?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronic obstructive pulmonary disease (COPD) is a major global healthcare problem. Studies vary widely in the reported frequency of mechanical ventilation in acute exacerbations of COPD. Invasive intubation and mechanical ventilation may be associated with significant morbidity and mortality. A good understanding of the airway pathophysiology and lung mechanics in COPD is necessary to appropriately manage acute exacerbations and respiratory failure. The basic pathophysiology in COPD exacerbation is the critical expiratory airflow limitation with consequent dynamic hyperinflation. These changes lead to further derangement in ventilatory mechanics, muscle function and gas exchange which may result in respiratory failure. This review discusses the altered respiratory mechanics in COPD, ways to detect these changes in a ventilated patient and formulating ventilatory techniques to optimize management of respiratory failure due to exacerbation of COPD.
Background
Chronic obstructive pulmonary disease (COPD), is the fourth leading cause of mortality in the world and in the US (CitationWorld Health Report 1998). COPD is a progressive disease and is associated with increasing frequency and severity of exacerbations. Clinical manifestations of acute exacerbations are highly variable ranging from being a mild event requiring only outpatient treatment to being a life threatening episode needing mechanical ventilatory support. It is reasonable to assume that worsening airway inflammation is the primary inciting event of COPD exacerbations and may be caused by bacteria, viruses, or environmental pollutants, including cigarette smoke (CitationSoler 1998; CitationBhowmik 2000; CitationSethi 2000).
The fundamental physiologic abnormality in acute exacerbation of COPD is worsening of expiratory airflow limitation and consequent dynamic hyperinflation. Dynamic hyperinflation increases the work of breathing, puts the respiratory muscles at a disadvantage (CitationOrozco-Levi 2003), as they have to breathe at higher functional residual capacity and can cause significant cardiac dysfunction (CitationBrochard 1995) leading to worsening hypoxemia with varying degree of hypercarbia and acidosis. The ensuing tissue acidosis further impairs ventilatory muscle function leading to the downward spiral of ventilatory failure.
Mechanical ventilation, either invasive or non-invasive, is a life saving measure in managing acute respiratory failure due to an acute exacerbation of COPD. However mechanical ventilation can be associated with a significant morbidity and mortality. A good understanding of the underlying pathophysiologic mechanisms in acute exacerbation of COPD is very important in optimizing ventilatory strategies. Most modern ventilators are programmed to display important waveforms like flow, pressure, volume, flow-volume loops, pressure volume loops and are capable of performing several maneuvers to detect changes in respiratory function and mechanics. These waveforms and maneuvers are very important tools which can be used in detecting dynamic hyperinflation, diagnosing complications before overt clinical signs develop and promote patient-ventilator synchrony.
Noninvasive versus invasive ventilation
Various methods of ventilatory support are available for the compromised patient including the traditional method of intubation and mechanical ventilation and noninvasive positive pressure ventilation (NPPV). Studies vary widely in their reported frequency of mechanical ventilation in acute exacerbations of COPD (CitationBrochard 1995). In one large multicenter cohort of patients admitted to 42 intensive care units across the US the frequency of mechanical ventilation was 47% (CitationSeneff 1995). Though experts have suggested using physiologic parameters in making a decision to intubate a patient these have not been tested in clinical studies. The decision to intubate is largely based on clinical judgment. Indications include deteriorating gas exchange despite medical management, cardiorespiratory arrest, severe respiratory distress (as evidenced by tachypnea, nasal flaring, accessory muscle recruitment, tracheal tug, recession of the suprasternal and intercostal spaces, pulsus paradoxus, diaphoresis) and altered level of consciousness.
In many cases of acute respiratory failure due to COPD, NPPV can give the same benefits as the standard intubation and mechanical ventilation without the complications that are usually associated with the latter. As a result patients treated with NPPV have shorter stay, lower morbidity and incur lesser costs (CitationHonrubia 2005). A Cochrane Database systematic review by CitationRam and colleagues (2004) concluded that NPPV improved mortality, decreased the need for intubation and reduced the treatment failures.
Among all the parameters pH is, perhaps, the most important variable in predicting the success or failure of NPPV. In an observational study of 1033 consecutive patients treated with NPPV for COPD exacerbation, a Glasgow coma score <11, APACHE II score ≥29, respiratory rate ≥30, and pH < 7.25 at admission was associated with a 70% risk of intubation (CitationConfalonieri 2005). In this study, after 2 hours of NPPV, the main factor influencing the outcome was the pH value: if pH < 7.25 the odds ratio for failure was 21. In another prospective study by CitationShameem and colleagues (2005), a pH of less than 7.26 at admission resulted in a high failure rate of NPPV (100 out of 150 patients eventually needing intubation). Though it is clear that, lower the pH the higher the rate of NPPV failures, there is no consensus on the cutoff number. It is reasonable to predict that a pH value of less than 7.2 at admission will have a high NPPV failure rates. However a pH value should not be used as the only deciding factor rather should be used in conjunction with other factors like patient’s mental status, comorbid conditions, patient’s code status etc.
There may be occasions when the patients are on the borderline. In such situations an intubation may be preferred early when facilities for closely monitoring the NPPV patients or emergency intubation are not available. On the other hand there may be situations where a NPPV may have to be used despite a critically ill patient, for eg, if patient is terminally ill, patient has ‘Do Not Intubate’ status.
Patient selection is an important factor for the success of NPPV. Early initiation is associated with better outcomes (CitationCelikel 1998). Generally patients who are younger, cooperative, with moderate hypercarbia (PCO2 45 to 90), moderate acidosis (pH 7.1 to 7.3) are the ones who will benefit the most (CitationEvans 2001). Patients who have failed or are unable to tolerate noninvasive ventilation, or have contraindications () to noninvasive ventilation should receive intubation and mechanical ventilation.
Table 1 Contraindications to NPPV
Respiratory physiology
In normal subjects, in the absence of respiratory effort, the lung will come to lie at the point of the functional residual capacity (FRC) or relaxation volume (Vrel). The point at which this occurs is determined by a balance between the inward elastic recoil of the lung and the equal and opposite outward recoil of the respiratory cage (mostly due to muscle tone). The intrapleural pressure (Ppl) at this point is −3 to −5 cm water. To generate a respiratory movement two factors must be overcome:
Resistance
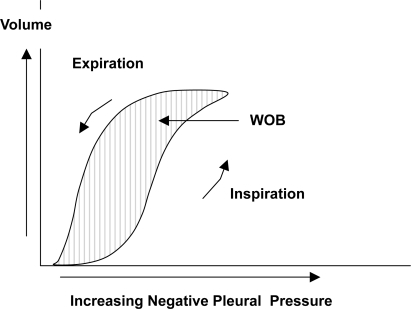
Resistance of the airways is described as obstruction to airflow provided by the conducting airways, resulting mainly from the larger airways (down to division 6–7). This is because the cross sectional area of the upper airways is much smaller compared to the smaller airways as the smaller airways are so many in numbers. The cross sectional area expands with each division of the airway and at generation 16 it is about 300 cm2 compared to 2.5 cm2 at the trachea. This results in a decrease of both airway resistance and air flow velocity. Airway resistance to flow is present during both inspiration and expiration and the energy required to overcome it represents the actual work of breathing (WOB) ().
Compliance
In a clinical setting this refers to the combined compliance of the lung and chest wall. It is the volume change per unit pressure change. When compliance is low, more effort is required to inflate the lungs. Compliance also varies depending on the degree of inflation, which is usually a sigmoid shaped curve in normal subjects ().
Respiratory mechanics in COPD
The two primary pathophysiologic changes that contribute to the development of respiratory distress and acute respiratory failure in patients with obstructive lung disease are:
Increased airway resistance: Patients with COPD have increased expiratory airflow resistance. In COPD, the alveolar attachments that normally keep the smaller airways open via radial traction are lost. This leads to airway narrowing and collapse especially during expiration. In normal subjects, during passive exhalation the intrapleural pressure is negative. In COPD the intrapleural pressure may be positive during exhalation due to recruitment of expiratory muscles. As exhalation occurs, the airway resistance increases further due to compression from the surrounding positive intrapleural pressure. This causes the airway segment to collapse. Soon after the collapse occurs the intraalveolar pressure is transmitted to the collapsed segment and the airway reopens because Palv exceeds Ppl (CitationBernasconi 1998; CitationWest 2000).
In acute exacerbations the already narrowed airways may be further compromised by increased secretions, mucosal swelling and peribronchial inflammation. The time constant for lung emptying is therefore prolonged and end expiratory lung volume is dynamically increased. Furthermore, during an exacerbation, patients tend to adopt a rapid shallow breathing pattern which further limits the time available for lung emptying, thus promoting greater dynamic hyperinflation (DHI) in a vicious cycle. In fact, any acute increase in ventilation (such as occurs with anxiety or transient hypoxaemia) can be associated with DHI in flow limited patients.
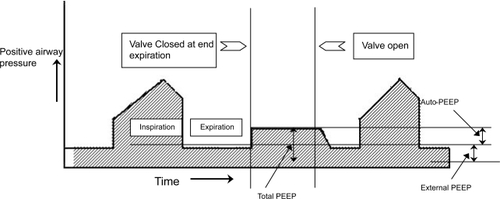
Dynamic hyperinflation: In the presence of increased expiratory airflow resistance the time available (expiratory time) to empty the inspired volume may not be sufficient. The next inspiration may start before the completion of the expiration leading to air trapping. Thus the respiratory system is unable to return to its normal relaxation volume at the end of expiration. This results in a new resting state where the FRC is greater than the Vrel. This condition of air trapping is otherwise called DHI. The DHI results in positive alveolar pressure at the end of expiration also referred to as auto-PEEP (positive end expiratory pressure) (CitationRossi 1995; CitationRanieri 1996). Initially the auto-PEEP may be beneficial to keep the airways open and thereby reducing the airway resistance. However auto-PEEP has many disadvantages:
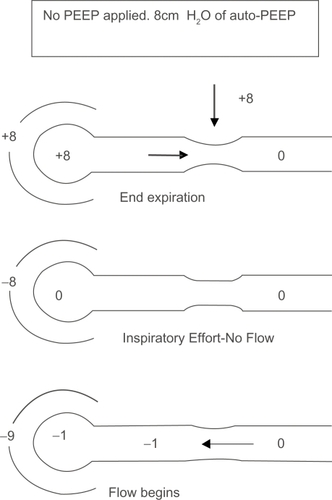
Increased work of breathing (WOB): Tidal breathing during an exacerbation in a patient with COPD may be shifted upwards closer to total lung capacity (TLC) as a consequence of DHI. Although this optimizes expiratory flows, it has the deleterious effect of forcing the respiratory system to operate on the flatter part of the compliance curve where progressive pressure increases generate smaller incremental volume changes. In other words, for the airflow to begin, the intrapleural pressure must fall below zero so the lung expands and the air flows in. In the presence of auto-PEEP, the intrapleural pressure generated must be more than the amount of auto-PEEP for airflow to begin which imposes a substantial inspiratory burden (CitationRoussos 1982; CitationFluery 1985; CitationTobin 1998) ().
In presence of DHI the lungs are operating at a higher than normal FRC. This causes the inspiratory muscles to operate at shorter than normal lengths. This places the respiratory muscles, in particular the diaphragm at a considerable mechanical disadvantage as they now have to operate on the flatter part of the compliance curve (CitationBraun 1982; CitationSmith 1987). As the diaphragm is lower in the chest wall during hyperinflation its ability to descend further during inspiration is impaired.
Excessive PEEP can compromise cardiac function in several ways. The increased intrathoracic pressure can lead to decreased venous return and decrease left ventricular compliance (CitationMatthay 1980; CitationVizza 1998; CitationScharf 2002). Also DHI increases pulmonary capillary resistance by compressing alveolar capillaries which leads to an increase in the right ventricular after load (CitationMahler 1984; CitationOswald-Mammosser 1991). These changes can lead to hypotension especially soon after intubation when other factors like sedatives and hypovolumia may be coexisting.
Regions of hyperinflated lung may compress adjacent areas of normal lung and adversely affect ventilation/perfusion relationships (CitationBrandolese 1993; CitationRossi 1995).
DHI and auto-PEEP may predispose patients to barotrauma – Pneumothorax, pneumomediastinum and pneumoperitoneum may occur (CitationPepe 1982).
Failure to recognize auto-PEEP and adjust for it can lead to inappropriate treatment:
- Misinterpretation of central venous and pulmonary artery catheter pressure measurements (CitationPepe 1982).
- Erroneous calculations of static respiratory compliance: the true value of static compliance will be underestimated in the presence of auto-PEEP (CitationRoussos 1982).
Figure 2A Effect of auto-PEEP on work of breathing (WOB). In the presence of airflow obstruction the alveoli remain inflated at end expiration. This results in alveolar pressure greater than atmospheric pressure. Without any inspiratory effort intra-pleural pressure equals alveolar pressure. A negative pressure greater than the auto-PEEP is required for the airflow to begin.
Dynamic hyperinflation can occur in the absence of airflow limitation in mechanically intubated patients. The causes are usually due to rapid respiratory rate, high tidal volumes, inspiratory time more than expiratory time, small bore endotracheal and ventilatory tubes (CitationScott 1986; CitationIotti 1997). Sometimes auto-PEEP without DHI can exist in patients who have excessive expiratory muscle activity (CitationLessard 1995).
Management
Ventilatory strategies
The ventilatory strategies are aimed at correcting the gas exchange abnormality, identifying and preventing DHI. The minute volume should be adjusted to pH and not to avoid over-ventilation with consequent to the PaCO2 alkali loss and reduced renal compensation. Continuous displays of ventilator waveforms are very useful in detecting and monitoring the lung mechanics. Clinical signs are important in detecting respiratory distress either in spontaneously breathing or those receiving assisted ventilation. Tachypnea, tachycardia, hypertension, or hypotension; decrease in arterial oxygen saturation; use of accessory muscles of breathing; reduction or absence of breath sounds over a region of the lung; wheezing; rib-cage abdominal asynchrony; paradoxical movement of the abdominal wall during inspiration; cyanosis; inability to trigger the ventilator; and apnea may all point to the need for urgent interventions or ventilator adjustments (CitationTobin 1988). Often times the waveforms can detect the abnormalities before the clinical signs are evident and therefore play an important role in the management of intubated patient.
Initial ventilator settings and the mode used is usually dependent on operator and local practices. In general low tidal volumes of 6 to 10 ml/Kg, FiO2 of 1.0, no added PEEP, respiratory of 10 to 14/minute and an inspiratory flow of 80 to 100 L/minute with square wave form are considered ideal. Ventilator-trigger sensitivity should be minimal as in the presence of auto-PEEP patient may not be able to generate enough negative pressure or flow (CitationDerenne 1988; CitationSchmidt 1989).
Effects of expiratory time, minute ventilation and expiratory flow rates on DHI
The important determinants of DHI are minute ventilation, tidal volume, time for expiration (TE), and severity of airway obstruction. Tuxen and Lane (CitationTuxen 1987) studied the various settings for minute ventilation, tidal volume and time for expiration on DHI. Minute ventilation was the most important determinant of DHI. A decrease in TE (by decreasing peak flows) while keeping the minute ventilation and tidal volume constant significantly increased end expiratory volume (VEE) suggesting air trapping. A similar effect was noted with decrease of TE by increasing respiratory rate. However if the decrease in TE was caused by an increase in tidal volume and decrease in respiratory rate while keeping a constant minute ventilation only a small insignificant increase in VEE was noted. It is therefore important to recognize that a simple adjustment of Inspiratory: Expiratory (I:E) ratio is not sufficient. The adjustment to the absolute expiratory time and the minute ventilation are more important in reducing the amount of air trapping.
Expiratory flow is also important to prevent DHI (CitationHubmayr 1990). With lower expiratory flow rate it takes longer to empty the tidal volume; the next breath takes place before the lungs return to normal resting state.
In general, adapting the following measures can reduce auto-PEEP:
Provide the longest expiratory phase that is possible.
Reduce patient ventilatory demand and minute ventilation.
Reduce airflow resistance by bronchodilators and steroids.
Effect of peak and plateau pressure
An elevated peak airway pressure seems to pose an increased risk for barotrauma. Several authors have suggested that peak pressures should be kept less than 50 cm water. However peak airway pressure does not reflect the pressure at the alveolar level especially in patients with airflow obstruction. It has been shown that peak airway pressure does not correlate with complications (CitationWilliams 1992; CitationSlutsky 1993). The plateau pressure is a better measurement as it reflects the true alveolar pressure. This has a greater correlation with DHI and risk for barotraumas. In patients with COPD the goal is to keep the plateau pressure less than 30 cm water to minimize barotrauma. This may be achieved by maintaining low tidal volume, low minute ventilation and correcting auto-PEEP.
Adding PEEP
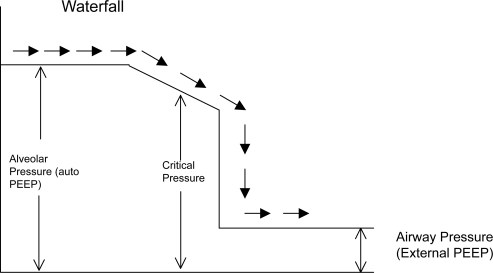
The presence of auto-PEEP acts as a threshold load for patient’s inspiratory effort (CitationFernandez 1988) (). To alleviate the breathing efforts that auto-PEEP imposes on the respiratory muscles an external PEEP can be applied. It might seem that applying external PEEP is detrimental when there is already positive pressure at end expiration. This seeming paradox can be explained by analogy to a stream with waterfall (CitationTobin and Lodato 1989) (). In this analogy the flow of water (airflow) is not affected until the downstream (external PEEP) rises above the critical pressure needed to constrict the airway. Above this level the external PEEP can increase upstream pressure and cause worsening DHI. The external PEEP should be kept below 75% to 85% of auto-PEEP to avoid any worsening of hyperinflation or circulatory compromise (CitationPetrof 1990; CitationGeorgopoulus 1993; CitationRanieri 1993).
Figure 2B Applying external PEEP helps reduce WOB. Effect of adding external PEEP. Extrinsically applied PEEP reduces the amount of negative pressure needed to generate airflow.
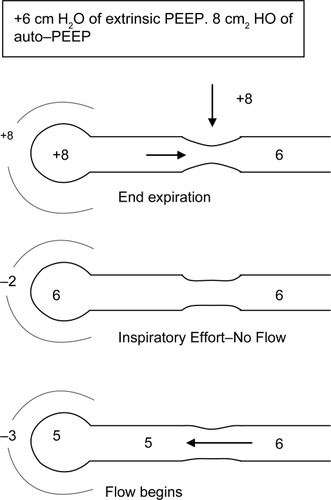
Figure 3 The waterfall analogy to explain the rationale behind applying external PEEP equal to or less than auto-PEEP. The waterflow (airflow) is not affected until the downstream water (external PEEP) reaches the critical pressure. External PEEP applied at the airway will not worsen auto-PEEP if it does not exceed critical pressure.
In a patient with auto-PEEP, if the ventilator is set to deliver patient initiated breaths external PEEP can help in two ways. Firstly external PEEP decreases the inspiratory threshold thereby decreasing the work of breathing (CitationSmith 1988). Secondly, it acts as a stent for the collapsible airways thereby increasing expiratory flow rates (CitationTobin et al 1986) (much like purse-lip breathing in nonintubated patient). However it is important to note that in a well sedated, non-hypoxic patient receiving controlled mechanical ventilation external PEEP may not benefit even in the presence of auto-PEEP unless indicated for reasons other than DHI.
Identification and measurement of auto-PEEP
Static auto-PEEP
Static auto-PEEP can be measured only in patients without active respiratory effort. This can be obtained using the end-expiratory hold control on the ventilator. This maneuver provides time to equilibrate lung units that have different regional auto-PEEPs with the ventilator. The auto-PEEP can then be calculated by subtracting the external PEEP from the total PEEP ().
Dynamic auto-PEEP
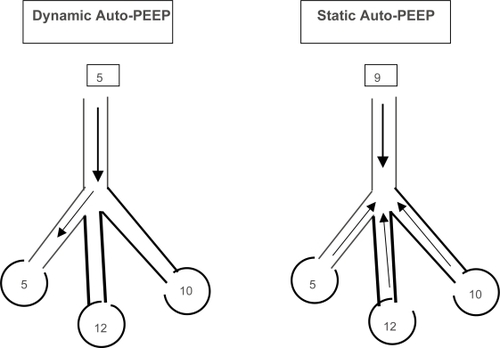
In spontaneously breathing patients, auto-PEEP is determined by simultaneously recording esophageal pressure and airflow tracings. Dynamic auto-PEEP is measured at end-expiration as the negative deflection of esophageal pressure to the point of zero flow. The dynamic auto PEEP is usually lower than the static auto-PEEP because dynamic auto-PEEP reflects the end expiratory pressure of the lung units with short time constants and rapid expiration, while units with long time constants are still emptying (CitationHaluszka 1990; CitationHernandez 1994; CitationZakynthinos 1997; CitationYounes 2000) (). Expiratory muscle activity, abdominal muscle activity and airway narrowing can all cause inaccurate measurements of auto-PEEP (CitationNinane 1992; CitationMaltais 1994).
Figure 5 Static and Dynamic auto-PEEP. In Dynamic auto-PEEP inspiratory flow begins as soon as the airway pressure is greater than the lung region with lowest auto-PEEP ie, shortest time constant (Dynamic auto-PEEP in this example is 5). In static auto PEEP end expiratory occlusion allows for equilibration of lung regions and the auto-PEEP measured is an average of all regions (Static auto-PEEP in this example is 10).
Measurement of resistance and compliance
These values are obtained by rapidly occluding the expiratory port at the end of inspiration. This produces a rapid fall in peak pressure and after 3–5 seconds the pressures at the ventilator and alveoli equilibrates at which point the pressure curve plateaus off ().
Figure 6 Measurement of resistance and compliance. The valve is occluded at end inspiration and the airway pressure declines from a peak (Ppeak) to a plateau when there is zero airflow. Airflow rates can be measured simultaneously.
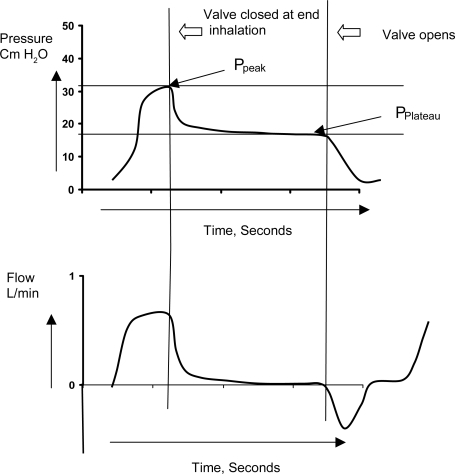
The difference between the peak pressure and plateau pressure (Pplat) gives the total resistance of the respiratory tract. Compliance is calculated by using the formula
Measurement of trapped volume
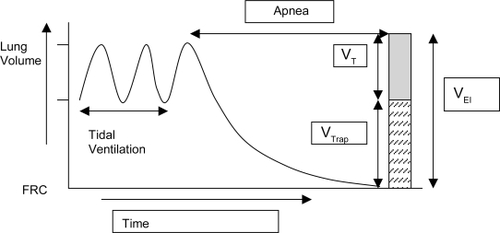
The patient needs to be paralyzed and prolonged apnea needs to be employed. The total exhaled volume is measured from the end of inspiration until there is no visually detectable change in volume (). The total volume of exhaled gas (called the volume at end-inspiration) is the sum of the tidal volume and any trapped gas above functional residual capacity. The need for paralyzing the patient to obtain this measurement is an important problem with the routine use of this technique. Moreover, to accurately measure the volume at end-inspiration, the equipment needs to be sensitive to very low flow rates. The practicality and the clinical relevance of doing this maneuver are debatable for the bedside management of patients.
Figure 7 Measurement of trapped volume. In normal subjects the lung volume returns to functional residual capacity (FRC). However in the presence of dynamic hyperinflation the end expiratory volume remains higher than FRC and tidal breathing occurs at higher lung volumes. After a tidal breath prolonged apnea is instituted and the volume of exhaled air is measured. Subtracting the tidal volume (VT) from the volume at end inspiration (VEI) gives the amount of trapped volume (VTrap).
Weaning
When to begin weaning is mostly dependent on physician’s clinical judgment. Weaning should begin once the cause of the exacerbation is adequately treated and the patient is hemodynamically stable. Physiologic parameters like minute ventilation (<15 L), respiratory rate (<30), tidal volume (>325 ml), dynamic compliance (>22), static compliance (>33), rapid shallow breathing index (<105), maximum inspiratory pressure (<–15) have some utility in predicting the patient’s ability to sustain spontaneous ventilation (CitationFiastro 1988; CitationJabour 1991; CitationYang 1991; CitationEli 1996). Daily spontaneous breathing trail (SBT) is one way of identifying patients stable to wean and it may reduce the number of ICU days (CitationEsteban 1995). Pressure support ventilation (PSV) weaning is used to decrease mechanical ventilatory support gradually. While PSV has not been shown to be superior to SBT (CitationBrochard 1994) it has advantages over synchronized intermittent mandatory ventilation (SIMV) mode of weaning (CitationBrochard 1994; CitationNava 1998). Weaning to a NPPV has been studied in several small studies (CitationGirault 1999; CitationChen 2001; CitationHill 2000; CitationFerrer 2003). A meta-analysis of these five studies, with 171 patients in total, intubated predominantly for COPD concluded that the use of NPPV to facilitate weaning was associated with promising but insufficient evidence of net clinical benefit at present (CitationBurns 2006).
The key points of optimal ventilatory techniques are summarized in .
Table 2 Key points on ventilatory techniques to optimize mechanical ventilation in COPD
Sedation
Sedation is an important part of mechanical ventilation. The Society of Critical Care Medicine has published the Clinical Practice Guidelines for the Sustained Use of Analgesia and Sedation (CitationJacobi 2002). It is important to distinguish between pain, agitation and delirium. The level of pain should be assessed through subjective observation of pain-related behaviors and as reported by the patient. If amalgesics are needed fentanyl, morphine or hydromorphone are the recommended agents. Sedation for agitated patient must be provided only after causative factors like pain are adequately treated. Benzoiazepines (Lorazepam, Midazolam) and propofol are the preferred agents. The titration of the sedative dose to a defined endpoint is recommended with systematic tapering of the dose or daily interruption. The potential for opioid, benzo-diazepine and propofol withdrawal should be considered after prolonged use. For delirious patients haloperidol is the preferred agent.
General management
The medical management of acute exacerbations includes identifying and treating the cause of the acute exacerbation, administration of antibiotics for infection, bronchodilators, steroids, maintaining adequate oxygenation, helping secretion clearance, preventing the complications of immobility and adequate nutrition.
Antibiotics
Antibiotic therapy for moderate acute exacerbations of chronic bronchitis and emphysema should be directed at S. pneumoniae, H. influenzae and M. catarrhalis, which are the most common pathogens. A meta-analysis (CitationSaint 1995) of nine clinical trials demonstrated the benefit of antibiotic therapy in the management of COPD.
Steroids
Short courses of systemic corticosteroids may provide important benefits in patients with exacerbations of COPD a more rapid increase in FEV1, fewer withdrawals, and a significantly shorter hospital stay (CitationDavies 1999; CitationNiewoehner 1999).
Bronchodilators
Careful use of bronchodilators has shown to improve symptoms and airflow limitation (CitationCooper 2005; CitationSin 2005). The use of combined beta2 agonists and anticho-linergic agents has been found to provide small additional bronchodilation compared with the use of either medication alone (CitationBone 1994). Intermittent nebulizer operation is more efficient for aerosol delivery than is continuous aerosol generation, because it minimizes aerosol waste during exhalation (CitationDhand 2004).
Conclusion
Acute exacerbation of COPD is a commonly occurring clinical entity. Ventilatory support in a compromised patient can be a life saving measure; however mechanical ventilation can be associated with significant morbidity and mortality. It is important for a physician to be familiar with the patho-physiology and respiratory mechanics in COPD and an understanding of expiratory airflow obstruction and dynamic hyperinflation helps in choosing the most appropriate ventilatory settings. Modern ventilators routinely display several flow, pressure and volume waveforms which allows the clinician to recognize changes in a patient’s condition at the bedside before clinical signs become overt. These waveforms and graphics are invaluable and must be routinely monitored so that abnormal patterns could be identified sooner leading to early interventions.
Notes
Note
There are no financial/other disclosures/conflicts of interest to report by the authors.
References
- BernasconiMPloysongsangYGottfriedSB1998Respiratory compliance and resistance in mechanically ventilated patients with acute respiratory failureIntensive Care Med1454753
- BhowmikASeemungalTARSapsfordRJ2000Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbationsThorax551142010639527
- BoneRBoyarsMBraunS1994In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent aloneChest1051411198181328
- BrandoleseRBroseghiniCPoleseG1993A: effects of intrinsic PEEP on pulmonary gas exchange in mechanically-ventilated patientsEur Respir J6358638472826
- BraunNMTAroraNSRochesterDF1982Force-length relationship of the normal human diaphragmJ Appl Physiol: Respirat Environ Exercise Physiol5340512
- BrochardLManceboJWysockiM1995Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary diseaseN Engl J Med333817227651472
- BrochardLRaussABenitoS1994Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilationAm J Respir Crit Care Med1508967921460
- BurnsKEAdhikariNKMeadeMO2006A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilationCan J Anaesth533051516527798
- CelikelTSungurMCeyhanB1998Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failureChest1141636429872200
- ChenJQiuDTaoD2001Time for extubation and sequential noninvasive mechanical ventilation in COPD patients with exacerbated respiratory failure who received invasive ventilationZhonghua Jie He He Hu Xi Za Zhi2499100(English abstract only).11802949
- ConfalonieriMGarutiGCattaruzzaMS2005A chart of failure risk for noninvasive ventilation in patients with COPD exacerbationEur Respir J253485515684302
- CooperCBTashkinDP2005Recent developments in inhaled therapy in stable chronic obstructive pulmonary diseaseBMJ330640415774995
- DaviesLAngusRMCalverleyPM1999Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trialLancet3544566010465169
- DerenneJPFleuryBParienteR1988Acute respiratory failure of chronic obstructive pulmonary diseaseAm J Respir Rev138100633
- DhandR2004Basic techniques for aerosol delivery during mechanical ventilationRespir Care496112215165296
- EliEWBakerAMDunaganDP1996Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneouslyN Eng J Med3351864
- EstebanAFrutosFTobinNJ1995A comparison of four methods of weaning patients from mechanical ventilationN Eng J Med332345
- EvansTAlbertRAngusD2001Noninvasive Positive Pressure Ventilation in acute respiratory failureAm J Respir Crit Care Med1632839111208659
- FernandezRBenitoSBlanchL1988Intrinsic PEEP: a cause of inspiratory muscle ineffectivityIntensive Care Med155123068269
- FerrerMEsquinasAArancibiaF2003Noninvasive ventilation during persistent weaning failure. A randomized controlled trialAm J Respir Crit Care Med16870612689847
- FiastroJFHabibMPShonBY1988Comparison of standard weaning parameters and the mechanical work of breathing in mechanically ventilated patientsChest94233
- FlueryBMurcianoDTalamoC1985Work of breathing in patients with chronic obstructive pulmonary diseaseAm Rev Respi Dis131822
- GeorgopoulosDGiannouliEPatakasD1993Effects of extrinsic positive end-expiratory pressure on mechanically ventilated patients with chronic obstructive pulmonary disease and dynamic hyperinflationIntensive Care Med191972038366227
- GiraultCDaudenthunIChevronV1999Noninvasive ventilation as a systematic extubation and weaning technique in acute on chronic respiratory failure. A prospective, randomized controlled studyAm J Respir Crit Care Med160869210390384
- HaluszkaJChartrandDAGrassinoAE1990Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis141119472111105
- HernandezPNavalesiPMaltaisF1994Comparison of static and dynamic measurements of intrinsic PEEP in anesthetized catsJ Appl Physiol762437427928868
- HillNSLinDLevyM2000Noninvasive positive pressure ventilation (NPPV) to facilitate extubation after acute respiratory failure: a feasibility studyAm J Respir Crit Care Med161A263(abstract)
- HonrubiaTGarcia LopezFJFrancoN2005Noninvasive vs. conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trialChest12839162416354864
- HubmayrRDAbelMDRehderK1990Physiologic approach to mechanical ventilationCrit Care Med18103132403503
- IottiGAOliveiMCPaloA1997Unfavorable mechanical effects of heat and moisture exchangers in ventilated patientsIntensive Care Med233994059142578
- JabourERRabilDMTruwitJD1991Evaluation of a new weaning index based on ventilatory endurance and the efficiency of gas exchangeAm Rev Respir Dis1445311892291
- JacobiJ2002Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adultCrit Care Med301194111902253
- LessardMRLofasoFBrochardL1995Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patientsAm J Respir Crit Care Med15156297842221
- MahlerDABrentBNLokeJ1984Right ventricular performance and central hemodynamics during upright exercise in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis13072296388444
- MaltaisFReissmannHNavalesiP1994Comparison of static and dynamic measurements of intrinsic PEEP in mechanically ventilated patientsAm J Respir Crit Care Med1501318247952559
- MatthayRABergerHJDaviesRA1980Right and left ventricular exercise performance in chronic obstructive pulmonary disease: radionucleotide assessmentAnn Intern Med9323497406373
- NavaSAmbrosinoNCliniE1998Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trialAnn Intern Med12872189556465
- NiewoehnerDEErblandMLDeupreeRH1999Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study GroupN Engl J Med3401941710379017
- NinaneVRypensFYernaultJC1992Abdominal muscle use during breathing in patients with chronic airflow obstructionAm Rev Respir Dis14616211385684
- Orozco-LeviM2003Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation?Eur Respir J22Suppl 464151s
- Oswald-MammosserMApprillMBachezP1991Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous typeRespiration58304101792422
- PepePEMariniJJ1982Occult positive end–expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effectAm Rev Respir Dis126166707046541
- PetrofBJLegareMGoldbergP1990Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic pulmonary diseaseRev Respir Dis1412819
- RamFSFPicotJLightowlerJ2004Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary diseaseCochrane Database of Systematic ReviewsCD004104DOI:10.1002/14651858.CD004104.pub3
- RanieriVMGiulianiRCinnellaG1993Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilationAm Rev Respir Dis1475138420430
- RanieriVMGrassoSFioreT1996Auto-positive end-expiratory pressure and dynamic hyperinflationClin Chest Med17379948875002
- RossiAPoleseGBrandiG1995Intrinsic positive end-expiratory pressureIntensive Care Med21522367560497
- RossiASantosCRocaJ1995Effects of PEEP on VA/Q mismatching in ventilated patients with chronic airflow obstructionAm J Respir Crit Care Med1515627842221
- RoussosCMacklemPT1982The respiratory musclesN Engl J Med307786977050712
- SaintSBentSVittinghoffE1995Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysisJAMA273957607884956
- ScharfSMIqbalMKellerC2002Hemodynamic characterization of patients with severe emphysemaAm J Respir Crit Care Med1663142212153963
- SchmidtGAHallJB1989Acute or chronic respiratory failure: assessment and management of patients with COPD in the emergency settingJAMA2613444532657124
- ScottLRBensonMSPiersonDJ1986Effect of inspiratory flowrate and circuit compressible volume on auto-PEEP during mechanical ventilationRespir Care3110759
- SeneffMGWagnerDPWagnerRP1995Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary diseaseJAMA274185277500534
- SethiS2000Infectious etiology of acute exacerbations of chronic bronchitisChest1175 Suppl 2S3805
- ShameemMBhargavaRAhmadZ2005Identification of preadmission predictors of outcome of noninvasive ventilation in acute exacerbation of chronic obstructive pulmonary diseaseIndian J Crit Care Med92004
- SinDDMcAlisterFAManSF2005Contemporary management of chronic obstructive pulmonary disease: scientific reviewBMJ330640415774995
- SlutskyAS1993Mechanical ventilation – ACCP consensus conferenceChest10418338252973
- SmithJBellemareF1987Effect of lung volume on in vivo contraction characteristics of human diaphragmJ Appl Physiol6218939003597263
- SmithTCMariniJJ1988Impact of PEEP on lung mechanics and work of breathing in severe airflow obstructionJ Appl Physiol651488993053583
- SolerNTorresAEwingS1998Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilationAm J Respir Crit Care Med15714985059603129
- TobinMJ1988aRespiratory monitoring in the intensive care unitAm Rev Respir Dis1381625423144222
- TobinMJ1988bRespiratory muscles in diseaseClin Chest Med9263863292127
- TobinMJLodatoRF1989PEEP, auto-PEEP, and waterfallsChest96449512670461
- TobinMJPerezWGuentherSM1986The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilationAm Rev Respir Dis1341111183789513
- TuxenDVLaneS1987The effects of ventilatory pattern on hyperinflation airway pressures, circulation in mechanically ventilation of patients with severe air flow obstructionAm Rev Respir Dis1368723662241
- VizzaCDLynchJPOchoaLL1998Right and left ventricular dysfunction in patients with severe pulmonary diseaseChest113576839515827
- WestJB2000Mechanics of breathing Respiratory physiology, the essentials6th edLippincottPhiladelphia79102
- WilliamsTJTuxenDVScheinkestelCD1992Risk factors for morbidity in mechanically ventilated patients with acute severe asthmaAm Rev Respir Dis1466071519836
- World Health Report 1998 Life in the 21st Century: a vision for allWorld Health OrganizationGeneva
- YangKLTobinMJ1991A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilationN Eng J Med3241445
- YounesM2000Dynamic intrinsic PEEP (PEEPi, dyn). Is it worth saving? (editorial)Am J Respir Crit Care Med1621608911069783
- ZakynthinosSGVassilakopoulosTZakynthinosE1997Accurate measurement of intrinsic positive end-expiratory pressure: how to detect and correct for expiratory muscle activityEur Respir J1052299072979