Abstract
COPD is underdiagnosed and its early assessment is problematic. It has been suggested that symptomatic smokers with normal FEV1/FVC (Stage 0 COPD, GOLD criteria) can develop COPD in the future. Potential early biomarkers in COPD include the matrix metallo-proteinases (MMPs). It is not yet known, whether alterations in MMP expression are associated with smoking alone or with the risk of developing COPD. In this cross-sectional study MMP-8, MMP-9 and MMP-12 were determined from induced sputum and plasma by ELISA, immunocytochemistry, zymography, and/or Western blot in non-smokers (n = 32), smokers with symptoms (Stage 0, GOLD criteria) (n = 23) or without symptoms (n = 23). Only MMP-8 differentiated Stage 0 COPD from non-symptomatic smokers (p = 0.02). MMP-9 levels were significantly elevated in the induced sputum of non-symptomatic smokers and Stage 0 COPD (p = 0.01, p < 0.001) compared to non-smokers, but did not differ between the two subgroups of smokers. MMP-12 was higher only at Stage 0 compared to non-smokers (p = 0.04). MMP-8, MMP-9 and MMP-12 immunoreactivity was localized in macrophages and neutrophils, especially in smokers. MMP-8 levels correlated significantly with the small airway flow parameters (MEF50, MEF25) (p = 0.005 and p = 0.0004) and markers of neutrophil activation (myeloperoxidase, lactoferrin). In conclusion MMP-8 may differentiate Stage 0 from healthy smokers.
Introduction
Chronic obstructive pulmonary disease (COPD) is leading cause of death worldwide, it also causes significant morbidity and disability (CitationCalverley and Walker 2003). COPD is underdiagnosed and its early assessment is problematic. Even the early stages of the disease with normal lung function parameters (FEV/FVC >0.7) possess inflammatory changes and structural abnormalities in the airways and lung parenchyma (CitationHogg et al 2004). The current international COPD classification, GOLD criteria (CitationPauwels et al 2001), takes these problems into consideration since there may be an apparent risk for COPD development in symptomatic smokers who have normal lung function parameters. The GOLD classification categorizes symptomatic subjects to have GOLD Stage 0 COPD. The usefulness of Stage 0 in predicting COPD development is still unclear (CitationVestbo and Lange 2002). However, recent studies do indicate that Stage 0 has importance, at least in predicting long-term mortality (CitationEkberg-Aronsson et al 2005; CitationMannino 2006; CitationStavem et al 2006). At present no biomarker has been found to differentiate these at-risk individuals from healthy cigarette smokers. Potential markers not only need to be evaluated in terms of disease development but they may also be implemented in smoking cessation protocols.
Biomarkers of tissue damage/remodeling in COPD include matrix metalloproteinases (MMPs) (CitationSaetta et al 2001; CitationShapiro 2002; CitationBarnes 2004). MMPs are activated by many different factors including cigarette smoke and oxidative stress (CitationRajagopalan et al 1996; CitationShapiro 2002; CitationNelson and Melendez 2004; CitationKinnula 2005; CitationKinnula et al 2005; CitationRahman and Adcock 2006). Markers of oxidative stress have been detected not only in COPD but also in cigarette smokers and subjects with Stage 0 COPD (CitationRytila et al 2006).
Several MMPs including MMP-1, MMP-2, MMP-8, MMP-9 and MMP-12 are elevated both in experimental emphysema and human COPD, especially MMP-8, MMP-9 and MMP-12 have been associated with COPD (CitationHautamaki et al 1997; CitationBeeh et al 2003; CitationVernooy et al 2004; CitationCulpitt et al 2005; CitationDemedts et al 2006; CitationElkington and Friedland 2006). The levels of these MMPs and TIMP-1 (the major endogenous inhibitor of MMP-8 and MMP-9) have not been earlier compared in non-smokers, non-symptomatic and symptomatic smokers (Stage 0 COPD). Knowing the effects of cigarette smoking on many signaling cascades, accumulation and activation of the inflammatory cells, and increased oxidative stress, it is likely that there is some increase/activation of MMPs in chronic smokers without airway flow limitation.
Induced sputum is a validated non-invasive method for the assessment of airway/tissue inflammation/injury in COPD (CitationDjukanovic et al 2002). MMP-8, MMP-9, MMP-12 and TIMP-1 (a major endogenous inhibitor of MMP-8 and MMP-9) were investigated in induced sputum and plasma samples of healthy non-symptomatic smokers with normal lung function parameters by GOLD criteria and patients with GOLD Stage 0. MMP levels were also correlated with the levels of induced sputum neutrophils and macrophages, lactoferrin, myeloperoxidase (MPO) and nitrotyrosine to further characterize the association between these MMPs and the oxidative stress and inflammatory profile of the lung.
Materials and methods
Subjects
This cross-sectional study population included three groups: non-smoking healthy controls (NS) (n = 32), non-symptomatic smokers (S) (n = 23, mean 24 pack-years) and symptomatic smokers (chronic symptoms such as cough and phlegm, but no airway obstruction), ie, GOLD stage 0 (n = 23, mean 37 pack-years) (Table ).
Table 1 Patient characteristics
Of the non-smoking subjects, 20 were never-smokers and 12 ex-smokers (mean 15.7 pack-years) who had stopped smoking at least 20 years earlier. For comparison, MMPs and TIMP-1 were also analyzed from stable COPD patients (Stage I–III) (n = 10, mean 47 pack-years), (FEV1 62% of predicted, DLCO/VA 66% of predicted). The staging was based on clinical symptoms assessed with the St George’ s Respiratory Questionnaire, and airflow parameters on spirometry, according to the GOLD classification (CitationAmerican Thoracic Society (ATS) 1995; CitationPauwels et al 2001). Three GOLD stage 0 patients had been prescribed β2-agonists and inhaled corticosteroid therapy. The medications of the COPD Stage I–III patients were as follows: 4 patients received β2-agonists, inhaled corticosteroid and anticholinergics; 4 patients received β2-agonists plus inhaled corticosteroid; 1 received β2-agonists and anticholinergics. None were receiving systemic corticosteroid therapy.
Exclusion criteria included allergies, asthma, a history of respiratory disease other than COPD, or respiratory infection less than 8 weeks before entering the study. The study was approved by the Ethics Committee of Helsinki University Hospital, Helsinki, Finland with written consent and registered (http://www.hus.fi/clinicaltrials).
Pulmonary function tests
Flow-volume spirometry was performed with a pneumotachograph based spirometer connected to a computer (Medriko M 904, Kuopio, Finland) (CitationViljanen 1982). The pulmonary diffusing capacity for carbon monoxide (DLCO) and static lung volumes were measured with a single breath method according to the European Respiratory Society.
Sputum induction
Sputum was induced by inhalation of hypertonic saline as recommended by the ERS Task Force and processed for differential counts of inflammatory cells as previously described (CitationRytila et al 2000; CitationDjukanovic et al 2002). Briefly, samples were treated by adding an equal volume, based on the weight of the sample, of dithioerythritol (DTE, Sigma, Germany) or phosphate-buffered-saline (PBS). The supernatant was frozen at −80 ºC for biochemical and immunological analyses. Cell viability was assessed by Trypan blue in a Burker chamber. Cytocentrifuge slides were prepared by Cytospin at 450 rpm for 6 min. One slide from each patient was stained with May-Grunwald-Giemsa-staining (Merck, Germany) for cell differential counts. A total of 400 cells were counted from each slide. The samples having less than 70% of squamous epithelial cells were accepted for further investigations. The slides were frozen at −20 ºC.
MMP-8, MMP-9, MMP-12, TIMP-1, and lactoferrin by ELISA
MMP-8, MMP-9, TIMP-1 and lactoferrin levels in sputum supernatant and plasma samples were determined by commercially available ELISA kits (Amersham Biosciences, Cardiff, UK) according to the manufacturers’ instructions. MMP-12 was measured in induced sputum and plasma samples by a custom-made ELISA, as previously described (CitationDemedts et al 2006). The detection limits were: 0.032 ng/ml for MMP-8, 0.6 ng/ml for MMP-9, 0.05 ng/ml for MMP-12, 1.25 ng/ml for TIMP-1 and 1.6 ng/ml for lactoferrin.
Immunocytochemical staining of the MMPs, TIMP, MPO and nitrotyrosine
MMP-8, MMP-9, MMP-12, TIMP-1, MPO and nitrotyrosine were also assessed by immunocytochemistry. The cytospin samples were treated with Ortho Permeafix (Ortho Diagnostic Systems Inc., UK). The endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in PBS and for immunostaining, Zymed ABC Histostain-Plus Kit was used according to the manufacturers’ protocol. The samples were incubated with polyclonal rabbit anti-human MMP-8 (CitationHanemaaijer et al 1997), MMP-9 (NeoMarkers, Fremont, CA), TIMP-1 (Chemicon, Temecula, CA, USA), MPO (LabVision Corp., Fremont, US) or nitrotyrosine (Upstate Lake Placid, NY, USA) or monoclonal MMP-12 (R&D Systems Inc., Minneapolis, US) antibody, and negative control samples with Zymed Rabbit or Mouse (MMP-12) isotype Control and PBS overnight at 4 ºC, and stained with AEC (Zymed Laboratories Inc., South San Francisco, CA) and thereafter with Mayer’s hematoxylin. MMP-8-, MMP-9-, MMP-12-, TIMP-1-, MPO- and nitrotyrosine positive and negative cells were counted (400 cells/cytospin).
MMP-9 by zymography and Western blot analysis
Sputum supernatant (60 μg protein) was incubated with Laemmli’s sample running buffer. After electrophoresis on 8% sodium dodecyl sulphate-polyacrylamide gels (SDS SDSPAGE) containing 1 mg/ml gelatine (Sigma, St. Louis, MO, USA), as a substrate, the gels were processed and stained with 0.1% Coomassie Brilliant Blue R250 as described (CitationSorsa et al 1997) for the detection of 92 kD MMP-9 (gelatinase B).
For the Western blot analysis, sputum supernatants were lyophilized (total protein content 40 μg per well in 15 μL volume). After 12% SDS-PAGE, the loaded samples were electrotransferred to nitrocellulose membranes (Schleicher & Shuell, Dassel, Germany). The membranes were first incubated with polyclonal rabbit anti-human MMP-9 (Calbiochem, Darmstadt, Germany) and after washing with 0.1% TTBS, treated with the secondary antibody (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA). The membranes were developed and quantified for MMP-9 by the Bio-Rad Model GS-700 Imaging Densitometer and AnalystTM program (CitationSorsa et al 1997).
Statistical analysis
All statistical analyses were performed with the SPSS 10.0 software program (SPSS Inc., Chicago, IL, USA). As the data were not normally distributed, non-parametric tests were used for all comparisons. Data for individual variables from the several groups were first analyzed by the Kruskal-Wallis test followed by the Mann-Whitney U-test. Correlations between variables were determined with the Spearman rank correlation coefficient. A p-value of <0.05 was considered significant.
Results
Lung function characteristics of non-smokers, healthy smokers and subjects with Stage 0 COPD
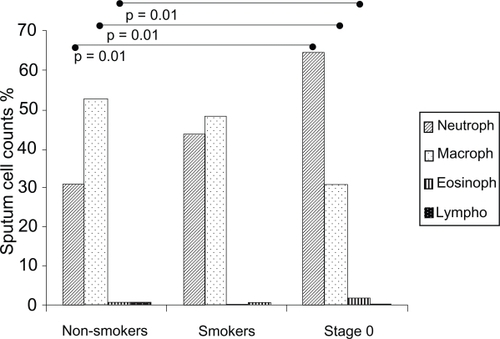
Lung function characteristics of the subjects are shown in Table . All the subjects had normal airway function according to GOLD criteria (post bronchodilator FEV1/FVC >0.7). The cell profile of the induced sputum specimens in Figure reveals the greater percentage of neutrophils and the lower percentage of macrophages in symptomatic smokers (Stage 0) than in non-smokers. Asymptomatic smokers and Stage 0 exhibited a higher total number of neutrophils (p = 0.005 and p = 0.001) than non-smokers.
The levels of MMPs and TIMP-1 in the induced sputum and plasma samples by ELISA
Subjects with Stage 0 COPD had higher sputum MMP-8 levels than the non-symptomatic smokers and non-smokers (p = 0.02 and p < 0.0001, Figure ). MMP-9 was higher in Stage 0 and non-symptomatic smokers than in non-smokers (p < 0.0001 and p = 0.01), but no significant difference existed between non-symptomatic smokers and those at Stage 0 (p = 0.062, Figure ). Stage 0 patients had higher MMP-12 than non-smokers (p = 0.04), but the difference between nonsymptomatic smokers and Stage 0 patients was not significant (p = 0.14, Figure ) There were no differences in TIMP-1 levels between the subgroups (Figure ).
Figure 2 MMP-8 (A), MMP-9 (B), MMP-12 (C) and TIMP-1 (D) levels by ELISA in the induced sputum from non-smokers, asymptomatic smokers and GOLD Stage 0. Mean values are shown with horizontal bars. The levels of MMP-8 were increased in Stage 0 when compared with asymptomatic smokers (p = 0.02) and non-smokers (p < 0.0001). The levels of MMP-9 were higher in Stage 0 (p < 0.0001) and asymptomatic smokers (p = 0.01) than in non-smokers. The MMP-12 levels were higher in Stage 0 than in non-smokers (p = 0.04) and there were no significant differences in TIMP-1 levels between the groups.
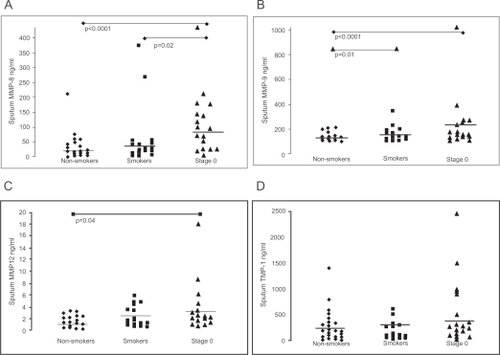
To confirm earlier findings on the elevation of MMP-8 and MMP-9 in COPD, their levels were also determined by ELISA in the induced sputum of Stage I–III COPD patients (n = 10). MMP-8 and -9 were significantly elevated in COPD compared to healthy smokers (MMP-8: p < 0.0001; MMP-9: p = 0.01). We also recently found this to be the case with MMP-12 (CitationDemedts et al 2006).
Plasma MMP-8 levels did not differ between the subgroups (p = 0.225). Plasma MMP-9 concentrations of smokers were higher than those of non-smokers (p = 0.04), but the difference between smokers without or with symptoms (Stage 0) was not significant (p = 0.62). Plasma MMP-12 levels were significantly higher in Stage 0 patients when compared to the non-symptomatic smokers and non-smokers (p = 0.008). Plasma TIMP-1 levels between the subgroups did not differ.
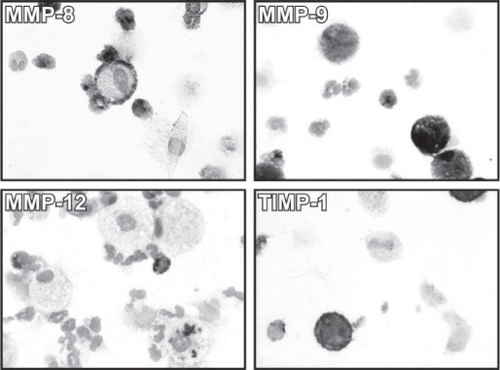
Localisation of MMPs and TIMP-1 in the alveolar macrophages and neutrophils
MMP-8, MMP-9 and TIMP-1 were localized in the neutrophils and macrophages. MMP-12 was mainly detected in the macrophages but could also be found in some neutrophils (). Some ciliated epithelial cells exhibited weak MMP-8 and TIMP-1 immunoreactivity. Generally, the reactivity was similar in healthy smokers and those at Stage 0, whereas the expression in non-smokers was very weak (data not shown).
Figure 3 Immunocytochemistry for MMP-8 (A), MMP-9 (B), MMP-12 (C) and TIMP-1 (D) on the cytospins of induced sputum cells from a GOLD Stage 0 smokers. Clear immunoreactivity for each MMP was detectable in macrophages and neutrophils from all patient groups, especially from smokers and those at GOLD Stage 0.
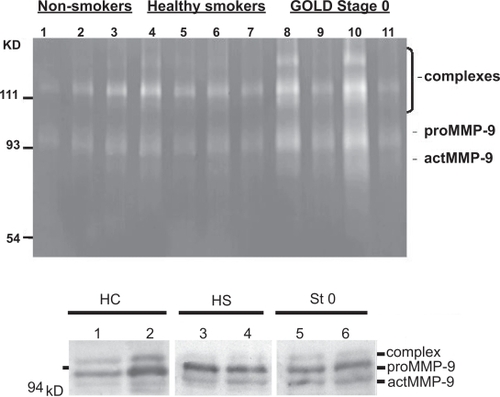
Detection of active MMP-9 by zymography and Western blotting
ELISA detected similar MMP-9 levels in healthy smokers and Stage 0 with individual variation. Therefore MMP-9 was also evaluated by zymography and Western blotting. However, even these methods exhibited extensive variability. Zymography revealed MMP-9 gelatinolytic activity (92 kD pro-MMP-9 and 77–82 kD active MMP-9) both in healthy smokers and those at Stage 0 with no major difference between the groups (Figure ). MMP-9 activation has been assessed also from the bands obtained from Western blot analysis (CitationSorsa et al 1997). The present results by densitometry were, however, variable (14% increase in the suggested MMP-9 active form in smokers compared to the non-smokers, n = 16) (n.s.) (Figure ).
Figure 4 Zymography and Western blotting analysis of MMP-9 in the sputum specimens. Representative zymography of 11 specimens (A): Gelatinolytic activity at 92 kD (pro-MMP-9) and 77–82 kD (activated MMP-9) of non-smoking controls (lanes 1–3), cigarette smokers (lanes 4–7), and subjects at GOLD stage 0 (lanes 8–11). Individual variation occurred, but generally MMP-9 was activated in smokers and especially those at GOLD Stage 0. Representative Western blotting of 6 specimens (B): HC healthy non-smoking control (lanes 1–2), HS healthy smoker (lanes 3–4), St 0 Stage 0 (lanes 5–6). There was individual variation, when calculated from 16 specimens no significant differences between the groups could be seen. Molecular weight standards at the left.
MMP/TIMP ratios in the sputum and plasma
No difference was found in the MMP-8/TIMP-1, MMP-9/TIMP-1 or MMP-12/TIMP-1 ratios in the induced sputum of Stage 0, smokers and non-smokers. In contrast, the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratio in plasma was substantially higher in healthy smokers when compared to non-smokers (p = 0.009 and p = 0.02). Patients with Stage 0 had a higher MMP-12/TIMP-1 ratio in plasma than non-symptomatic smokers and non-smokers (p = 0.04 and p = 0.005).
Correlation of MMPs and TIMP-1 with inflammatory cells and markers of oxidative stress
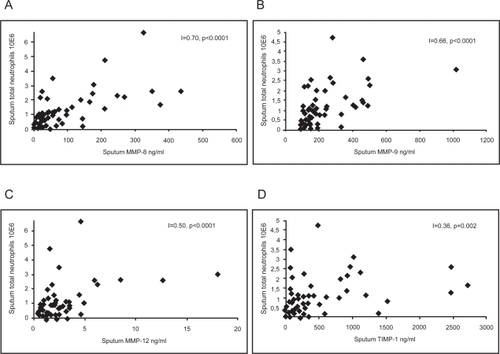
MMP-8, MMP-9, MMP-12 and TIMP-1 levels in the induced sputum, assessed by ELISA, generally correlated positively with the total number and percentage of neutrophils (MMP-8: p = 0.0001 and p = 0.0002; MMP-9: p < 0.0001 and p < 0.0001; MMP-12 p < 0.0001 and p = 0.129; TIMP-1: p = 0.002 and p = 0.004) (Figure ). In addition, MMP-8 and MMP-9 levels negatively correlated with the percentage of macrophages (p = 0.02 and p = 0.0003). MMP-12 levels correlated positively with the total number of macrophages (p = 0.008).
Figure 5 Correlation between sputum MMP-8 (A) (n = 70; r = 0.70; p < 0.0001), MMP-9 (B) (n = 69; r = 0.66; p < 0.0001), MMP-12 (C) (n = 56; r = 0.50; p < 0.0001), and TIMP-1 (D) (n = 68; r = 0.36; p = 0.002) levels and total number of neutrophils.
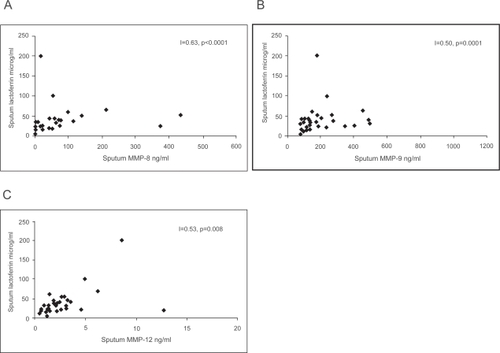
Mean lactoferrin levels in induced sputum of GOLD Stage 0, smokers and non-smokers were 59.7 ± 18.0, 36.3 ± 11.0 and 28.7 ± 3.42 μg/ml (p = 0.071) and in plasma 772 ± 121, 761 ± 155 and 772 ± 121 ng/ml (p = 0.616), respectively. MMP-8, MMP-9 and MMP-12 correlated with sputum lactoferrin (Figure ) and MMP-8 and MMP-9 correlated with the levels of MPO positive cells (r = 0.55, p = 0.004 and r = 0.49, p = 0.01) but not with nitrotyrosine.
Correlation between the levels of MMPs and lung function parameters
The purpose of this study was to evaluate the significance of MMPs at the early stages of COPD with normal lung function parameters based on GOLD criteria (FEV/FVC). Special interest was with the airflow parameters in the small airways (MEF50, MEF25). When the various MMPs were correlated with the lung function parameters of all smokers with or without COPD (GOL Stage 0) there was highly significant correlation with all lung function parameters (Table ).
Table 2 Correlation between the levels of sputum MMPs and lung spirometric variables
Discussion
The present study suggests that MMP-8 can differentiate symptomatic smokers (Stage 0), ie, those who may be at risk for COPD development (CitationPauwels et al 2001; CitationWillemse et al 2005; CitationMannino 2006; CitationStavem et al 2006) from non-symptomatic chronic smokers. MMP-8 also correlated with the lung function parameters, in particular the correlations with the small airway flow parameters were highly significant. Also MMP-9 and MMP-12 were already elevated in the induced sputum of Stage 0 COPD but neither MMP-9 nor MMP-12, with the exception of plasma MMP-12, appeared to differentiate healthy smokers from Stage 0 patients. MMPs can be activated by ROS, and this study also found a significant association especially between the levels of MMP-8 and MMP-9 and neutrophils and markers of their activation (lactoferrin, MPO). However, as in biological samples in general, there was considerable individual variation in the levels of MMPs and TIMP-1 in both the induced sputum and the plasma specimens.
One problem in assessing MMPs in cigarette smokers has been the wide variation in the smoking history and time elapsed from smoking cessation of the control group (CitationCataldo et al 2000; CitationKang et al 2003; CitationCulpitt et al 2005). We included a representative group of non-smokers and smokers, who were either symptom-free or exhibiting symptoms. Based on this study only MMP-8 could differentiate symptomatic smokers from healthy chronic smokers with normal FEV1/FVC ie, without major airway obstruction. The levels of MMP-8, MMP-9 and MMP-12 in the induced sputum of ex-smokers and lifelong non-smokers were similar suggesting that the effects of smoking on MMP-8, MMP-9 and MMP-12 levels may be reversible. Like in many biological measurements there was wide variation in MMP-values within the groups with some outliners in each group. These values and the clinical characteristics of the subjects were carefully re-evaluated. To confirm the elevation of these MMPs in COPD as earlier published (CitationVernooy et al 2004; CitationCulpitt et al 2005; CitationMercer et al 2005), additional measurements were conducted with the sputum specimens of Stage I–III COPD patients (CitationDemedts et al 2006), detecting significantly higher levels of these MMPs in COPD subjects in comparison to healthy smokers.
Some previous studies have shown MMP-9 elevation in chronic cigarette smokers when compared to non-smokers (CitationLim et al 2000; CitationKang et al 2003), but no comparisons were made between asymptomatic and symptomatic smokers. Our recent study found higher MMP-12 levels in the induced sputum of COPD patients when compared to smokers and non-smokers (CitationDemedts et al 2006), but again nonsymptomatic and symptomatic smokers were not compared. Our present results with MMP-8 and Stage 0 are in line with previous suggestions about the special role of MMP-8 in the development of emphysema (CitationSegura-Valdez et al 2000). Since MMP-9 levels as assessed by ELISA were very similar both in non-symptomatic smokers and individuals at Stage 0, its activation was further investigated by zymography and Western blot analysis which showed extensive interindividual variability. MMP-12 levels were higher in patients with Stage 0 than in non-smokers but there was no difference between healthy smokers and individuals at Stage 0. Also MMP-12 showed high variability in the Western blotting analysis (not shown). This does not signify that MMP-12 has no relevance in COPD development (CitationRussell et al 2002b; CitationDemedts et al 2006), but the fact that MMP-12 is already increased in smokers without airway obstruction.
MMP-8, MMP-9 and MMP-12 are known to be expressed in alveolar macrophages (CitationRussell et al 2002a; CitationVernooy et al 2004; CitationBracke et al 2005; CitationMolet et al 2005) and MMP-8 and MMP-9 in neutrophils (CitationCataldo et al 2001; CitationBarnes et al 2003; CitationBeeh et al 2003; CitationTakafuji et al 2003; CitationVernooy et al 2004; CitationCulpitt et al 2005; CitationGueders et al 2005). These previous observations were confirmed here and the expressions were similar in healthy and symptomatic smokers. Although MMP-12 is produced by macrophages, its presence or absence in neutrophils has not been reported (CitationChurg et al 2003; CitationBracke et al 2005; CitationMolet et al 2005). In the present study some neutrophils clearly exhibited MMP-12 immunoreactivity. The relative functional role of alveolar macrophages and neutrophils as cellular sources of these MMPs in COPD is still unclear. The present findings support the importance of neutrophils as all MMPs correlated with neutrophils and markers of neutrophil activation.
MMPs were also elevated in the plasma of smokers, in agreement with previous observations (CitationNakamura et al 1998) and with the fact that COPD is a systemic disease, but also with the fact that MMPs are associated with other smoking-related systemic diseases (CitationJones et al 2003; CitationMacCallum 2005; CitationWouters 2005). This is the first study to report the elevated plasma MMP-12 levels in symptomatic smokers, but the relative role of all of these MMPs as systemic markers in COPD progression remains to be clarified.
TIMP-1 is a major endogenous inhibitor of MMP-8 and -9 and shown to be elevated in COPD (CitationBeeh et al 2003; CitationOwen et al 2003; CitationCulpitt et al 2005; CitationHigashimoto et al 2005). The sputum MMP-8/TIMP-1 and MMP-9/TIMP-1 ratio did not change significantly in smokers, pointing to a role for TIMP-1 as a natural defense mechanism in preventing tissue destruction.
The conformation of latent MMPs is maintained by thiol interactions between Cys-residues in the prodomain and the zinc atom present in the catalytic site of all MMPs. Disruption of this interaction is thought to represent the critical step in initiating the process of MMP autoactivation. ROS are known to reversibly react with -SH groups, such as those involved in preserving MMP latency (CitationRajagopalan et al 1996). Recent studies from our laboratory and others have indicated that cigarette smoke can elevate the oxidant burden also in healthy humans without airway obstruction (CitationRytila et al 2006). The present study not only found increased levels of MMPs, especially MMP-8 in healthy smokers and those with symptoms (Stage 0), but also found a significant correlation between MMP-8, MMP-9 and the levels of MPO and lactoferrin, both of which are neutrophil-derived markers of oxidant generation. These findings further support the hypothesis that especially neutrophil derived oxidants, may significantly activate/induce the tissue destructive MMPs already in the lungs of healthy cigarette smokers. We did not find any association between MMPs and nitrotyrosine immunoreactivity showing also the complexity between smoking, oxidative stress and the expression of oxidized/nitrosylated proteins in sputum inflammatory cells.
In conclusion, MMPs, especially MMP-8, may function as sensitive biomarkers in COPD development. The relative role of various MMPs as markers of COPD and its progression remains to be clarified in future longitudinal investigations.
Acknowledgements
Tiina Marjomaa, Merja Luukkonen and Ritva Keva are acknowledged for their excellent technical assistance, Antero Kokkonen and the Jaakko Pöyry Company for helping us in the recruitment of the non-smokers and smokers. We are very grateful to every subject who agreed to participate in this study.
This work was supported by the Sigrid Juselius Foundation, The Finnish Antituberculosis Association Foundation, Jansson Foundation, the Helsinki University Hospital (HUCH-EVO), the Academy of Finland and the ERS COPD Travel Grant for Best Posters 2005.
References
- American Thoracic Society (ATS)1995Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med152S771217582322
- BarnesPJ2004Mediators of chronic obstructive pulmonary diseasePharmacol Rev565154815602009
- BarnesPJShapiroSDPauwelsRA2003Chronic obstructive pulmonary disease: molecular and cellular mechanismsEur Respir J226728814582923
- BeehKMBeierJKornmannO2003Sputum matrix metalloproteinase-9, tissue inhibitor of metalloprotinease-1, and their molar ratio in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and healthy subjectsRespir Med97634912814147
- BrackeKCataldoDMaesT2005Matrix metalloproteinase-12 and cathepsin D expression in pulmonary macrophages and dendritic cells of cigarette smoke-exposed miceInt Arch Allergy Immunol1381697916192742
- CalverleyPMWalkerP2003Chronic obstructive pulmonary diseaseLancet36210536114522537
- CataldoDMunautCNoelA2000MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary diseaseInt Arch Allergy Immunol1232596711112863
- CataldoDMunautCNoelA2001Matrix metalloproteinases and TIMP-1 production by peripheral blood granulocytes from COPD patients and asthmaticsAllergy561455111167375
- ChurgAWangRDTaiH2003Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha releaseAm J Respir Crit Care Med1671083912522030
- CulpittSVRogersDFTravesSL2005Sputum matrix metalloproteases: comparison between chronic obstructive pulmonary disease and asthmaRespir Med997031015878486
- DemedtsIKMorel-MonteroALebecqueS2006Elevated MMP-12 protein levels in induced sputum from patients with COPDThorax6119620116308335
- DjukanovicRSterkPJFahyJV2002Standardised methodology of sputum induction and processingEur Respir J Suppl371s2s12361359
- Ekberg-AronssonMPehrssonKNilssonJA2005Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitisRespir Res69816120227
- ElkingtonPTFriedlandJS2006Matrix metalloproteinases in destructive pulmonary pathologyThorax612596616227332
- GuedersMMBalbinMRocksN2005Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammationJ Immunol17525899716081833
- HanemaaijerRSorsaTKonttinenYT1997Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycyclineJ Biol Chem2723150499395486
- HautamakiRDKobayashiDKSeniorRM1997Requirement for macrophage elastase for cigarette smoke-induced emphysema in miceScience277200249302297
- HigashimotoYYamagataYIwataT2005Increased serum concentrations of tissue inhibitor of metalloproteinase-1 in COPD patientsEur Respir J258859015863647
- HoggJCChuFUtokaparchS2004The nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med35026455315215480
- JonesCBSaneDCHerringtonDM2003Matrix metalloproteinases: a review of their structure and role in acute coronary syndromeCardiovasc. Res598122314553821
- KangMJOhYMLeeJC2003Lung matrix metalloproteinase-9 correlates with cigarette smoking and obstruction of airflowJ Korean Med Sci18821714676438
- KinnulaVL2005Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseasesThorax6069370016061713
- KinnulaVLFattmanCLTanRJ2005Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapyAm J Respir Crit Care Med1724172215894605
- LimSRocheNOliverBG2000Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10Am J Respir Crit Care Med16213556011029344
- MacCallumPK2005Markers of hemostasis and systemic inflammation in heart disease and atherosclerosis in smokersProc Am Thorac Soc2344316113467
- ManninoDM2006GOLD Stage 0 COPD: Is it Real? Does it Matter?Chest1303091016899823
- MercerPFShuteJKBhowmikA2005MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbationRespir Res615116372907
- MoletSBelleguicCLenaH2005Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary diseaseInflamm Res5431615723202
- NakamuraTEbiharaIShimadaN1998Effect of cigarette smoking on plasma metalloproteinase-9 concentrationClin Chim Acta27617379764735
- NelsonKKMelendezJA2004Mitochondrial redox control of matrix metalloproteinasesFree Radic Biol Med377688415304253
- OwenCAHuZBarrickB2003Inducible expression of tissue inhibitor of metalloproteinases-resistant matrix metalloproteinase-9 on the cell surface of neutrophilsAm J Respir Cell Mol Biol292839412663332
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med16312567611316667
- RahmanIAdcockIM2006Oxidative stress and redox regulation of lung inflammation in COPDEur Respir J282194216816350
- RajagopalanSMengXPRamasamyS1996Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stabilityJ Clin Invest98257298958220
- RussellRECulpittSVDeMatosC2002aRelease and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol26602911970913
- RussellREThorleyACulpittSV2002bAlveolar macrophagemediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteasesAm J Physiol Lung Cell Mol Physiol283L8677312225964
- RytilaPRehnTIlumetsH2006Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPDRespir Res76916646959
- RytilaPHLindqvistAELaitinenLA2000Safety of sputum induction in chronic obstructive pulmonary diseaseEur Respir J1511161910885433
- SaettaMTuratoGMaestrelliP2001Cellular and structural bases of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1631304911371392
- Segura-ValdezLPardoAGaxiolaM2000Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPDChest1176849410712992
- ShapiroSD2002Proteinases in chronic obstructive pulmonary diseaseBiochem Soc Trans309810212023833
- SorsaTSaloTKoivunenE1997Activation of type IV procollagenases by human tumor-associated trypsin-2J Biol Chem27221067749261109
- StavemKSandvikLErikssenJ2006Can Global Initiative for Chronic Obstructive Lung Disease Stage 0 Provide Prognostic Information on Long-term Mortality in Men?Chest1303182516899828
- TakafujiSIshidaAMiyakuniY2003Matrix metalloproteinase-9 release from human leukocytesJ Investig Allergol Clin Immunol13505
- VernooyJHLindemanJHJacobsJA2004Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPDChest12618021015596677
- VestboJLangeP2002Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease?Am J Respir Crit Care Med1663293212153965
- ViljanenAA1982Reference values for spirometric, pulmonary diffusing capacity and body pletysmographic studiesScan J Clin Invest42suppl 159150
- WillemseBWten HackenNHRutgersB2005Association of current smoking with airway inflammation in chronic obstructive pulmonary disease and asymptomatic smokersRespir Res63815850494
- WoutersEF2005Local and systemic inflammation in chronic obstructive pulmonary diseaseProc Am Thorac Soc2263316113466