Abstract
Measurement of inspiratory capacity (IC) as a marker of dynamic lung hyperinflation has been shown to correlate with dyspnea and exercise performance in stable COPD, and is therefore of potential utility in the management of this condition. We have examined whether similar relationships exist during acute exacerbations of COPD and asthma in order to determine whether there is a role for IC monitoring in acute management of these conditions. Eight patients with COPD and ten with asthma requiring hospital admission for acute exacerbations were studied with spirometry (including IC) at admission and at discharge and had concurrent self-perceived resting dyspnea ratings recorded. Over the admission there were significant improvements in resting dyspnea for the COPD group only, and improvements in spirometric indices in the asthma group only. No significant correlations were found between changes in dyspnea and changes in IC, in terms of acute responses to bronchodilator and in response to treatment over the hospital admission. These data suggest that dynamic hyperinflation during acute exacerbations of COPD and asthma is not as sensitive an indicator of resting dyspnea as in stable disease. A role for IC monitoring in the management of acute exacerbations of these diseases has not been identified.
Introduction
Demonstration of airflow obstruction is an essential requirement in the diagnosis of chronic obstructive pulmonary disease (COPD). The forced expiratory volume in one second (FEV1) is the spirometric index most commonly used to detect the presence of COPD, to characterize its severity and to determine bronchodilator response (CitationATS 1991). Although FEV1 is a strong prognostic indicator in COPD, it correlates poorly with both dyspnea level and limitation to exercise performance (CitationO’Donnell et al 1999), arguably the symptoms which are of most relevance to patients.
As well as being defined by the presence of airflow obstruction, COPD is characterized by dynamic hyperinflation of the lungs. Recent studies suggest that improvements in spirometric indices of hyperinflation in COPD are more closely related to dyspnea relief than are other more commonly-used measurements of airway caliber such as the FEV1 (CitationO’Donnell et al 1998; CitationO’Donnell et al 1999; CitationTaube et al 2000; CitationBoni et al 2002). Traditionally, the more complex measurements of total lung capacity (TLC) and functional residual capacity (FRC) have been used to detect and quantify hyperinflation. However, the inspiratory capacity (IC) provides an indirect measurement of end expiratory lung volume and is a technically simpler test.
Inspiratory capacity has been shown to increase significantly in ‘irreversible’ COPD (CitationPellegrino et al 1998; CitationTantucci et al 1998; CitationO’Donnell et al 2001), even in the absence of improvement in FEV1 (CitationTantucci et al 1998; CitationO’Donnell et al 2001) indicating that functional improvement is possible, independent of changes in maximal flow. Furthermore, increases in IC following bronchodilator are significantly correlated with improvements in dyspnea at rest (CitationO’Donnell et al 1999; CitationTaube et al 2000; CitationDi Marco et al 2003), with dyspnea during exercise (CitationO’Donnell et al 1998; CitationO’Donnell et al 1999), and with exercise performance (CitationO’Donnell et al 1999; CitationDiaz et al 2000). Monitoring IC has also been useful in describing changes in dynamic hyperinflation in response to oxygen therapy in COPD (CitationAlvisi et al 2003), and in response to induced bronchoconstriction in asthma (CitationLougheed et al 1993; CitationTantucci et al 1999).
These studies suggest that the measurement of IC could potentially be incorporated into the assessment and management of COPD as a simple, objective and clinically relevant measurement of disease status. The majority of previous studies investigating the utility of IC measurements in COPD have been performed in stable disease. The question of the applicability and usefulness of IC measurements during acute exacerbations of COPD has received little attention. We wished to determine if there is a role for IC measurement in the clinical management of acute exacerbations of COPD. In addition, given the relationship between IC and dyspnea observed in induced bronchoconstriction in asthma (CitationLougheed et al 1993; CitationTantucci et al 1999), IC measurement may also have a role in the management of asthma exacerbations.
The aims of the present study were (1) to investigate the relationships between dyspnea and IC during acute exacerbations of COPD and asthma, and (2) to assess the relationship between changes in IC and in dyspnea over the course of a hospital admission for an exacerbation of COPD or asthma.
Methods
Subjects
Adults requiring hospital admission for management of acute exacerbations of either asthma or COPD were prospectively recruited within 24 hours of admission. All subjects had received standard bronchodilator and corticosteroid therapy in the Accident and Emergency Department. Exclusion criteria were inability to reliably perform spirometry or to comprehend the use of the Borg dyspnea scale. The study protocol was approved by our institution’s medical research ethics committee and all subjects gave written informed consent.
Respiratory function tests
Respiratory function tests were performed using a computerized spirometry system (Sensormedics Vmax, Yorba Linda, USA) on two separate occasions: (1) within 24 hours of admission and (2) immediately prior to discharge from hospital. At least 4 hours abstinence from short-acting beta-2 agonists and anticholinergic therapy was required prior to each set of tests. A minimum of one minute of quiet tidal breathing was allowed on the mouthpiece-flowmeter assembly prior to each of at least three measurements of inspiratory capacity (IC) and slow vital capacity (VC). Best VC and average of three IC measurements matching to within 10% were used for analysis. Following these measurements, maximal flow volume curves were performed in accordance with ATS recommendations (CitationATS 1995). All spirometric testing was repeated 10 minutes after supervised administration of 200 μg of salbutamol delivered by metered dose inhaler via spacer. Resting dyspnea scores using the modified Borg scale (CitationBorg 1982) were obtained immediately prior to baseline and again immediately prior to post bronchodilator respiratory function testing. All baseline testing was performed within 24 hours of admission, and all tests were repeated in an identical fashion at the time of hospital discharge.
Statistical analyses
Student’s t-test was used for statistical analysis of paired comparisons and linear correlation used to detect relationships between variables. A p value of <0.05 was considered statistically significant.
Results
Twelve subjects with asthma and nine with COPD were recruited for the study and performed baseline measurements, however complete data was obtained in ten subjects with asthma and in eight with COPD. Reasons for non-completion were, in all cases, unwillingness to repeat spirometric measurements at the time of discharge. Only results from those subjects who completed the study are included in this report.
Subject characteristics are shown in . Subjects in the asthma group were younger and predominantly female. The COPD group was predominantly male and had a higher pack year history of cigarette smoking.
Table 1 Admission characteristics for all subjects completing the study. Values quoted are means (range). Statistical difference between groups indicated by * (p ≤ 0.001) or # (p < 0.0001)
Acute changes after bronchodilator
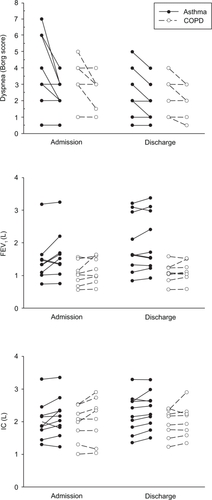
show the respiratory function data at both visits to the laboratory (within 24 hours of admission and at the time of discharge) for the asthma and COPD groups respectively. There were statistically significant acute improvements in dyspnea following bronchodilator for both patient groups on both visits. In addition, there were small acute bronchodilator improvements in VC at admission and in IC at discharge in the asthmatic group. No other parameter achieved statistically significant change following bronchodilator. shows spirometric and dyspnea values for individual patients.
Table 2a Mean (SEM) respiratory function and dyspnea data for patients with asthma. Baseline to post bronchodilator (Post BD) t-test comparisons are indicated by the p-value in the table
Table 2b Mean (SEM) respiratory function and dyspnea data for patients with COPD. Baseline to post bronchodilator (BD) t-test comparisons are indicated by the p-value in the table
Changes over the admission
Respiratory function data for both groups at discharge are shown in . Post-bronchodilator values were considered to provide more appropriate data for comparison over the course of the admission. The asthma group showed significant improvements in all measures of respiratory function (p = 0.05 for FEV1, p = 0.05 for VC, p = 0.04 for FEV1/VC and p = 0.006 for IC), whereas the COPD group showed no statistically significant changes. In contrast, resting dyspnea scores did not alter over the course of the admission in the asthma group, however there was a small improvement in resting dyspnea in the COPD group (p = 0.04).
Comparisons between asthma and COPD
Degree of airflow obstruction as measured by the FEV1/VC ratio was more severe in the COPD group compared with the asthma group: 42% versus 63% at admission, 42% versus 70% at discharge (p < 0.0001 for both). Although there was no statistical difference between the admission FEV1 for the asthma and COPD groups, at discharge the FEV1 was significantly higher in the asthma group (p = 0.016). Thus, notable differences were observed between the two patient groups in the changes in measured indices over the course of the admission. Spirometric indices improved in the asthma group, and dyspnea scores improved in the COPD group.
Correlations between parameters
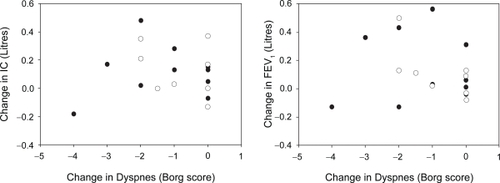
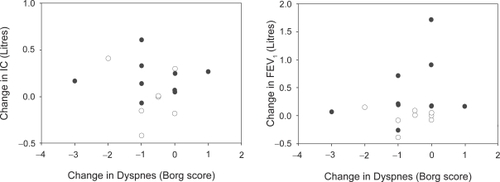
Acute changes in lung function parameters and in dyspnea scores following inhaled bronchodilator are shown in and reveal no significant relationships in either patient group. Data obtained at time of discharge similarly showed no significant correlation between these measurements. There was also no significant correlation between changes in these measures over the course of the admission ().
Discussion
We report an absence of correlation observed between change in dyspnea score and change in IC over the course of a hospital admission for treatment of exacerbations of asthma or COPD.
The theoretical reasoning that reductions in end expiratory lung volume should lead to improved lung mechanics and decreased work of breathing in COPD has been supported by a number of clinical studies over the past decade (CitationO’Donnell et al 1998, Citation1999; CitationTaube et al 2000; CitationBoni et al 2002). These studies have demonstrated that measures of lung hyperinflation correlate more strongly with dyspnea at rest and during exercise and with exercise performance than does FEV1. This work suggests that the emphasis in monitoring disease status in COPD should be shifted away from measures of airway caliber and function to measures of the degree of lung hyperinflation. Whilst functional residual capacity (FRC) and other lung volumes (such as residual volume and total lung capacity) have traditionally been used to describe lung hyperinflation, their measurement requires sophisticated, expensive instrumentation which is not always readily accessible. Relying on the assumption that total lung capacity does not change, IC can be used to provide a surrogate estimate of lung hyperinflation and requires no more than a simple spirometer for its measurement. Assessing changes in IC has been proposed as an important predictor of therapeutic response to bronchodilator therapy in COPD (CitationTantucci et al 1998; CitationO’Donnell et al 1999, Citation2001; CitationDuranti et al 2002).
For the COPD group, there was no change in the mean IC over the admission and no correlation between change in IC and change in dyspnea. However it was noted that the COPD patients who showed the largest improvement in IC over the admission (410 ml increase) also showed the largest improvement in resting dyspnea (−2 in Borg score). It has been suggested that an improvement in IC of equal to or greater than 10% of the predicted value corresponds with a real improvement in exercise performance (CitationO’Donnell et al 1999). Four of the ten patients in the asthma group and two of eight COPD patients exhibited such a change in IC over the course of the admission. However there were no significant differences in the changes in dyspnea scores between these ‘responders’ and the rest of the groups. This also applies to those who had at least a 10% of predicted acute improvement in IC in response to bronchodilator (two of ten and two of eight at admission, and two of ten and one of eight at discharge for asthma and COPD respectively).
Given that IC has been shown to provide a simple measure of hyperinflation which correlates with symptoms of dyspnea and exercise intolerance, it is not unreasonable to expect that IC should provide a useful index to monitor disease state during acute exacerbations of COPD. Our data show that over the course of an admission for treatment of disease exacerbations there was a measurable improvement in all spirometric indices in the asthma group, but in none for the COPD group. This is a not unexpected result for a measure of ventilatory function, given the nature of the two diseases – at least partly reversible obstruction for asthma, but essentially fixed airways disease for COPD.
Our data show that IC did not change significantly over an average 3 day hospital admission for COPD exacerbation. This is a similar finding to that of CitationParker et al (2005) who did not find any significant change over 7 days of recovery in a group of 20 COPD patients with less severe exacerbations, most of which did not require hospitalization. They did however find significant improvements in IC at 14 days which coincided with dyspnea improvements.
The observed improvement in resting dyspnea in the COPD group despite the lack of measurable physiological change may relate to the known euphoriant effect of corticosteroids in this patient group. There may also have also been a potential ‘I am being discharged therefore I must be feeling better’ phenomenon, however an improvement in dyspnea was not observed in the asthmatic group where a similar effect could be expected. Dyspnea in COPD is likely multifactorial in origin, with hyperinflation being only one of several mechanisms which may induce this symptom. Other factors which may impact on the cognitive sensation of dyspnea include the mechanical status of the chest (including degree of airflow obstruction) and the degrees of integration of respiratory afferent activity, respiratory motor drive, affective state, attention, experience and learning which exist in the individual patient (CitationO’Donnell DE et al 2007).
In our asthma group, self-perceived dyspnea on discharge from hospital did not improve despite the improvement in ventilatory function. In asthma, the perception of dyspnea during induced bronchoconstriction is known to be diminished in the presence of baseline airflow obstruction (CitationBurdon et al 1982). This is possibly due to a developing adaptation to poor lung function during periods of worsening asthma (CitationSalome et al 2002). At the time of admission when our subjects with asthma are clearly in a state of suboptimal asthma control, it is not unreasonable to expect poor perception of dyspnea and therefore dyspnea ratings somewhat unrelated to airway caliber. There is also evidence that perception of dyspnea improves with steroid therapy (CitationSalome et al 2002) and given that systemic corticosteroid therapy provides the mainstay of inpatient asthma management, our asthma patients are probably better perceivers of bronchial status at discharge compared with at admission. It is therefore not surprising that dyspnea on discharge from hospital did not improve despite improvement in ventilatory function.
The utility of IC as a measure of lung hyperinflation has been shown to be closely related to dyspnea during methacholine-induced bronchoconstriction in asthma (CitationLougheed et al 1993; CitationTantucci et al 1999). Its use in assessing bronchodilator response has received less interest in asthma than in COPD, presumably because of the utility of monitoring FEV1 in this disease. In one study in stable, mild asthma there was no correlation between change in dyspnea with change in FRC following bronchodilator (CitationLavietes et al 2001), and a similar finding in another study during acute exacerbations of asthma (CitationRosi et al 2002). Our data support these findings and differ from those assessing relationships between dyspnea and IC during induced bronchoconstriction (CitationLougheed et al 1993; CitationTantucci et al 1999). The differences in nature of onset of bronchoconstriction could explain these contrasting findings – acute bronchoconstriction from a relatively well preserved baseline is quite different from the slowly progressing deterioration that often precedes an exacerbation. Symptom perception is likely to be heightened in the former case whereas an adaptation or tolerance to impaired function may develop in the latter.
The lack of association between a change in IC and a change in dyspnea was not simply due to a lack of statistical power. Based on the data in the current study and assuming an α (significance) of 0.05 and a β (power) of 0.8, 78 subjects with asthma and 51 with COPD would have had to be tested to find a statistically significant association. These large numbers suggest the test has little clinical utility during exacerbations of COPD or asthma.
In summary, we have not been able to demonstrate any relationship between patient-perceived dyspnea and spirometric measurements of ventilatory function during acute exacerbations of asthma or COPD. In particular, changes in inspiratory capacity in these two disease states were not found to correlate with changes in dyspnea either as an acute response to bronchodilator, or in response to changes elicited during a hospital admission. A role for IC monitoring in the management of acute exacerbations of COPD and asthma has not been identified.
References
- AlvisiVMirkovicTNesmeP2003Acute effects of hyperoxia on dyspnea in hypoxemia patients with chronic airway obstruction at restChest12310384612684291
- [ATS] American Thoracic Society1991Lung function testing: selection of reference values and interpretative strategies [comment]American Review of Respiratory Disease1441202181952453
- [ATS] American Thoracic Society1995Standardization of Spirometry, 1994 updateAm J Respir Crit Care Med1521107367663792
- BoniECordaLFranchiniD2002Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at restThorax575283212037229
- BorgGA1982Psychophysical bases of perceived exertionMedicine and Science in Sports and Exercise14377817154893
- BurdonJGJuniperEFKillianKJ1982The perception of breathlessness in asthmaAm Rev Respir Dis12682587149447
- Di MarcoFMilic-EmiliJBoveriB2003Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPDEuropean Respiratory Journal21869412570114
- DiazOVillafrancaCGhezzoH2000Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at restEuropean Respiratory Journal162697510968502
- DurantiRFilippelliMBianchiR2002Inspiratory capacity and decrease in lung hyperinflation with albuterol in COPDChest12220091412475840
- LavietesMHMattaJTierskyLA2001The perception of dyspnea in patients with mild asthmaChest1204091511502637
- LougheedMDLamMForkertL1993Breathlessness during acute bronchoconstriction in asthma. Pathophysiologic mechanismsAmerican Review of Respiratory Disease148145298256884
- O’DonnellDEForkertLWebbKA2001Evaluation of bronchodilator responses in patients with “irreversible” emphysemaEuropean Respiratory Journal189142011829096
- O’DonnellDELamMWebbKA1998Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary diseaseAmerican Journal of Respiratory and Critical Care Medicine1581557659817708
- O’DonnellDELamMWebbKA1999Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary diseaseAmerican Journal of Respiratory and Critical Care Medicine160542910430726
- O’DonnellDEBanzettRBCarrieri-KohlmanV2007Pathophysiology of dyspnea in chronic obstructive pulmonary diseaseProc Am Thorac Soc41456817494725
- ParkerCMVoducNAaronSD2005Physiological changes during symptom recovery from moderate exacerbations of COPDEur Respir J26420816135722
- PellegrinoRRodarteJRBrusascoV1998Assessing the reversibility of airway obstructionChest1141607129872196
- RosiELaniniBRonchiMC2002Dyspnea, respiratory function and sputum profile in asthmatic patients during exacerbationsRespiratory Medicine967455012243322
- SalomeCMReddelHKWareSI2002Effect of budesonide on the perception of induced airway narrowing in subjects with asthmaAm J Respir Crit Care Med165152111779724
- TantucciCDuguetASimilowskiT1998Effect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease patientsEuropean Respiratory Journal127998049817148
- TantucciCEllaffiMDuguetA1999Dynamic hyperinflation and flow limitation during methacholine-induced bronchoconstriction in asthmaEuropean Respiratory Journal1429530110515404
- TaubeCLehnigkBPaaschK2000Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1622162010903244