Abstract
This was a multicenter, randomized, double-blind within device, parallel-group, dose-ranging study. COPD patients (n = 202; 86% male; mean age: 61 years) were randomized to receive tiotropium 1.25 μg, 2.5 μg, 5 μg, 10 μg, or 20 μg Respimat® SMI (a novel, propellant-free device); tiotropium 18 μg HandiHaler®; placebo Respimat®; or placebo HandiHaler® for 3 weeks. The primary endpoint was trough FEV1 on Day 21. Other assessments included FVC, PEFR, rescue medication use, safety, and pharmacokinetics. In general, all active treatments improved the primary and secondary endpoints on Day 21 (steady state) compared with placebo. Tiotropium 5 μg Respimat®, 20 μg Respimat®, and tiotropium 18 μg HandiHaler® were statistically significantly higher than placebo for the primary endpoint (mean change in trough FEV1 was 150 mL (both Respimat® doses) versus 20 mL (placebo Respimat®); p < 0.05; and 230 mL (HandiHaler®) versus −90 mL (placebo HandiHaler®); p ≤ 0.001). The urinary excretion (up to 2 hours post-dose) of tiotropium 5–10 μg Respimat® was comparable with tiotropium 18 μg HandiHaler®; the overall incidence of adverse events was comparable across treatment groups. Tiotropium 5 and 10 μg Respimat® improve lung function in COPD patients and appear to be comparable with tiotropium 18 μg HandiHaler®.
Introduction
Tiotropium is well established as a first-line maintenance treatment in patients with chronic obstructive pulmonary disease (COPD) (CitationATS 1995; CitationGOLD 2005; CitationSaberi and O’Donnell 2005). Once-daily administration of this antimuscarinic agent provides 24-hour bronchodilation via prolonged blockade of muscarinic M3-receptors. At present, tiotropium is delivered via HandiHaler® (Boehringer Ingelheim, Ingelheim am Rhein, Germany) a dry-powder, breath-actuated inhaler, and this drug/delivery combination has many supporting clinical data (CitationNiewoehner et al 2000; CitationCasaburi et al 2002; CitationVincken et al 2002; CitationO’Donnell et al 2004; CitationMaltais et al 2005). Previous dose-ranging studies have shown that tiotropium 18 μg is the optimal dose for use with HandiHaler® and this is the marketed dose with this device (CitationMaesen et al 1995).
More recently, an innovative delivery device, the Respimat® Soft Mist™ Inhaler (SMI), has been developed (Boehringer Ingelheim, Ingelheim am Rhein, Germany). This multi-dose, propellant-free device, is powered by a compressed spring inside the inhaler (CitationDalby et al 2004; CitationZierenberg 1999), and the dose is loaded when the lower half of the device is turned through 180 degrees. This draws drug into the micropump, which is then released when the patient presses the dose-release button. The aerosol from the Respimat® SMI has a much lower velocity and longer duration than that observed with pressurized metered-dose inhalers (pMDIs), and the fine particle fraction (<5.8 μm) in Respimat® SMI accounts for 66% of aqueous drug solution (CitationHochrainer et al 2005; CitationZierenberg 1999). As a result, Respimat® SMI improves lung drug deposition, reduces oropharyngeal deposition, and may require a lower dose of drug than that normally used with either dry powder inhalers (DPIs) or pMDIs (CitationNewman et al 1998; CitationNewman 1999; CitationPitcairn et al 2005).
Against this background, the aim of this dose-ranging study was to establish the doses of tiotropium that are most efficacious with Respimat® SMI in patients with COPD. The maximum dose used in this study was tiotropium 20 μg. It was hypothesized that a lower dose would be needed than that used with HandiHaler® due to higher lung deposition with the Respimat® SMI device.
Materials and methods
Study design
This was a 3-week, multicenter, randomized, double-blind within device, parallel-group, active- and placebo-controlled, dose-ranging study (Study #205.127), which was performed in 15 centers across France. The study was designed to determine the dose(s) of tiotropium that were efficacious with Respimat® SMI, and to compare these doses with the marketed tiotropium 18 μg HandiHaler® in patients with COPD. Five different doses of tiotropium inhalation solution (1.25 μg, 2.5 μg, 5 μg, 10 μg, and 20 μg) were chosen for use with Respimat® SMI. Tiotropium 18 μg HandiHaler® was used as the active control. Placebo HandiHaler® and placebo solution Respimat® SMI were used as placebo controls with each respective device. The study was conducted from March 1998 to April 1999. It was performed in accordance with the Declaration of Helsinki, to the requirements of Good Clinical Practice principles and local regulations, and was sponsored by Boehringer Ingelheim.
Inclusion and exclusion criteria
Males or females aged ≥40 years with a diagnosis of COPD (30% ≤ FEV1 ≤65% of predicted normal value [CitationQuanjer et al 1993] and FEV1/FVC ≤70%), and a smoking history ≥10 pack-years were eligible. Patients with a history of asthma, rhinitis, atopy, a clinically significant disease, or a lower respiratory tract infection or exacerbation within the last 6 weeks were excluded. Pregnant women, nursing women, and women of childbearing potential not using contraception were also excluded. All patients provided written, informed consent to participate.
Cromolyn, nedocromil sodium, anticholinergics, antihistamines, hydroxyzine, astemizole, long-acting beta-agonists, other investigational drugs and regular use of daytime oxygen therapy were not allowed during the study. Any rescue medication considered appropriate by the physician, inhaled corticosteroids, and oral corticosteroids if stabilized within the 6 weeks prior to study entry (ie, ≤10 mg prednisone per day or equivalent) were allowed. Short-acting beta-agonists and oral theophylline were permitted if withdrawn prior to clinic visits.
Medication restrictions
Prior to the screening visit (Visit 1), long-acting inhaled beta-agonists were not permitted for 48 hours, short-acting inhaled anticholinergics were not permitted for 8 hours, and long-acting anticholinergics were not permitted for 12 hours prior to Visit 1. Short-acting oral beta-agonists were not permitted for 18 hours, long-acting oral beta-agonists were not permitted for 36 hours, oral anticholinergics (7 days), oral antihistamines (48 hours), oral hydroxyzine (96 hours), and astemizole (3 months). Tiotropium had never previously been administered to patients using Respimat® SMI.
Some medications were allowed throughout the study period, but had to be withdrawn prior to clinic visits; these agents were as follows (the withdrawal time period is indicated in brackets): short-acting inhaled beta-agonists (8 hours); short-acting oral theophylline (24 hours); and oral slow-release theophylline (48 hours). No washout period was required for inhaled corticosteroids; they could be continued throughout the study provided the dosage was constant during the 6 weeks prior to Visit 1.
Methods
Following screening (Visit 1), eligible patients were randomized to receive tiotropium 1.25 μg, 2.5 μg, 5 μg, 10 μg, or 20 μg delivered via the Respimat® SMI, tiotropium 18 μg delivered via the HandiHaler®, placebo Respimat® SMI, or placebo HandiHaler® for 3 weeks. Study drug was administered once daily (two puffs via Respimat® SMI or one capsule via HandiHaler®) in the morning between 8:00 and 10:00 hours. The study was double-blind within device, ie, there was no blinding between Respimat® SMI and HandiHaler®.
The primary endpoint was the change from baseline (Day 0; Visit 2) in morning pre-dose (ie, trough) FEV1 on Day 21 (Visit 5), ie, at steady-state. Trough FEV1 was calculated using the largest of three FEV1 readings measured during the last 2 hours of the 24-hour dosing interval. Secondary endpoints included FVC and rescue medication use. Daily peak expiratory flow rate (PEFR) measurements were recorded in diary cards. The patient was also asked if he/she was awakened during the night by coughing or shortness of breath.
All clinic spirometry measures were performed in accordance with ATS criteria (CitationATS 1995). All pulmonary function tests were conducted while the patient was in a seated position. At each time point, the spirometric manoeuvres were conducted in triplicate and the largest FEV1 and largest FVC were recorded after examining all of the acceptable curves. The timing of the baseline pulmonary function testing always started at the same time of day (07:00 hours ± 1 hour). A time window of 1 hour was permitted between the start (baseline pulmonary testing) on the first day (Day 0, Visit 2) and the start on the three other test days (Day 7 [Visit 3], Day 14 [Visit 4], and Day 21 [Visit 5]). The timing of subsequent hourly lung function testing during Visits 2, 3, 4, and 5 varied; a time window of 1 hour was permitted during these subsequent measures. FEV1 and FVC were measured at least 2 hours pre-dose and up to 4 hours (240 minutes) post-dose.
Adverse events were recorded throughout the trial. Changes in vital signs (electrocardiogram [ECG], heart rate, pulse rate, blood pressure) were recorded on test days (Days 0, 7, 14, and 21), and physical examinations and routine laboratory tests were recorded before and after the trial.
Pharmacokinetics
Urine samples were collected in two fractions lasting from −2 to 0 hours (± 5 minutes) pre-dose and from 0 to 2 hours (± 5 minutes) post-drug inhalation on Days 7, 14, and 21 (± 2 days). Diuresis was aided by the intake of 100 mL of water during both collection periods. Tiotropium concentrations were measured using a validated high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) assay. The assay had a limit of quantification of 10 pg/mL. The amount of tiotropium excreted unchanged in the urine (Ae) was determined pre- and post-dose; excretion data at steady state were evaluated between Days 7, 14, and 21 (± 2).
Analysis
The primary analysis was designed to test whether each dose of tiotropium Respimat® SMI was more effective than placebo. The primary endpoint and secondary clinic spirometry endpoints were evaluated by an analysis of covariance (ANCOVA) with baseline data as a covariate and treatment effect as a factor. Descriptive statistics were used for other secondary efficacy variables, safety, and pharmacokinetic data. All treated patients were included in the safety analysis. The intent-to-treat (ITT) population consisted of all patients who had been randomized to treatment, received at least one dose of drug, and had at least one valid baseline and one post-treatment evaluation. Using a standard deviation for average FEV1 post-drug administration of 0.17 L, a total of 192 patients (24 patients per treatment group) was planned to detect a 150 mL difference in mean FEV1 response between tiotropium Respimat® SMI and placebo at 5% level of significance with at least 80% power using a two-tailed t-test.
Results
Patient characteristics and disposition
A total of 294 patients were screened, and 202 eligible patients were randomized to treatment. Patient characteristics are shown in . The treatment groups were well matched in terms of age, COPD duration, lung function, and tobacco consumption (comparative data not shown). The majority were male (85.6%), the mean age was 60 years, the mean baseline FEV1 (% predicted) was 43.6%, the mean COPD duration was 10.5 years, and the mean tobacco consumption was 44.7 pack-years. Concomitant medication use was similar between treatment groups; 79.7% used beta-agonists, 62% used inhaled steroids, and 27.2% used theophylline. A total of 11 patients (5.4%) discontinued prematurely (3 for worsening of disease under study; 2 for worsening of pre-existing disease; 3 for other adverse events (AEs); 1 was not compliant; and 2 withdrew their consent).
Table 1 Baseline characteristicsTable Footnote* of randomized patients (n = 202)
Efficacy endpoints
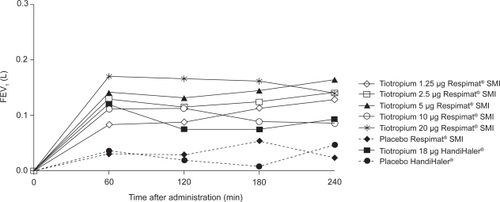
The primary endpoint, trough FEV1, was statistically significantly improved following treatment with tiotropium 5 μg Respimat® SMI, 20 μg Respimat® SMI, and tiotropium 18 μg HandiHaler® compared with placebo (p < 0.05) (). Tiotropium 10 μg Respimat® SMI showed a similar numerical advantage over placebo as that observed with the 5 μg and 20 μg Respimat® SMI doses; however, the difference approached, but did not reach, statistical significance (p = 0.06).
Table 2 MeanTable Footnote‡ (SEM) primary and secondary trough FEV1 measurements
Mean FEV1 was sustained above placebo for all active treatments throughout the study; the improvements following treatment on Day 21 (steady state) are shown in . At other time points, only the tiotropium 5 μg Respimat® SMI and tiotropium 18 μg HandiHaler® doses reached statistical significance on both Days 7 and 14 (largest: p < 0.05) (). FVC also improved after treatment with tiotropium Respimat® SMI and HandiHaler® compared with placebo. On Day 21, the greatest improvements in FVC were observed with the tiotropium 5 μg and 20 μg Respimat® SMI dose and with tiotropium 18 μg HandiHaler® (). All active treatments improved morning and evening PEFR on Day 21 compared with placebo (largest: p < 0.05) (). Rescue medication use declined in all active treatment groups, and with the exception of tiotropium 2.5 μg Respimat® SMI, the mean decrease for each treatment group was statistically different from placebo (p < 0.05). A trend in favor of active treatment over placebo was observed for nocturnal awakenings (data not shown).
Table 3 MeanTable Footnote‡ (SEM) secondary measurements during steady state
Pharmacokinetics
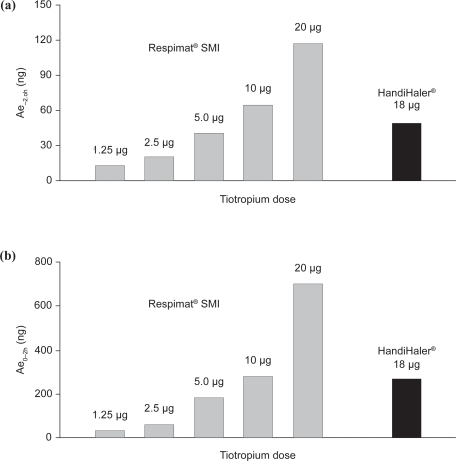
The urinary excretion of tiotropium Respimat® SMI at steady state, measured from −2 hours to 0 hours pre-dose and from 0 to 2 hours post-dose on Day 21, was dose-dependent as shown in . For both the pre- and post-dose measures, the urinary excretion of tiotropium 5–10 μg Respimat® SMI was comparable with that of tiotropium 18 μg HandiHaler®.
Safety
During the 3-week treatment period, 27.7% (56/202) of randomized patients reported AEs. The overall incidence of AEs was comparable across all active treatment groups and placebo. The most frequently reported AEs are shown in . Dry mouth was more common in the active treatment groups at doses higher than 5 μg. Eight patients withdrew from the study due to an AE: AV block (one patient on tiotropium 2.5 μg Respimat® SMI); COPD exacerbations (one patient from each of the tiotropium 2.5 μg Respimat® SMI, tiotropium 10 μg Respimat® SMI, placebo Respimat® SMI, and placebo HandiHaler® groups); dyspnea (one patient on tiotropium 18 μg HandiHaler®); pneumonia (one patient on tiotropium 1.25 μg Respimat® SMI); and hematuria (one patient on tiotropium 2.5 μg Respimat® SMI). Six patients had serious adverse events (SAEs) (only one was considered to be study related: hematuria [tiotropium 2.5 μg Respimat® SMI]). There were no patient deaths during the trial. There were no clinically relevant changes in laboratory values (hematology, biochemistry), vital signs, or ECG.
Table 4 Total and individual adverse events (AEs) reported in ≥5% of patients in any treatment group
Discussion
The aim of this dose-ranging study was to identify the doses of tiotropium delivered from the Respimat® SMI that were effective and well tolerated, and were most comparable with tiotropium 18 μg HandiHaler®. All active doses used with Respimat® SMI improved the primary endpoint (trough FEV1 after 3 weeks of treatment), and reduced rescue medication use compared with placebo. In general, tiotropium 5 and 10 μg Respimat® SMI were most comparable to tiotropium 18 μg HandiHaler® in terms of some efficacy measures and pharmacokinetics. The tiotropium 20 μg dose did not offer additional efficacy advantages over those observed with 5 and 10 μg, and the systemic exposure was much higher compared with tiotropium 18 μg HandiHaler®. As a result, this higher dose (20 μg) was not investigated further in phase III studies of tiotropium using Respimat® SMI.
Previous studies (CitationNewman et al 1996, Citation1998), which compared flunisolide delivery from Respimat® SMI with a metered dose inhaler (MDI), showed that significantly more flunisolide was delivered to the lungs with the Respimat® SMI device. In one study the lung deposition was almost twice that observed with an MDI (45% versus 26%, respectively) (CitationNewman et al 1998). Similar findings have been observed when Respimat® SMI was compared with a dry powder inhaler (DPI); the lung deposition of budesonide was 46%–57% with Respimat® SMI compared with 14%–33% from a Turbuhaler DPI used with fast and slow peak inhaled flow rates (CitationPitcairn et al 2005).
These findings therefore show that the Respimat® SMI device offers increased lung deposition and lower oropharyngeal deposition, compared with other devices; this benefit may be irrespective of the drug used. This selective delivery pattern could therefore improve the therapeutic ratio of inhaled medications when delivered via Respimat® SMI. It will also enable a lower nominal dose of drug to be used with Respimat® SMI. In one study, a 50% reduction in dose of ipratropium/fenoterol was appropriate with Respimat® SMI to achieve similar therapeutic efficacy to an MDI (CitationKilfeather et al 2004).
In the present study, all active treatments were well tolerated. Dry mouth was more common on active treatment than placebo, which was not surprising given the anticholinergic effect of tiotropium; this has also been shown in previous studies (CitationVincken et al 2002; CitationBrusasco et al 2003; CitationKoumis and Samuel 2005). The incidence of dry mouth was lower in all tiotropium doses below 10 μg; for example, 5 μg Respimat® SMI group compared with tiotropium 10 μg and tiotropium 18 μg HandiHaler® (4% versus 11.5% and 8%, respectively), which is suggestive of dose dependency. Based on the principles of seamless transition from phase II to phase III studies, it is prudent to carry forward one or two “best” doses (CitationBretz et al 2006). Phase III trials have further examined tiotropium 5 and 10 μg doses for use with Respimat® SMI. Dry mouth is unlikely to be an issue with the lower tiotropium dose without compromising efficacy in these clinical studies.
In conclusion, based on the safety, efficacy, and pharmacokinetics data obtained in this study, the 5 and 10 μg doses of tiotropium administered with Respimat® SMI can reasonably be considered as the best choice of safe and effective doses and are, in general, the most comparable doses with the active comparator, tiotropium 18 μg HandiHaler®.
Acknowledgements
The authors would like to thank the following for their participation: Alain Patat, Arnaud Beck, Jean Pierre Kantelip, Christophe Pison, Patricia Molinier Cournot, Michel Febvre, Benoit Wallaert, Regine Rouzier Panis, Jean-Marie Grosbois, Jean-François Muir, Violette Leclerc. The authors would like to acknowledge PAREXEL for their writing assistance.
Disclosures
This work was sponsored by Boehringer Ingelheim Ltd.
Demetri Pavia is an employee of Boehringer Ingelheim Ltd.
References
- [ATS] American Thoracic Society1995Standardization of spirometry: 1994 updateAm J Respir Crit Care Med1521107367663792
- BretzFSchmidliHKonigF2006Confirmatory seamless phase II/III clinical trials with hypotheses selection at interim: general conceptsBiom J486233416972714
- BrusascoVHodderRMiravitllesM2003Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax5839940412728159
- CasaburiRMahlerDAJonesPW2002A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J192172411866001
- DalbyRSpallekMVoshaarT2004A review of the development of Respimat® Soft MistTM InhalerInt J Pharm2831915363496
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Executive summaryBethesda, MDNational Institutes of Health Updated 2006. URL: http://www.ersnet.org/lrPresentations/copd/files/main/contenu/pages/full_text.pdf (last accessed 11 June 2007)
- HochrainerDHolzHKreherC2005Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalersJ Aerosol Med182738216181002
- KilfeatherSAPonitzHHBeckE2004Improved delivery of ipratropium bromide/fenoterol from Respimat Soft Mist Inhaler in patients with COPDRespir Med983879715139567
- KoumisTSamuelS2005Tiotropium bromide: a new long-acting bronchodilator for the treatment of chronic obstructive pulmonary diseaseClin Ther273779215922812
- MaesenFPVSmeetsJJSledsensTJH1995Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD)Eur Respir J81506138575576
- MaltaisFHamiltonAMarciniukD2005Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest12811687816162703
- NewmanSP1999Use of gamma scintigraphy to evaluate the performance of new inhalersJ Aerosol Med12Suppl 1S253110623338
- NewmanSPBrownJSteedKP1998Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicationsChest113957639554631
- NewmanSPSteedKPReaderSJ1996Efficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizerJ Pharm Sci8596048877887
- NiewoehnerDERiceKCoteC2000Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med14331726
- O’DonnellDEFlugeTGerkenF2004Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J238324015218994
- PitcairnGRReaderSPaviaD2005Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbohaler dry powder inhalerJ Aerosol Med182647216181001
- QuanjerPHTammelingGJCotesJE1993Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory SocietyEur Respir J Suppl165408499054
- SaberiFO’DonnellDE2005The role of tiotropium bromide, a long-acting anticholinergic bronchodilator, in the management of COPDTreat Respir Med42758116086600
- SchuermannWSchmidtmannSMoroniP2005Respimat Soft Mist Inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfactionTreat Respir Med4536115725050
- VinckenWvan NoordJAGreefhorstAP2002Improved health outcomes in patients with COPD during 1-year treatment with tiotropiumEur Respir J192091611871363
- ZierenbergB1999Optimizing the in vitro performance of RespimatJ Aerosol Med12Suppl 1S192410623337