Abstract
The objective of this in-vitro study was to determine whether mixtures of three nebulizable drugs are physicochemically compatible. Drug combinations were prepared by mixing the content of one respule Flutide® forte “ready to use” (fluticasone propionate) with 2 milliliter Atrovent® LS (ipratropium bromide) and 0.5 milliliter Sultanol® inhalation solution (albuterol sulfate). Test suspensions were stored at room temperature and exposed to normal laboratory light for 5 hours. Concentrations of fluticasone-17-propionate, ipratropium bromide, and albuterol sulfate were determined by using stability-indicating high-performance liquid chromatography assays with ultraviolet detection. Physical compatibility was determined by measuring pH and osmolality. Main outcome measures were the drug concentrations of the active components of the mixtures. All drug concentrations retained nearly 100% of the initial drug concentrations after mixing and storage in glass containers at room temperature. Osmolality and pH of the mixtures exhibited no significant changes and no visible changes of the mixtures were detectable over the inspection period. Mixtures of fluticasone propionate, ipratropium bromide, and albuterol sulfate inhalation drug products were shown to be physicochemically compatible over a period of 5 hrs. In order to avoid contamination and microbiological instability, mixing should only take place immediately before administration. Further investigations are needed to determine whether or not drug delivery is affected by mixing the nebulizer suspensions and to ensure that simultaneous nebulization is recommendable.
Introduction
For patients suffering from airway diseases, inhalation of aerosolized medications is a mainstay of therapy. Especially for patients with poor inhalation pattern, eg, small children and patients suffering from chronic obstructive pulmonary disease (COPD), nebulization is the preferred method of administration. Nebulizers convert drug solution/suspension by ultrasound or a jet stream of compressed air into an aerosol. Aerosolized droplets or particles should be 1 to 5 μm diameter in size, to ensure that the droplets reach bronchioles (CitationBoe et al 2001).
Drug substances commonly used for inhalation therapy in COPD are: Dornase alfa (recombinant human deoxyribonuclease); antibiotics, ie, tobramycin or colistin, corticoidsteroids, ie, budesonide or fluticasone propionate; and bronchodilators, ie, albuterol sulfate and ipratropium bromide. The patients often need to inhale multiple doses of several nebulizable drugs daily. Each nebulization procedure takes about 15 minutes. Thus patients tend to mix drug solutions or suspensions for simultaneous nebulization. In order to help patients to make the best use of their inhalation drugs, knowledge of the compatibilitiy of drug solutions and suspensions for oral inhalation is a prerequisite. However the available data are limited (CitationKamin et al 2006).
Known data prove the compatibility and stability of ipratropium and albuterol inhalation solutions mixed together (CitationJacobson et al 1995; CitationNagtegaal et al 1997). Also mixtures of budesonide nebulizer suspension with ipratropium or albuterol formulations for oral inhalation were shown to be compatible (CitationSmaldone et al 2000b; CitationGronberg et al 2001; CitationMcKenzie et al 2004). To our knowledge compatibility information about mixtures of fluticasone nebulizer suspension with ipratropium and/or albuterol nebulizer solutions is not yet available.
Drug combinations were prepared in accordance with the product information and clinical practice by mixing the content of one respule Flutide® forte “ready to use” with 2 mL Atrovent® LS and 0.5 mL Sultanol® inhalation solution. Each 2 mL respule Flutide® forte “ready to use” contains 2.0 mg fluticasone-17-propionate (CitationFachinformation 2004a) in addition to polysorbate 20, sorbitan laurate, sodium dihydrogen phosphate dihydrate, disodium hydrogenphosphate, sodium chloride, and water for injection used as excipients. Atrovent® LS was withdrawn from a multiple unit container containing benzalkonium chloride (0.1 mg/mL) as a preservative (CitationFachinformation 2005). Additional excipients are disodium edetate and hydrochloric acid 3.6% to adjust pH value. From the different commercially available albuterol nebulizer solutions, we used Sultanol® inhalation solution for our studies. This multiple unit container also contains benzalkonium chloride as a preservative and sulphuric acid 10% to adjust pH value (CitationFachinformation 2004b).
Test suspensions were stored at room temperature and exposed to light. Concentrations of fluticasone-17-propionate, ipratropium bromide, and albuterol sulfate were determined by using stability-indicating high-performance liquid chromatography (HPLC) assays with ultraviolet detection. Physical compatibility was determined by measuring pH and osmolality. The results can be used to inform patients and healthcare personnel, if mixing of fluticasone propionate, ipratropium bromide, and albuterol sulfate formulations in nebulizer cups and simultaneous inhalation is feasible.
The data presented here have in part been published previously in abstract form (CitationSchwabe et al 2005).
Study aim
The objective of this study was to determine whether mixtures of the three nebulizable drugs fluticasone propionate (Flutide® forte “ready to use”), ipratropium bromide (Atrovent® LS), and albuterol sulfate (Sultanol® inhalation solution) are physicochemically compatible.
Methods
Sample preparation
All tests were performed with the commercially available nebulizer suspension Flutide® forte “ready to use” and the nebulizer solutions Atrovent® LS and Sultanol® inhalation solution. Mixtures were prepared in 10 mL glass containers with glass stoppers by mixing 2.0 mL of Flutide® forte “ready to use” (withdrawn from a 2 mL respule containing 2 mg fluticasone-17-propionate) with 2.0 mL of Atrovent® LS (withdrawn from a 20 mL multiple unit container containing 261 μg/mL ipratropium bromide × 1 H2O equivalent to 250 μg ipratropium bromide) and 0.5 mL of Sultanol® inhalation solution (withdrawn from a 10 mL multiple unit container containing 6 mg/mL albuterol sulfate equivalent to 5 mg/mL albuterol). For each HPLC assay, three test suspensions were prepared, gently mixed, and stored at room temperature under ambient light conditions (mixed daylight and normal laboratory fluorescent light). 450 μL samples or 1 mL samples were withdrawn from each test suspension for the determination of fluticasone-17-propionate or for the simultaneous determination of ipratropium bromide and albuterol sulfate, respectively, immediately after mixing and after 5 hours of storage. Samples were diluted in glass containers to a nominal volume of 10 mL by adding mobile phase (see ) or a mixture of acetonitrile/mobile phase 1:3 (see ), for determination of fluticasone-17-propionate or ipratropium bromide and albuterol sulfate, respectively, and resolved to a clear solution by shaking.
2.0 mL Flutide® forte “ready to use” only and 2.0 mL of Atrovent® LS plus 0.5 mL Sultanol® inhalation solution diluted with 2 mL 0.9% NaCl were assayed as control samples. Control samples were stored in the 10 mL glass containers and additionally in 13 mL polystyrene containers.
HPLC assays
Drug concentrations were determined by different assay methods. The assays were conducted on an HPLC system consisting of a Hewlett Packard HP series 1050 autosampler, a HP series 1050 on-line degasser, a HP series 1050 pump and a HP series 1050 UV detector MWD. In both assays injection volume was 40 μL and run time was 15 min. All assays were performed in triplicate. Data acquisition and integration were performed with the Hewlett Packard Software HPLC ChemStation (version Rev.A.02.05). Peak areas were used for quantification.
Samples with drug concentrations ≥ 90 % (mean) of the initial concentrations taken at time zero were defined as chemically compatible with regard to the drug substance determined.
Determination of fluticasone-17-propionate concentration
Fluticasone-17-propionate concentrations were determined by adapting the HPLC method of the European Pharmacopoeia monograph (CitationPharmacopoeia Europea 2005). The assay conditions are summarized in .
Chromatograms of Sultanol® inhalation solution or albuterol sulfate solution prepared from reference substance were assayed under the same conditions and showed a peak assigned to albuterol sulfate (retention time ~4.7 minutes), which did not interfere with the peak of fluticasone-17-propionate. Ipratropium bromide and benzalkonium chloride were not detectable by this assay.
The stability-indicating nature of the fluticasone assay was confirmed by analyzing base degraded solutions (NaOH 1 mol/l, 75 °C for 5 hrs) of Flutide® forte “ready to use” suspension, 1 mg/mL fluticasone-17-propionate suspension (prepared from reference substance) and 1.65 mg/mL albuterol sulfate solution (prepared from reference substance). Peaks of degradation products of fluticasone-17-propionate (retention times of 2–5 min) were clearly separated from the parent drug peak. The resultant chromatogram of albuterol sulfate solution showed no interference of the degradation products of albuterol sulfate with the peak of fluticasone-17-propionate.
The linearity of the method was evaluated at eight concentrations injected in triplicate (varying from 40% to 110% of Flutide® forte “ready to use”). The calibration curve constructed from plots of peak area versus fluticasone-17-propionate concentration was linear and the correlation coefficient was 0.9987.
Assay precision was determined with Flutide® forte “ready to use”. Solutions containing 20 μg/mL fluticasone-17-propionate were prepared and analyzed on the same day (“intra-day precision”) or on seven different days (“inter-day precision”).
Determination of ipratopium bromide and albuterol sulfate concentrations
Analysis of ipratropium bromide and albuterol sulfate concentrations were performed simultaneously using a HPLC method described previously by Citationvan den Bemt et al (1997). The assay conditions are summarized in .
Chromatograms of benzalkonium chloride solution (prepared from reference substance) assayed under the same conditions, showed a peak of benzalkonium chloride (retention time ~7 min) at the detection wavelength 205 nm, which did not interfere with the peaks of ipratropium bromide or albuterol sulfate. Fluticasone-17-propionate was not detectable with this assay.
The assay was validated as stability-indicating by analyzing forced-degraded ipratropium bromide and albuterol solutions. 2 mL Atrovent® LS mixed with 0.5 mL Sultanol® inhalation solution and solutions of the reference substances ipratropium bromide × 1 H2O (224 μg/mL) or albuterol sulfate (1.2 mg/mL) were degraded at 70–75 °C for 3–6 hrs with NaOH 1 mol/L or HCl 1 mol/L or H2O2 35%. The resultant chromatograms indicated that the degradation products were clearly separated from the parent drug peaks.
The linearity of the method was evaluated at eight concentrations injected in triplicate (varying from 14% to 130% of Atrovent® LS and Sultanol® inhalation solution). The calibration curve constructed from plots of peak areas versus concentrations of ipratropium bromide or albuterol sulfate was linear and the correlation coefficients were 0.9999 and 0.999, respectively.
Assay precisions were determined with Atrovent® LS and Sultanol® inhalation solution. Solutions containing 11.11 μg/mL or 55.56 μg/mL ipratropium bromide or albuterol sulfate were prepared and analyzed on the same day (“intra-day precision”) or on eight different days (“inter-day precision”).
Physical compatibility
Osmolality and pH of the mixtures and of the mixture components (Flutide® forte “ready to use”, Atrovent® LS, Sultanol® inhalation solution) were determined. Mixtures were tested after 1–1.5 hrs and 6 hrs of storage. Values of pH were measured with Merck pH test strips accurate for pH 4 to 7. Osmolality was determined via the freezing depression method with an osmometer (Osmomat −030, Gonotec GmbH, Germany). Test suspensions were visually inspected with the unaided eye for any changes over the entire test period.
Results
Mixtures of the three nebulizable drugs are defined as physicochemically compatible, when stability (decomposition of 10% or less) of each active ingredient and no change in osmolality, pH values and physical appearance are proven. According to this definition mixtures of Flutide® forte “ready to use” with Atrovent® LS and Sultanol® inhalation solution were designated to be physicochemically compatible over a period of 5 hrs.
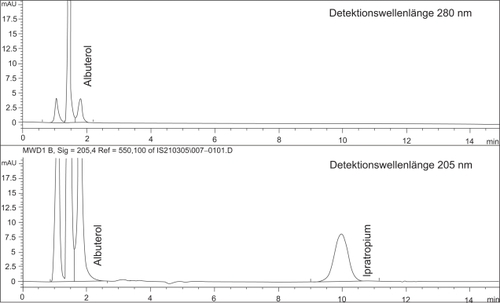
Drug concentrations of fluticasone-17-propionate, ipratropium bromide and albuterol sulfate retained nearly 100% of the initial drug concentrations after mixing and storage in glass containers at room temperature for 5 hours. Results of the HPLC assays are summarized in and . Measured variations of the concentrations fell within the range of the relative standard deviation of the method. No additional peaks of degradation products were detectable in the chromatograms of either assay (see and ).
Figure 1 Example of a chromatogram of the HPLC-determination of fluticasone-17-propionate in a diluted sample of the admixture of Flutide® forte Fertiginhalat “ready to use” 2,0 mg/2 ml with 2,0 ml Atrovent® LS and 0,5 ml Sultanol® after 5 h storage at room temperature.
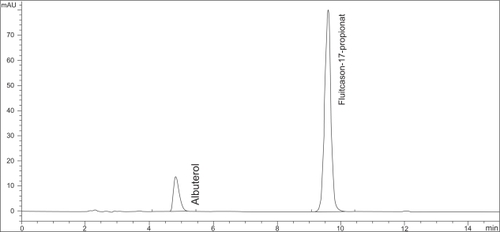
Figure 2 Example of a chromatogram of the simultaneous HPLC-determination of ipratopium and albuterol in a 1:10 diluted sample of the admixture of Flutide® forte “ready to use” 2.0 mg/2 ml with 2.0 ml Atrovent® LS and 0.5 ml Sultanol® after 5 h storage at room temperature.
The peak at retention time ~ 1 minute is designated to excipients in the nebulizable drugs.
Results of osmolality and pH measurements are shown in . The pH values of each of the nebulizable drug products and the mixture were in the range of 4 to 6, which corresponds to the specific limits set for nebulizer suspensions/solutions in the Pharmacopoeia Europea (pH 3–8.5). The ready to use Flutide® forte nebulizer suspension and Atrovent® LS inhalation solution were isotonic, whereas Sultanol® inhalation solution was hypotonic. Osmolality and pH of the mixtures exhibited no significant changes after 6 hrs of storage. No visible changes of the mixtures were detectable over the inspection period.
Table 4 Osmolality and pH values of the pure nebulizer suspension/solutions Flutide® forte “ready to use”, Atrovent® LS and Sultanol® Inhalationslösung (Inhalation solution) and mixtures of these 3 nebulizable drugs, stored under ambient light conditions at room temperature
Each of the drug products (Flutide® forte “ready to use”, Atrovent® LS, Sultanol®) tested was found to be compatible with and stable in the glass containers under the storage conditions chosen. However Flutide® forte “ready to use” proved incompatible with the polystyrene containers tested. Fluticasone-17-propionate concentrations declined to 90% of the nominal concentrations immediately after transfer of the nebulizer suspension to the polystyrene containers. The concentrations further declined during the test period of 25 hrs. Moreover the transparent polystyrene containers became opaque after 25 hrs of storage.
Discussion
The HPLC assays used were adapted from the literature and additionally validated to be stability indicating. Standard deviations of the results were acceptable although the suspension character of the inhalation mixtures made sampling more difficult. Prior to each sampling procedure the mixtures had to be homogenized in a standardized manner. In order to determine the ipratropium bromide and albuterol sulfate concentrations of the mixtures, samples of test suspensions had to be diluted with a mixture of acetonitrile and mobile phase in order to dissolve fluticasone-17-propionate. The chromatograms of these samples showed two peaks assigned to albuterol sulfate (retention times ~ 1.4 min., ~ 1.8 min). This phenomenon was probably due to the solution behaviour of albuterol sulfate in the solvent used. None of the chromatograms of forced-degraded albuterol solutions showed a peak with the same retention time. For the sake of simplicity only the peak area of the 10-fold increased peak at the retention time ~ 1.4 minutes was used for quantification of albuterol sulfate. This procedure was justified by the proven constant ratio of the peak areas.
Values of pH and osmolality are important factors influencing the tolerability of nebulizable drugs. Inhalation of acidic and/or hypoosmolar inhalation formulations may induce bronchoconstriction and coughing (CitationSchoeffel et al 1981; CitationElwood et al 1982; CitationEschenbacher et al 1984; CitationMann et al 1984; CitationBoulet et al 1987; CitationFine et al 1987; CitationBalmes et al 1988; CitationBeasley et al 1988; CitationO’Callaghan et al 1989). Nebulized formulations having pH values in the lower range of the established Ph. Eur. limits and osmolarities lower than 150 mOsm/L (no limits defined in the Ph. Eur.) may cause intolerance reactions. The observed hypotonicity of Sultanol® inhalation solution is of no clinical relevance, because this drug product is prescribed to be diluted with isotonic 0.9% sodium chloride solution prior to inhalation (CitationFachinformation 2004b). Mixing of the three components proved to be favourable for the resulting pH (ie, pH 6) and the resulting osmolality. In addition pH and osmolality remained unchanged over the test period indicating physical compatibility. No physical signs of change, such as colour changes or non-resuspendibility, were found with visual inspection at any point in time. However, formation of a precipitate might be difficult to detect, as Flutide® itself is formulated as a suspension.
Further investigations are necessary to determine the compatibility of Flutide® nebulizer suspension with nebulizer cups consisting of special plastic materials. We suggest that the substantial loss of fluticasone propionate observed in polystyrene containers was an effect of sorption. As Flutide® is marketed in polyethylene containers, sorption must be material specific and further experimental studies are necessary to decide whether different polystyrene types act in different ways. This issue will be investigated in the future.
The compatibility and physicochemical stability of the three drug admixture supports its clinical use for oral inhalation. However, simultaneous nebulization of mixtures of nebulizable medications can affect drug delivery of the components by eg, altering the aerosolized droplet size distribution. Due to the increased charge volume and the constant dead volume of the nebulizer, nebulization of mixtures can be more efficient. If nebulization is continued until the nebulizer runs dry, total mass output and inhaled mass of nebulized drug is increased (CitationClay et al 1983; CitationSmaldone et al 2000a; CitationMcKenzie et al 2002). The increase of nebulization duration is compensated by the convenience of not having to clean, reassemble and refill the nebulizer as necessary for consecutive nebulization.
Mixing drug products generally decreases concentrations of active ingredients and excipients, thus diminishing the bronchoconstrictive effects of excipients such as benzalkonium chloride or disodium edetate (CitationBeasley et al 1988). Decreased concentrations of preservatives may also lead to reduced microbiological stability of the mixtures. Therefore mixtures should be prepared directly before nebulization and surplus quantities should not be stored.
Conclusion
The time-consuming combination therapy of airway diseases with nebulizable corticosteroids and bronchodilators cries out for simultaneous instead of consecutive oral inhalation. Mixtures of fluticasone propionate (Flutide® forte “ready to use”), ipratropium bromide (Atrovent® LS) and albuterol sulfate (Sultanol® inhalation solution) inhalation drug products were shown to be compatible and physicochemically stable over a period of 5 hours in glass containers. In order to avoid contamination and microbiological instability, mixing should only take place immediately before administration. Further investigations are needed to determine whether drug delivery is affected by mixing the nebulizer suspension/solutions and to ensure that simultaneous nebulization is recommendable.
Materials
Flutide® forte “ready to use” 2.0 mg/2 mL: GlaxoSmithKline GmbH and Co. KG, Germany, lots: GR0002 and GR0025
Atrovent® LS: Boehringer Ingelheim Pharma GmbH and Co. KG, Germany, lots: 433418A, 433238A and 433812A
Sultanol® inhalation solution: GlaxoSmithKline GmbH and Co. KG, Germany, lots: C109486, C112951, C153571 and C158218
Fluticasone-17-propionate: grant by GlaxoSmithKline GmbH and Co. KG, Germany, lot: CCI 18781 GOD/65793
Ipratropium bromide Monohydrate: grant by Boehringer Ingelheim GmbH and Co. KG, Germany, lot: 1013218
Albuterol: catalog number 701154, Fagron GmbH and Co.KG, Germany, lot: 0401A030
Benzalkonium chloride: catalog number 700174-0002, Synopharm, Germany, lot: 0301A004
Sodium chloride 0.9%, preservative free: catalog number 2350548, B. Braun Petzold GmbH, Germany, lot: 4191C12 and 4411C12
NaOH 1 mol/l: catalog number 1.09137.1000, Merck, Germany, lot: OC411207
HCl 1 mol/l: catalog number 1.09057.1000, Merck, Germany, lot: OC408082
Water HPLC Gradient Grade: catalog number 4218, Mallinckrodt J.T. Baker, Germany, lots: 0433810014, 0413810002, 0501310012 and 0511710023
Acetonitrile: catalog number 9128, Promochem, Germany, lot: LC 301314, LC 146312 and LC 395414
Monobasic ammonium phosphate: catalog number 1.01126.0500, Merck, Germany, lot: A502226507
Phosphoric Acid 85%: catalog number 1.00573.1000, Merck, Germany, lot: K32782273 350
Methanol HPLC Grade: catalog number M/4056/17, Fisher Scientific, Germany, lots: 0443093, 0553512 and 0560085
KOH: catalog number 1.05021.0250, Merck, Germany, lot: B314921 326
Triethylamine: catalog number A3845, 0025, Applichem, Germany, lot: 4E000109
Filter 0.45 μm: catalog number FHLC04700, Millipore, Germany, lot: H3SN59888
Gass containers: catalog number Lenz 3.0214.13, VWR International GmbH, Germany
Polystyrene containers: catalog number 55.468.001, Sarstedt, Germany
Spezialindikator pH 4.0–7.0: catalog number 109542, VWR International GmbH, Germany
Conflict of interest
Flutide® forte “ready to use” and a grant for conducting these experiments were kindly provided by GlaxoSmithKline GmbH and Co. KG, but the company had absolutely no role in the content or conduct of the experiments. All authors negate any financial or other relationship in conjunction with this study that may lead to a conflict of interests.
Acknowledgements
The authors wish to thank Dr. Frank Erdnüß for his help in preparing this manuscript.
References
- BalmesJRFineJMChristianD1988Acidity potentiates bronchoconstriction induced by hypoosmolar aerosolsAm Rev Respir Dis1383592849338
- BeasleyRRaffertyPHolgateST1988Adverse reaction to the non-drug constituents of nebuliser solutionsBr J Clin Pharmacol2528383358893
- BoeJDennisJHO’DriscollBR2001European Respiratory Society guidelines on the use of nebulizersEur Respir J182284211510796
- BouletLPLegrisCThibaultL1987Comparative bronchial responses to hyperosmolar saline and methacholine in asthmaThorax4295383438883
- ClayMMPaviaDNewmanSP1983Assessment of jet nebulizers for lung aerosol therapyLancet2592946136746
- ElwoodRKHoggJCParéPD1982Airway response to osmolar challenge in asthma (abstract)Am Rev Respir Dis125617065512
- EschenbacherWLBousheyHASheppardD1984Alteration in Osmolality of inhaled aerosols cause bronchoconstriction and cough, but absence of a permanent anion causes cough aloneAm Rev Respir Dis129211156696320
- Fachinformation2004aFlutide® “ready to go“ 0.5 mg/2.0 mL Suspension, Flutide® forte “ready to go” 2.0 mg/2 mL Suspension, 08/2004 GlaxoSmithKline GmbH and Co. KG, München.
- Fachinformation2004bSultanol® “ready to go”, Sultanol® forte “ready to go”, Sultanol® inhalation solution, 04/2004 GlaxoSmithKline GmbH and Co. KG, München.
- Fachinformation2005Atrovent® LS, 04/2005 Boehringer Ingelheim Pharma GmbH and Co. KG, Ingelheim am Rhein.
- FineJMGordonTThompsonJE1987The role of titratable acidity in acid aerosol-induced bronchoconstrictionAm Rev Respir Dis135826303551704
- GronbergSMagnussonPBladhN2001Chemical Compatibility of Budesonide Inhalation Suspension (Pulmicort™) with other nebulization productsAm J Respir Crit Care Med163A588
- JacobsonGAPetersonGM1995Stability of ipratropium bromide and salbutamol nebuliser admixturesInt J Pharm Pract316973
- KaminWSchwabeAKrämerI2006Inhalation solutions-which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizersJ Cyst Fibros542051316678502
- MannJSHowarthPHHolgateST1984Bronchoconstriction induced by ipratropium bromide in asthma: relation to hypotonicityBMJ2894696235889
- McKenzieJGronbergSCruz RiveraM2002Budesonide inhalation suspension can be nebulized with solutions containing albuterol sulphate [poster]Chest122Suppl183S
- McKenzieJECruz-RiveraM2004Compatibility of budesonide inhalation suspension with four nebulizing solutionsAnn Pharmacother389677215084687
- NagtegaalJEDe JongADe WaardWJ1997Formulation and shelf life of two solutions for inhalationZiekenhuisfarmacie13239
- O’CallaghanCMilnerADSwarbrickA1989Paradoxical bronchoconstriction in wheezing infants after nebulised preservative free isoosmolar ipratopium bromideBMJ299143334
- Pharmacopoeia Europea20055th Ed. Deutscher Apotheker Verlag, Stuttgart, Govi-Verlag-Pharmazeutischer Verlag, Eschborn.
- SchoeffelRGAndersonSDAltounyanRE1981Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and salineBMJ2831285876794821
- SchwabeAKrämerIKaminW2005Physico-chemical compatibility of ipratropium, albuterol and fluticasone nebulizer solutionsDPhG-Jahrestagung, Mainz, 05.-08.10.2005; Abstract ISBN 3-00-016844-3.
- SmaldoneGCMcKenzieJCruz RiveraM2000aEffect of nebulizer volume on drug delivery: budesonide inhalation suspensionAm J Respir Crit Care Med161A36
- SmaldoneGCMcKenzieJCruz RiveraM2000bBudesonide inhalation suspension is chemically compatible with other nebulizing formulations [abstract]Chest11898S
- van den BemtPMLAde BijlGLangenMCJ1997Validation of two methods for the analysis of salbutamol/ipratropium inhalation fluidZiekenhuisfarmacie13925