Abstract
Chronic obstructive pulmonary disease (COPD) represents a major global cause of disability and death. COPD is currently the fourth most common global cause of death and also exerts an enormous toll on patients quality of life. The present database analysis aimed to identify clinical trials using fixed combination therapies that have assessed the impact on the patients quality of life. Within the different studies, questionnaires including the George’s Respiratory Questionnaire (SGRQ) the Chronic Respiratory Disease Questionnaire (CRDQ) and the Clinical COPD Questionnaire (CCQ) were used and differing results in quality of life were obtained when combination therapies such as fluticasone/salmeterol or fluticasone/salmeterol were compared with monotherapies. While there were some differences in favor of combination therapies reported when the combination therapy was compared to inhaled steroid monotherapy there were no consistent differences when combination therapies were compared to bronchodilator monotherapies. Future trials will lead to a proof-of-principle stage concerning the use of combination therapies.
Introduction
Chronic obstructive pulmonary disease (COPD) has evolved as one of the major global causes of death and disability over the past decades (CitationHansen-Flaschen 2003). The global burden of disease studies reported in alarming increase in COPD prevalence (CitationMurray and Lopez 1997a, Citationb, Citationc). As with other respiratory diseases, COPD is related to exposure against air pollutants (CitationBoschetto et al 2006; CitationGroneberg-Kloft et al 2006). In 1990, the disease was classified as the sixth most common global cause of death. For the year 2020, it was predicted that COPD would become the third commonest cause of death irrespective of public-health intervention. Also, COPD was reported to become the fourth most important disease leading to disability by the year 2020 (CitationMurray and Lopez 1997a, Citationb, Citationc). WHO studies lead to the assumption that these predictions are likely to be correct since COPD had become the fourth commonest global cause of mortality (CitationMurray et al 2001). The National Heart, Lung, and Blood Institute reported for the US that COPD has increased in importance during the past two decades in contrast to all other causes of chronic disability (CitationNational Heart 2002). Concomitant with these increases, COPD is more often diagnosed by general practitioners as reported for the UK: Here, an increase in COPD diagnosis was reported for the period 1990–1997 (CitationSoriano et al 2000) which was paralleled by an increase in COPD-related hospital admissions (CitationCalverley and Walker 2003). In the context of disease management, acute exacerbations are important clinical events in COPD and largely contribute to a decline of quality of life. The life quality in patients with COPD as assessed, ie, by activities of daily living, emotional functioning, social-role functioning, and recreational pastimes was found to be impaired relative to healthy subjects on all dimensions (CitationMcSweeny et al 1982; CitationJones et al 1992; CitationKetelaars et al 1996; CitationHajiro et al 1998). Depression was the preponderant emotional disturbance reported, difficulties with home management and reduction in social interaction were the primary social-role deficits while sleep and rest, mobility, and a variety of recreational pastimes were also severely affected (CitationMcSweeny et al 1982). Patients with COPD also only have low expectations of medical care, and many of them do not complain despite substantial disease-associated disabilities (CitationRennard et al 2002). This reluctance to ask for an increased attention may be related to increased emotional disturbances such as depression (CitationMcSweeny et al 1982).
As COPD is characterized by a variable pathology among patients (CitationWelte and Groneberg 2006) and there are no specific “COPD-drugs” available, an international standardization of disease management has been difficult to establish over the past years. However, with an increasing significance of COPD concerning the global burden of disease, important guidelines to optimize disease management were proposed by several thoracic societies and international organizations in the past years (CitationSociety 1987; CitationSiafakas et al 1995; CitationFabbri et al 1998; CitationBalter et al 2003). The most prominent guidelines are the Global initiative for chronic Obstructive Lung Disease (GOLD) which was initiated by the WHO and the US National Heart, Lung, and Blood Institute (CitationPauwels et al 2001) and the international guidelines of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (CitationCelli and MacNee 2004).
Prior to assessing the impact of drug therapies it is crucial to define the disease entity since there have been various approaches towards the definition in the past decades. The clinical diagnosis of COPD largely relies on the patient’s history and abnormal lung function tests. As COPD is characterized by a variable pathology and the molecular mechanisms are not completely understood, it has been difficult to agree on a simply disease definition. First approaches based on epidemiological features of chronic cough and sputum production such as lasting of symptoms for 3 months over a period of at least 2 years (chronic bronchitis) or on pathological features such as the identification of emphysema in COPD airway tissues. However, these approaches did not prove to be efficient in the clinical management of the disease.
A keystone in the approach towards a rational disease definition is represented by the first reports that related disability and death in COPD to a progressive decrease in the forced expiratory volume in 1 second (FEV1) (CitationFletcher and Peto 1977; CitationPeto et al 1983).
It is now generally accepted that COPD diagnosis crucially relies on the presence of airflow obstruction defined as decreased FEV1 to FVC (forced vital capacity) or vital capacity ratio. However, it should also be critically remarked that lung function only partly correlates to other prognosis parameters. Therefore, the BODE (body-mass index, airflow obstruction, dyspnea, and exercise capacity index) score was developed (CitationCelli et al 2004).
Extending the basic functional features, the GOLD guidelines introduced persistent inflammation and the potential presence of noxious stimuli to the disease definition. With the persistent inflammation being an accepted feature of the disease, the question arises whether combination therapies with inhaled bronchodilators and steroids are superior to monotherapies with inhaled bronchodilators (). Therefore the present analysis aims to assess the effects of the fixed combination therapies budesonide/formoterol and fluticasone/salmeterol on the quality of life.
Table 1 Stage-specific therapy of stable COPD. Potential use of combination therapy with inhaled corticosteroid and bronchodilator instead of bronchodilator monotherapy
Methods
Sources
Clinical trials were identified by the use of the PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE and CINAHL. Also, hand-searching of pulmonary journals and meeting abstract booklets was performed. The terms “COPD” and “chronic obstructive pulmonary disease” were searched in combination with the terms (quality of life), (*steroid or steroid* OR corticosteroid*) and ((beta* and agonist*) and long*) or ((beta* and adrenergic*) and long*) and (formoterol and budesonide) or Symbicort and (fluticasone and salmeterol) or Seretide or Advair. The most recent search was run in July 2007.
Analysis
The analysis was performed by independently identifying abstracts of trials which appeared relevant. Then the full text of each study was used and trials were selected trials for inclusion in the analysis. After a preliminary assessment of all studies was performed to confirm the basic requirements, the methodological quality of the included studies was assessed using accepted Cochrane criteria with grade A: adequate concealment, grade B: uncertain, grade C: clearly inadequate concealment.
Results
Data selection
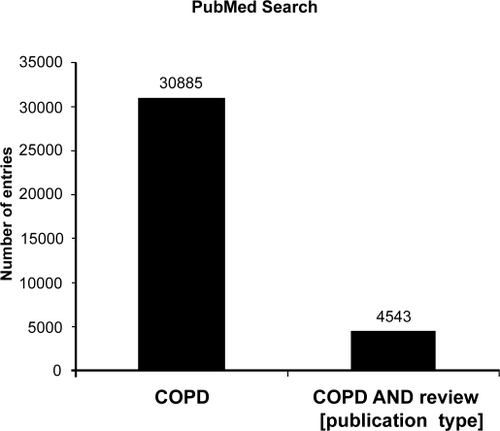
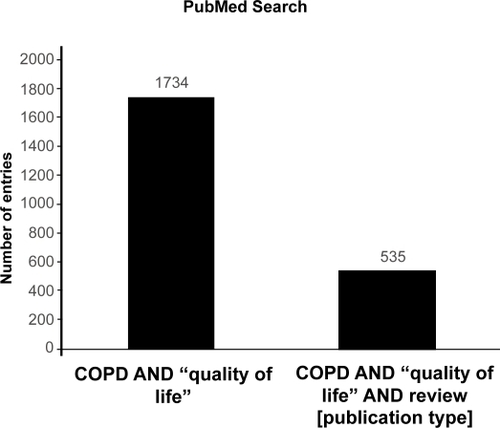
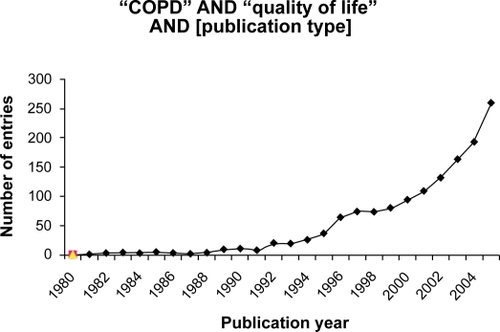
Using database searches a large number of entries related to COPD () and quality of life () was found that was further classified and analysed for publication dates () and the following study parameters.
Trial quality
All included trials had a randomized, double-blind, placebo controlled parallel group design. Methods of randomization were described only in one study (CitationMahler 2002). Method of blinding was not fully described in all trials. All included participants suffered from COPD, with variable definition of COPD and reversibility.
Health-related quality of life
Most studies assessed the quality of life either using the St. George’s Respiratory Questionnaire (SQRQ) or the Chronic Respiratory Disease Questionnaire (CRDQ), partly only reporting single domains. Concerning the analysis if the SQRQ it needs to be criticized that most of the studies only reported the mean values of the SQRQ scores. In this respect, it should have been important to know if there is a difference between the numbers of patients with an increase in the score of 4 units (cut-off point for significant improvement). Also, a separate analysis of single domains should not be performed.
Fluticasone/salmeterol
To assess the health-related quality of life the St. George’s Respiratory Questionnaire (SGRQ) (CitationCalverley et al 2003a; CitationWouters et al 2005), the Chronic Respiratory Disease Questionnaire (CRDQ) (CitationMahler et al 2002; CitationHanania et al 2003) and the Clinical COPD Questionnaire (CCQ) (CitationWouters et al 2005) were used. For the global SGRQ score one study (CitationCalverley et al 2003a) reported a significant difference favorable for a combination therapy in comparison to fluticasone monotherapy (score was 1.4 units lower) after 1 year of therapy. Also a significant difference favorable for a combination therapy in comparison to salmeterol monotherapy was found after 44 weeks in this study (score was 2.24 units lower) (CitationCalverley et al 2003a).
A further study reported a significant difference after 24 weeks of combination therapy (500 μg fluticasone) in the CDRQ total score and in the dyspnea score in comparison to fluticasone monotherapy (the total score was 4.8 units higher than the monotherapy score) (CitationMahler et al 2002). However, there were no differences in comparison to salmeterol monotherapy (CitationMahler et al 2002). The study using a lower dosage (250 μg fluticasone) (CitationHanania et al 2003) did not find any significant differences in comparison to monotherapy. The CRDQ total score increased after 24 weeks of therapy with combination therapy (250 and 500 μg fluticasone) with 10 units in comparison to baseline values. The dyspnea score increased with 3.9 (250 μg fluticasone) and 4.2 (500 μg fluticasone) units. For the CDSQ one study found a significant difference in favor of the combination therapy (CitationMahler et al 2002) in comparison to the fluticasone monotherapy. However, there were no differences in comparison to the salmeterol monotherapy. The same results were reported for the study with the lower dosage (CitationHanania et al 2003). Absolute differences in these scores in comparison to the baseline values were similar in both studies (CitationHanania et al 2003).
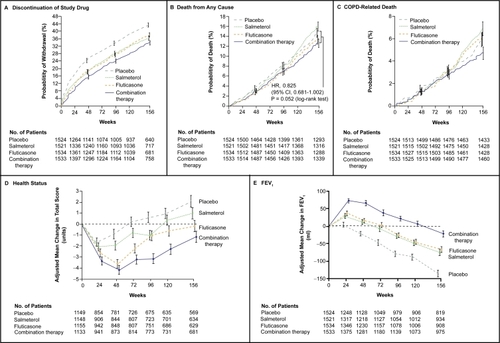
An important contribution towards the understanding of effects of combined therapies on quality of life is the TORCH study (CitationCalverley et al 2007). This randomized, double-blind trial compared salmeterol at a dose of 50 microg plus fluticasone propionate at a dose of 500 microg twice daily (combination regimen) with placebo, salmeterol alone, or fluticasone propionate alone for a period of 3 years. The primary outcome was mortality from any cause for the comparison between the combination regimen and placebo. While the reduction in death from all causes among patients with COPD in the combination-therapy group did not reach the predetermined level of statistical significance, significant benefits were present in all other outcomes measured (CitationCalverley et al 2007). Concerning the quality of life, the St. George’s Respiratory Questionnaire (SGRQ) was used. Total scores on the St. George’s Respiratory Questionnaire initially improved from baseline in all groups, with the greatest changes occurring in the combination-therapy group (mean score at baseline, 48.7, with a mean reduction of 3.0 units averaged over 3 years), as compared with the placebo group (a mean score of 48.4 at baseline, with an increase of 0.2 unit in the placebo group) (). Averaged over 3 years, the health status in the combination-therapy group was significantly better than in the groups receiving placebo, salmeterol alone, or fluticasone propionate alone (CitationCalverley et al 2007).
Figure 4 Outcomes in the TORCH study. In the combination regimen, salmeterol was administered at a dose of 50 μg and fluticasone propionate at a dose of 500 μg twice daily. Salmeterol alone was administered at a dose of 50 μg twice daily, and fluticasone propionate alone was administered at a dose of 500 μg twice daily. The effect of each study medication on health status (assessed according to changes in patients’ total scores on the St. George’s Respiratory Questionnaire). Vertical bars represent standard errors. Reproduced with permission from CitationCalverley PM, Anderson JA, Celli B, et al. 2007. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med, 356:775–89. Copyright © 2007 Massachusetts Medical Society. All rights reserved.
Budesonide/formoterol
When the combination budesonide/formoterol was assessed for effects on quality of life data from two studies demonstrated a significant effect in favor of budesonide/formoterol compared with placebo of −6.06 units on the SGRQ; 95%CI −7.90 to −4.22 (CitationSzafranski et al 2003; CitationCalverley et al 2003b; CitationNannini et al 2004). However, there appeared to be a high level of heterogeneity when these data were pooled (I2 71.8%) as indicated by a Cochrane analysis. Random effects modelling also generated a significant effect (weighted mean difference WMD −5.8 units; 95% CI −9.32 to −2.28. The improvement from baseline in the budesonide/formoterol group was 3.9 units (CitationSzafranski et al 2003). This was not dissimilar to change scores from post run-in treatment in the other study (CitationCalverley et al 2003b). Comparisons with monotherapies were not fully reported in the studies but Szafranski et al reported similar effects observed in patients treated with formoterol monotherapy (3.6 units) (CitationSzafranski et al 2003).
Discussion
COPD is characterized by slowly progressive development of a poorly reversible airflow limitation and inflammatory airway changes. Since it is estimated to be currently the fourth most common global cause of death and success of pharmacotherapy is still limited, new therapeutic regimen need to be evaluated. One of the most promising approaches should be the use of combined inhaled betamimetics and corticosteroids with the corticosteroids targeting the features of inflammation and mucus secretion and thus inhibiting further symptoms including airflow limitation and cough (). The present analysis assessed the effects of combination therapies on the quality of life. There were differing results concerning quality of life when fluticasone/salmeterol was compared with inhaled steroids alone while there were no significant differences present between fluticasone/salmeterol and long-acting beta-agonist in quality of life scores. The combination budesonide/formoterol was also shown to improve symptoms when compared with the budesonide monotherapy but not with formoterol. There were differing results in quality of life scores when budesonide/formoterol was compared with monotherapies.
Figure 5 Concept of the use of combined inhaled corticosteroids and betamimetics. While betamimetics target the symptom of bronchoconstriction corticosteroids should be beneficial in targeting inflammation and mucus secretion and thus abolishing further symptoms such as mucus-induced cough and airflow limitation.
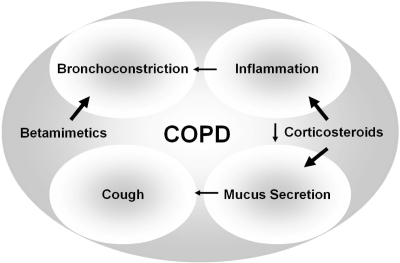
It can be assumed that the combination of inhaled corticosteroids and salmeterol led to benefits over monotherapies on certain clinical outcomes but not in others. Differences in the treatment protocols in the budesonide/formoterol combination studies may explain the differences between health status scores. Although differences between combination therapies and placebo were statistically signifcant, the difference from the study of Calverley et al suggested that the combination of inhaled steroids and betamimetics led to a clinically relevant advantage compared with placebo treatment (CitationCalverley et al 2003b). This difference may be interpreted with caution due to a pre-treatment with high dose prednisolone and formoterol, leading to an increase of 4.5 units in the SGRQ.
Future trials will lead to a proof-of-principle stage concerning the use of combination therapies. In these studies, combinations should be compared with separate administrations of long acting beta-agonists and inhaled corticosteroids. Of course, the design should be in a double-dummy design fashion in large scale multi-centre studies. These studies should also include an assessment of the patient compliance and the patient preference which has not been accomplished in the presently available studies. Also, pharmacoeconomic studies could be helpful to assist health agencies.
In summary, it can be stated that while there were some differences in favor of combination therapies reported when the combination therapy was compared with inhaled steroid monotherapy there were no consistent differences when combination therapies were compared to bronchodilator monotherapies. Future trials are needed to lead to a proof-of-principle stage.
Disclosures
AF and TW have received research funding from several pharmaceutical companies with an interest in COPD. They have spoken at meetings sponsored by companies including GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Altanta Pharma, Schering Plough, and Novartis.
References
- BalterMSLa ForgeJLowDE2003Canadian guidelines for the management of acute exacerbations of chronic bronchitisCan Respir J10Suppl B3B32B
- BoschettoPQuintavalleSMiottoD2006Chronic obstructive pulmonary disease (COPD) and occupational exposuresJ Occup Med Toxicol11116756686
- CalverleyPMAndersonJACelliB2007Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med3567758917314337
- CalverleyPMBoonsawatWCsekeZ2003bMaintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J229129
- CalverleyPPauwelsRVestboJ2003aCombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet36144956
- CalverleyPWalkerP2003Chronic obstructive pulmonary diseaseLancet36210536114522537
- CelliBRCoteCGMarinJM2004The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med35010051214999112
- CelliBRMacNeeW2004Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J239324615219010
- FabbriLCaramoriGBegheB1998Chronic obstructive pulmonary disease international guidelinesCurr Opin Pulm Med476849612669
- FletcherCPetoR1977The natural history of chronic airflow obstructionBMJ116458871704
- Groneberg-KloftBKrausTvan MarkA2006Analysing the causes of chronic cough: Relation to diesel exhaust, ozone, nitrogen oxides, sulphur oxides and other environmental factorsJ Occup Med Toxicol1616722555
- HajiroTNishimuraKTsukinoM1998Comparison of discriminative properties among disease-specific questionnaires for measuring health related quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med157785909517591
- HananiaNADarkenPHorstmanD2003The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPDChest1248344312970006
- Hansen-FlaschenJH2003Update in pulmonary medicineAnn Intern Med1383192512585830
- JonesPWQuirkFHBaveystockCMLittlejohnsP1992A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory QuestionnaireAm Rev Respir Dis145132171595997
- KetelaarsCAJSchlosserMAGMostertR1996Determinants of health-related quality of life in patients with chronic obstructive pulmonary diseaseThorax5139438658367
- MahlerDAWirePHorstmanD2002Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16610849112379552
- McSweenyAJGrantIHeatonRK1982Life quality of patients with chronic obstructive pulmonary diseaseArch Intern Med14247387065785
- MurrayCJLopezAD1997aAlternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease StudyLancet3491498504
- MurrayCJLLopezAD1997bGlobal mortality, disability, and the contribution of risk factors: Global Burden of Disease StudyLancet349143642
- MurrayCJLLopezAD1997cMortality by cause for eight regions of the world: Global Burden of Disease StudyLancet349126976
- MurrayCJLLopezADMathersCDSteinC2001The Global Burden of Disease 2000 Project: global programme on evidence for health policy discussionGenevaWHO, paper number 36.
- NanniniLCatesCJLassersonTJPooleP2004Combined corticosteroid and long acting beta-agonist in one inhaler for chronic obstructive pulmonary diseaseCochrane Database Syst RevCD00379415266502
- National Heart LaBI2002NHLBI morbidity and mortality chartbookBethesdaUS Department of Health and Human Services, Public Health Service
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med16312567611316667
- PetoRSpeizerFECochraneAL1983The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observationAm Rev Respir Dis1284915006614643
- RennardSDecramerMCalverleyPM2002Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International SurveyEur Respir J2079980512412667
- SiafakasNMVermeirePPrideNB1995Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J813984207489808
- SocietyAT1987Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986Am Rev Respir Dis136225443605835
- SorianoJBMaierWCEggerP2000Recent trends in physician diagnosed COPD in women and men in the UKThorax557899410950900
- SzafranskiWCukierARamirezA2003Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J21748112570112
- WelteTGronebergDA2006Asthma and COPDExp Toxicol Pathol57S23540
- WoutersEFPostmaDSFokkensB2005Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trialThorax60480715923248