cytokines interleukin-6 (IL-6), interleukin-1 receptor agonist (IL-1ra) and soluble-tissue necrosis factor receptors (s-TNF-R) (CitationPetersen and Pedersen 2005), whilst pro-inflammatory IL-6 may help to attenuate production of TNF-α (CitationStarkie et al 2003).
Since COPD patients demonstrate increased systemic inflammation at rest (Citationvan Helvoort et al 2005), it is theorized that exercise should be carefully prescribed to avoid intensifying the inflammatory state. Early data shows higher TNF-α and IL-6 in CF patients compared with healthy subjects. These differences persisted after moderate intensity exercise (CitationTirakitsoontorn et al 2001). Similarly, COPD patients are de-conditioned and exercise might induce a larger increase in these inflammatory mediators. Rabinovich et al demonstrated a significant increase in TNF-α after 11 minutes of constant work rate cycling exercise in severe COPD patients but not in healthy subjects (CitationRabinovich et al 2003). Individual cytokine response varies greatly, with intensity and duration of exercise (CitationPetersen and Pedersen 2005) body composition (CitationSchols et al 1996), age and gender (CitationTimmons et al 2006).
The paucity of research available regarding the acute exercise-induced cytokine response in COPD patients makes inferring relationships difficult. A significant reduction in baseline CRP has been demonstrated in healthy marathon runners after 9 months of training compared to untrained controls (CitationMattusch et al 2000). Greiwe and colleagues reported that following 6 months of resistance training, healthy older subjects had reduced muscle TNF-α, mRNA and protein levels whilst anabolism increased (CitationGreiwe et al 2001). Exercise in healthy subjects clearly confers anti-inflammatory benefits, but whether this also occurs in COPD, is at present unknown.
The purpose of this pilot study was to determine the cytokine response to a maximal walking test, and to determine whether there was any effect of a 7-week training and education programme on the response to fixed walking exercise.
Methods
Patients
Twenty patients with a known diagnosis of COPD were recruited through primary and secondary referral processes. COPD was confirmed with spirometry values consistent with obstructive airways disease (CitationThe COPD guidelines group of the standards of care committee of the british thoracic society 1999). Seventeen (10 males, 7 females) COPD patients (aged 55–82 years) with mild to very severe COPD (FEV1 94%–27% predicted) according to GOLD criteria (CitationBuist et al 2006), attended for an initial assessment. Six patients were present smokers, 10 patients were ex-smokers and 1 patient was described as a non-smoker, mean (SD) pack years 48.1 (17.8). Twelve (6 male, 6 female) COPD patients (aged 62–82 years) with mild to very severe COPD (FEV1 91%–22% predicted) according to GOLD criteria (CitationBuist et al 2006) completed pulmonary rehabilitation and the testing protocol. 3 patients were still smoking, 8 were ex-smokers, and 1 patient was described as a non-smoker. Exclusion criteria consisted of exacerbation necessitating any change in medication or significant worsening of symptoms within the 6 weeks prior to the first assessment, unstable angina or known cardiac disease, mobility limiting joint problems, known neurological disease and other inflammatory conditions such as rheumatoid arthritis. Medication was unaltered; no patients were receiving oral steroids at the time of the study. Full ethical approval was received from Merton & Sutton/Wandsworth COREC and patients gave informed written consent.
Primary outcomes
Exercise capacity was assessed using the incremental shuttle walk test (ISWT) which is a maximal, standardised, externally paced incremental exercise test (CitationSingh et al 1992). The test involves the patient walking between two cones placed 9 meters apart (hence a distance of 10 m) at a speed externally paced via the use of a tape recording. The speed of shuttles increases each minute until the patient can no longer match the pace or reaches a symptom limiting maximum. The instructions are standardised and delivered via the tape recording. One practice walk was performed a week prior to the ISWT. For safety reasons percutaneous oxygen saturation (SpO2) and heart rate (HR) were monitored throughout the test and patients were stopped if blood oxygen saturation fell to less than 80% or predicted HR max was reached.
Acute exercise induced cytokine response
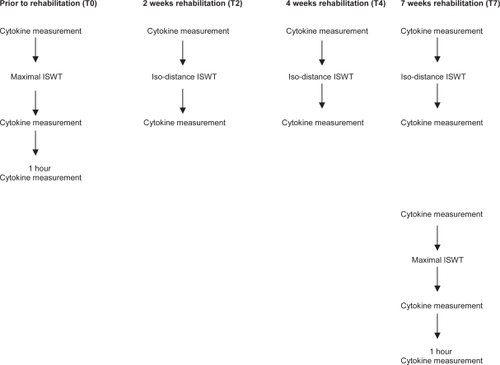
The acute exercise induced cytokine response was measured from venous blood collected from the cubital veins before ISWT and immediately post ISWT (T0). A further blood sample was taken after patients rested for 1 hour. Patients then entered a 7 week pulmonary rehabilitation programme. Pre and post ISWT blood tests were repeated using the isodistance measurements of ISWT (patients were instructed to stop at the level achieved at the initial assessment ISWT) at, 2 weeks (T2) and 4 weeks (T4) into the training period and at 7 weeks (T7), the end of the rehabilitation period.
After rehabilitation (T7) two walking tests were performed 2 days apart: a symptom-limited maximal ISWT and an iso-distance measurement based on the results of the initial walking test. The acute cytokine response was determined for both these walks. At T0 and T7 patients were asked to rest after the walking tests for 1 hour and bloods were taken again to evaluate further post-exercise responses (Figure ).
The rehabilitation programme
Patients attended outpatient pulmonary rehabilitation, which consisted of exercise and education sessions in accordance with BTS guidelines (CitationMorgan et al 2001), twice weekly for 7 weeks. If sessions were missed, additional attendance was organized. In addition to this, patients were required to complete 20 minutes of home exercise each day, five times a week. The exercise programme has been previously described (CitationGarrod et al 2004). Each rehabilitation session comprised one hour of exercise followed by an education session. Exercises were a mixture of functional aerobic and resistance activities-walking and cycling, sit to stands, step-ups and upper limb work.
Cytokine measurements
C-reactive protein (CRP) Interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) were determined from venous blood at the time points described above. Plasma was frozen at −80 °C within an hour of sampling. High sensitivity CRP, IL-6 and TNF-α were all measured using commercially available assays (Euro/DPC Ltd, Gwynedd, UK) and performed on an Immulite® automated analyser (Euro/DPC Ltd). The functional sensitivities of each of the assays were 0.2 mg/L−1, 2.0 pg/mL−1, and 1.7 pg/mL−1 for CRP, IL-6 and TNF-α respectively. The manufacturer claimed within within-assay and between-assay precisions to be less than 8% at the concentrations reported here for all three assays.
Spirometry
Spirometry was performed using a hand held Spirometer (Microloop ML 3535, Micromedical Ltd.,). The best of 3 attempts was taken and all researchers were trained in the use of spirometry according to American Thoracic Society guidelines (CitationThe COPD guidelines group of the standards of care committee of the British Thoracic Society 1999). Verbal encouragement was standardized for all tests.
Body composition
Percentage body fat was determined using bioelectrical-impedance methods with Tanita scales (Body Composition Analyzer, BC-418MA, Tanita, UK Ltd, The Barn, Philpots Close, Yiewsley, West Drayton, Middlesex, England, UB77RY). Fat mass was calculated using body fat percentage and body weight; fat free mass was calculated by subtracting fat mass from body weight. FFMI was calculated by FM in kg/(height in m2) and nutritional depletion was defined as BMI ≤21 kg.m2 and/or FFMI ≤15 (females) or ≤16 (males) kg.m2 (CitationSchols et al 1993).
Quadriceps muscle strength
Quadriceps muscle strength was tested using a hand held Myometer (SH-5001, Physio-Med Services, Glossop Brook Business Park, Surrey Street, Glossop, Derby, SK13 7AJ) with the patient seated and knee at 90° flexion.
Health status
Health status was assessed using the St. George’s Respiratory Questionnaire (SGRQ) (CitationJones et al 1992). This 50 item, disease-specific, self-complete questionnaire has been validated to measure health impairment in respiratory patients. Scores range from 100 ‘worst possible health status’ to 0 ‘best possible health status.’
Statistical analysis
Pre-exercise TNF-α data at T4 from 1 subject was removed as this subject had an abnormally high result, which was significantly lower at all other time points. The acute exercise induced cytokine response to a fixed bout of exercise (iso-ISWT) throughout the pulmonary rehabilitation programme was calculated by: rest cytokine value-post walk value for T0, T2, T4, T7 and analysis of variance (ANOVA) was used to identify change in this response. These data were normally distributed. Paired t tests were used to identify change in cytokine response to the maximal walking test before and after pulmonary rehabilitation. To identify differences in the plasma level of CRP, IL-6 and TNF- at rest, post and 1 hour post-maximal ISWT at T0 and T7, Friedman’s test was used (cytokine data was not normally distributed). Heterogeneity in the acute cytokine response at T0 was explored according to the acute cytokine response. Baseline characteristics for those that dropped out of rehabilitation were compared with those that completed using Mann Whitney un-paired test.
Results
Patient details
Baseline characteristics (Table ) of the patients who completed rehabilitation (n = 12) compared to those who did not (n = 5) were not significantly different apart from forced vital capacity (FVC) mean difference (95% CI) 1.7 (0.43 to 3.04)l and shuttle walk distance mean difference (95% CI) 197.8 (77.7 to 318.0)m. One female patient suffered from fat free mass depletion (FFMI 13.8kg.m2) and nutritional depletion (BMI 14.5 kg.m2). This female had a reduced CRP and IL-6 concentration pre and post exercise at T0 compared with the non-depleted group value (median (range) CRP pre: 0.82 vs 3.88 (12.34); CRP post: 0.51 vs 4.2 (13.0); IL-6 pre: 2.1 vs 2.3 (5.0); IL-6 post: 2.1 vs 3.2 (4.3)), whilst at T0, TNF-α pre exercise was similar but post exercise higher (TNF- α pre: 15.0 vs 6.8 (43.2); TNF-α post: 33.0 vs 6.8 (43.2)).
Table 1 Patient characteristics
Effects of rehabilitation
As expected there was a statistically significant effect of rehabilitation on maximal ISWT, mean difference (95% CI) 37.5 (10.4 to 64.6) m, although it is not clear how much of an improvement in distance is needed to be clinically significant (CitationThe Australian lung foundation and Australian physiotherapy association 2006). However, Quadriceps strength did not change significantly over time (p = 0.24) n/kg, nor did SGRQ Scores (p = 0.43).
Iso-distance cytokine response
Table shows the exercise induced cytokine response at equivalent work load (iso time ISWT) over 7 week rehabilitation period (n = 12). There were no significant differences over time. 8 patients were able to complete iso time ISWT’s over the 7 week training period, whilst 3 patients were not able to complete this distance at all times: patient 7 had a reduction of 20 m at 2 and 4 weeks; patient ‘s10 and 11 had a reduction of 20 m at week 7.
Table 2 Exercise induced cytokine response at equivalent work load (iso distance ISWT) over 7 week rehabilitation period (n = 12)
Maximal distance cytokine response
There was no significant difference in the acute cytokine response to a maximal bout of walking pre or post rehabilitation in these patients (Table ).
Table 3 Acute exercise induced cytokine response (before and after bout of walking) pre and post 7 week rehabilitation period
One hour post
At the one hour time point after the maximal exercise test, before and upon completion of rehabilitation, there was no significant difference in CRP, IL-6 or TNF-α (data not shown).
Heterogeneity in acute cytokine response
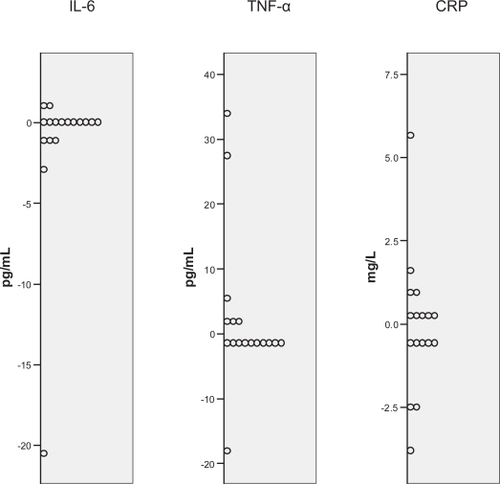
There was heterogeneity in the acute cytokine response to a maximal bout of walking between subjects at baseline (Figure ).
Dropouts compared with those that completed rehabilitation
Those that dropped out of rehabilitation demonstrated a significantly higher walking distance, mean difference (SD); 197.8(56.3) m (p = <0.05) and greater FVC; 1.7 (0.6) l (p = 0.01) at baseline.
Discussion
The main findings of this study strongly support the view that in response to a maximal bout of walking in COPD patients there is no significant acute cytokine response demonstrated. Although, in this pilot study our sample size is small, we note the large standard deviations for cytokine response and consistency of our results. This suggests that in this population of COPD patients, changes in cytokine levels as a result of maximal walking are likely to be small if present at all. This novel study design enabled us to assess possible change in cytokine response to iso-distance exercise throughout the training period. There was no modification to cytokine levels (or acute changes) over the 7-week period of rehabilitation. There was however, heterogeneity in the exercise induced cytokine response between subjects. A number of factors are known to influence the acute exercise induced cytokine response including body mass composition (CitationSchols et al 1996), age, gender (CitationTimmons et al 2006), carbohydrate load (CitationNieman et al 2005) and timing of exercise in relation to previous bouts of activity (CitationLi and Gleeson 2004). Unfortunately in this study we did not standardise for caffeine and carbohydrate ingestion prior to testing. We did however record body composition prior to the testing procedure but there was no significant difference in cytokine release according to percentage body fat. In one female patient, with lean mass and nutritional depletion, TNF-α release after the initial exercise bout was high, compared with the rest of the group who were not depleted. This finding is similar to that of other authors suggesting that muscle status may be an important determinant in the inflammatory response to exercise (CitationRabinovich et al 2003; Citationvan Helvoort et al 2005; Citationvan Helvoort et al 2006).
Detailed characterisation of patients prior to these evaluations will be necessary in order to enable us to eventually tease out possible influences on cytokine exercise responses.
In accordance with us, Rabinovich and colleagues recently showed that IL-6 levels remained unchanged in both 6 healthy subjects and 11 COPD patients after exercise (CitationRabinovich et al 2003).In this study steady state low intensity exercise (40% of maximal) was chosen as the stimulus and sustained for 11 minutes. However, interestingly, TNF-α levels increased significantly from baseline in the COPD patients, even persisting after 8 weeks training, but did not in the healthy controls (CitationRabinovich et al 2003). We had hypothesised, that as in healthy subjects, IL-6 would prove to attenuate exercise induced TNF-α levels (CitationStarkie et al 2003). However, we found little evidence of this in our study. Body composition was not assessed by Rabinovich and colleagues making comparison with their study difficult. It is feasible that in their study the population were depleted (CitationRabinovich et al 2003).
Van Helvoort in contrast, tested 16 COPD subjects with maximal incremental cycle ergometry and although circulating lymphocytes were significantly higher after exercise, there were no changes in CRP; whilst IL-6 and TNF-α were not measured (Citationvan Helvoort et al 2005). At present we cannot tell whether the exercise induced cytokine response in COPD differs from that of healthy subjects or whether it is merely a reflection of the fact that by necessity exercise intensities are considerably lower. A criticism of our study is that, unlike these previous studies, we did not include healthy control subjects; we were however predominantly interested in any modifying effects of rehabilitation in this group rather than comparisons.
These data support the now well established view that systemic inflammation is higher in patients with COPD (CitationGan et al 2004) particularly in muscle-wasted COPD patients, and that acute maximal exercise in non-muscle wasted COPD patients does not cause pronounced cytokine release (Citationvan Helvoort et al 2006). There is strong evidence in healthy subjects that CRP levels of higher that 3 mg/L−1 are highly associated with cardiovascular mortality and morbidity (CitationTorres and Ridker 2003). Our hypothesis, that pulmonary rehabilitation may modify pro-inflammatory mediators, as long term training in healthy subjects does (CitationJankord 2004), has not been demonstrated in this small pilot study. Five out of our 17 patients were unable to complete rehabilitation, (4 patients suffered an exacerbation and 1 refused after initial assessment). Our patients who dropped out of rehabilitation were able to walk significantly further than those who completed rehabilitation. This limits our study findings since these may be fitter patients who might have been expected to train at a higher intensity and endurance and therefore show a modifying effect of exercise on inflammatory markers. Thus before we conclude a lack of effect of training on inflammation in COPD subjects, further trials would do well to look at longer term training, possibly with increased resistive exercise (CitationGreiwe et al 2001) in patents with mild disease and in larger samples. In conclusion, this study is reassuring in that clinical pulmonary rehabilitation is unlikely to enhance systemic inflammation in non-muscle wasted COPD patients.
Acknowledgements
We gratefully acknowledge the financial support of St. George’s NHS Therapies Research Fund in the development of this work.
References
- BroekhuizenRWoutersEFCreutzbergEC2006Raised CRP levels mark metabolic and functional impairment in advanced COPDThorax61172216055618
- BuistASAnzuetoACalverleyP2006Global initiative for chronic obstructive lung disease Globabl strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global inititative for chronic obstructive disease (GOLD) report
- DebigareRCoteCHMaltaisF2001Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanismsAm J Respir Crit Care Med16417121711719314
- GanWQManSFSenthilselvanA2004Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysisThorax595748015223864
- GarrodRFordKDalyC2004Pulmonary rehabilitation: analysis of a clinical servicePhysiother Res Int91112015560668
- GreiweJSChengBORubinDC2001Resistance exercise decreases skeletal muscle tumor necrosis factor {alpha} in frail elderly humansThe FASEB Journal154758211156963
- JankordR2004Influence of physical activity on serum IL-6 and IL- 10 levels in healthy older menMedicine and science in sports and exercise365
- JonesPWQuirkFHBaveystockCM1992A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory QuestionnaireAm Rev Respir Dis145132171595997
- LacasseYBrosseauLMilneS2002Pulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Sys Rev3CD003793
- LiT-LGleesonM2004The effect of single and repeated bouts of prolonged cycling on leukocyte redistribution, neutrophil degranulation, IL-6, and plasma stress hormone responsesInternational Journal of Sport Nutrition & Exercise Metabolism145011615673097
- MorganMCalverleyPClarkC2001British Thoracic Society Statement on Pulmonary RehabilitationThorax568273411641505
- MattuschFDufauxBHeineO2000Reduction of the plasma concentration of C-reactive protein following nine months of endurance trainingInt J Sports Med2121410683094
- NiemanDCDavisJMHensonDA2005Muscle cytokine mRNA changes after 2.5 h of cycling: Influence of carbohydrateMedicine & Science in Sports & Exercise3712839016118573
- PetersenAMPedersenBK2005The anti-inflammatory effect of exerciseJ Appl Physiol9811546215772055
- RabinovichRFiguerasMArditeE2003Increased tumour necrosis factor alpha plasma levels during moderate-intensity exercise in COPD patientsEuropean Respiratory Journal217899412765422
- ScholsAMSoetersPBDingemansAMMostertRFrantzenPJWoutersEF1993Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitationAm Rev Respir Dis147115168484624
- ScholsAMBuurmanWAStaal van den BrekelAJ1996Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary diseaseThorax51819248795671
- SinghSJMorganMDScottS1992Development of a shuttle walking test of disability in patients with chronic airways obstructionThorax471019241494764
- StarkieROstrowskiSRJauffredS2003Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humansThe FASEB Journal17884612626436
- The Australian lung foundation and Australian physiotherapy association Accessed 20th January 2007. URL: http://www.pulmonaryrehab.com.au/index.asp?page=20
- The COPD guidelines group of the standards of care committee of the british thoracic society1999BTS guidelines for the management of chronic obstructive pulmonary diseaseThorax52S128
- TimmonsBWTarnopolskyMASniderDP2006Immunological changes in response to exercise: Influence of age, puberty, and genderMedicine & Science in Sports & Exercise3829330416531898
- TirakitsoontornPNussbaumEMoserC2001Fitness, acute exercise, and anabolic and catabolic mediators in cystic fibrosisAm J Respir Crit Care Med1641432711704591
- TorresJLRidkerPM2003Clinical use of high sensitivity C-reactive protein for the prediction of adverse cardiovascular eventsCurr Opin Cardiol18471814597888
- van HelvoortHAvan de PolMHHeijdraYF2005Systemic inflammatory response to exhaustive exercise in patients with chronic obstructive pulmonary diseaseRespir Med9915556715890510
- Van HelvoortHAHeijdraYFThijsHM2006Exercise-induced systemic effects in muscle-wasted patients with COPDMed Sci Sports Exerc3815435216960513