Abstract
COPD is a complex disease with multiple pathological components, which we unfortunately tend to ignore when spirometry is used as the only method to evaluate the disorder. Additional measures are needed to allow a more complete and clinically relevant assessment of COPD. The earliest potential risk factors of disease in COPD are variations in the genetic background. Genetic variations are present from conception and can determine lifelong changes in enzyme activities and protein concentrations. In contrast, measurements in blood, sputum, exhaled breath, broncho-alveolar lavage, and lung biopsies may vary substantially over time. This review explores potential markers of early disease and prognosis in COPD by examining genetic markers in the α1-antitrypsin, cystic fibrosis transmembrane conductance regulator (CFTR), and MBL-2 genes, and by examining the biochemical markers fibrinogen and C-reactive protein (CRP), which correlate with degree of pulmonary inflammation during stable conditions of COPD. Chronic lung inflammation appears to contribute to the pathogenesis of COPD, and markers of this process have promising predictive value in COPD. To implement markers for COPD in clinical practice, besides those already established for the α1-antitrypsin gene, further research and validation studies are needed.
Introduction
Chronic obstructive pulmonary disease is defined as a disease state characterized by airflow limitation that is not fully reversible; the airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases.Citation1 According to the WHO Global Burden of Disease Study, COPD is the fifth leading cause of death worldwide.Citation2 Projections from the WHO predict that COPD will continue to increase in the years to come, challenging the health services in countries where cigarette smoking is prevalent. Consequently, we need to improve the diagnostic and therapeutical tools against COPD.
COPD is a complex disease with multiple pathological components,Citation3–Citation6 which we unfortunately tend to ignore when spirometry is used as the only method to evaluate the disorder. Additional measures are needed to allow a more complete and clinically relevant assessment of COPD.Citation7–Citation9 This may enable better phenotyping of different types of the disease and help improve the evaluation of disease activity and efficacy of therapy. The earliest potential risk factors of disease in COPD are variations in the genetic background. Genetic variations are present from conception and can determine lifelong changes in enzyme activities and protein concentrations. In contrast, measurements in blood, sputum, exhaled breath, broncho-alveolar lavage, and lung biopsies may vary substantially over time. The best known inherited risk factor for early-onset COPD is genetically deficient plasma levels of α1-antitrypsin.
In this review we have explored potential markers of early disease and prognosis in COPD using data from the prospective epidemiological study, the Copenhagen City Heart Study,Citation10–Citation12 and information from two Danish National Registers covering all hospital discharges and causes of death in the country. The investigations are focused on genetic markers in the α1-antitrypsin and cystic fibrosis transmembrane conductance regulator (CFTR) genes, the two most important known genes for obstructive lung disease, and the MBL-2 gene in which functional polymorphisms have been associated with features of COPD in previous reports.Citation13,Citation14 Since COPD is defined by an abnormal inflammatory response to noxious particles or gases, elevated lung inflammation could also represent an early pathological event in COPD. Fibrinogen and C-reactive protein (CRP) correlate with degree of pulmonary inflammation during stable conditions of COPD,Citation15,Citation6 and could potentially be useful as markers of early disease and prognosis in COPD.
α1-antitrypsin deficiency and COPD
The Z allele substitutes lysine for glutamic acid at position 342 in the α1-antitrypsin gene, while the S allele substitutes valine for glutamic acid at position 264. The Z allele and to a lesser degree the S allele can cause α1-antitrypsin to polymerise in hepatocytes leading to reduced plasma levels of α1-antitrypsin and higher risk for COPD.Citation17 Severe α1-antitrypsin ZZ deficiency is the most important known genetic risk factor for COPD. Individuals with less severe α1-antitrypsin deficiency (MS, MZ, SZ genotypes) could also have an increased susceptibility for COPD. We compared plasma α1-antitrypsin level, decline in lung function, and risk of hospitalization for COPD in α1-antitrypsin MS, MZ, SZ genotypes versus. controls.Citation18,Citation19 We found a stepwise reduction in plasma α1-antitrypsin with increasing severity of the α1-antitrypsin genotype (). The MZ, SZ and ZZ genotypes were associated with reduced plasma α1-antitrypsin, greater annual FEV1 decline (ZZ only borderline), and with greater risks of spirometry-defined airway obstruction and COPD (MZ and SZ only borderline for airway obstruction) (). Although MS was associated with lower plasma α1-antitrypsin level, this genotype did not confer greater FEV1 decline or increased risk of airway obstruction and COPD.
Figure 1 Plasma α1-antitrypsin, FEV1 decline, and risk of airway obstruction or COPD by α1-antitrypsin genotype. Values are mean ± SEM, odds ratio [OR] (95% CI), or hazard ratio [HR] (95% CI). ***p < 0.001, **p < 0.01, *p < 0.05. Data derived from Dahl M, Tybjaerg-Hansen A, Lange P, Vestbo J, Nordestgaard BG. Ann Intern Med. 2002;136:270–279.Citation18
![Figure 1 Plasma α1-antitrypsin, FEV1 decline, and risk of airway obstruction or COPD by α1-antitrypsin genotype. Values are mean ± SEM, odds ratio [OR] (95% CI), or hazard ratio [HR] (95% CI). ***p < 0.001, **p < 0.01, *p < 0.05. Data derived from Dahl M, Tybjaerg-Hansen A, Lange P, Vestbo J, Nordestgaard BG. Ann Intern Med. 2002;136:270–279.Citation18](/cms/asset/416b1431-88eb-4398-bb7a-4c0246ada762/dcop_a_3106_f0001_b.jpg)
To put these results into an international context, we identified previous studies on COPD risk in intermediate α1-antitrypsin deficiency, aggregated the results using meta-analyses, and calculated summary risk estimates for COPD in MS, MZ, and SZ individualsCitation20,Citation21 (). Seventeen studies were included in meta-analyses on MS, 16 in meta-analyses on MZ, and 6 in meta-analyses on SZ. The summary odds ratio for MS was increased at 1.2 (1.0–1.4), the summary odds ratio for MZ was increased at 2.3 (1.6–3.4), and the summary odds ratio for SZ was increased at 3.3 (1.2–8.6). Previous publications indicate that the magnitude of the COPD risk in MZ differs by study design. Thus, we performed a meta-analysis for MZ stratified by study design. After stratification, the summary odds ratio for MZ was 1.5 (0.97–2.3) in cross-sectional studies and 3.0 (2.1–4.3) in case-control studies. The summary odds ratios for MS and SZ, and the summary odds ratio for MZ in cross-sectional studies, did not differ considerably from the results provided by the Copenhagen City Heart Study.Citation18–Citation21
Figure 2 Cross-sectional and case-control studies of COPD risk in protease inhibitor MS, MZ, and SZ heterozygotes versus MM individuals. Box sizes are proportional to inverse-variance weights (random effects model). Lines represent 95% confidence intervals. Adapted with permission from Dahl M, Hersh CP, Ly NP, Berkey CS, Silverman EK, Nordestgaard BG. Eur Respir J. 2005;26:67–76.Citation20 Copyright © 2005 European Respiratory Society Inc, and from Hersh CP, Dahl M, Ly NP, Berkey CS, Nordestgaard BG, Silver-man EK. Thorax. 2004;59:843–849.Citation21 Copyright © BMJ Publishing Group.
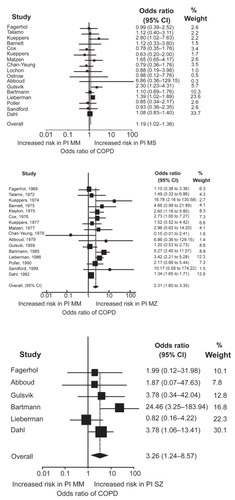
The meta-analyses suggest that risk of COPD is marginally elevated in MS and MZ individuals (in cross-sectional studies), whereas the more severe SZ genotype is an important risk factor for COPD. It is possible that MS, MZ, and SZ have greater risk estimates in subgroups of patients or in individuals with other additional risk factors for COPD, but further studies of very large populations are needed to determine if this is the case. We now offer an analysis in COPD patients that detects the S and Z alleles of the α1-antitrypsin gene with a simultaneous measurement of plasma α1-antitrypsin.Citation22 This will help clinicians to detect α1-antitrypsin-deficient individuals with high risk of COPD, and opens up for early smoking prevention counselling and potential therapy to individuals identified with α1-antitrypsin deficiency through this new analysis.
Cystic fibrosis F508del and COPD
Cystic fibrosis is characterized by progressive obstructive lung disease in homozygous individuals, and it is possible that heterozygotes for cystic fibrosis are at increased risk of less severe forms of obstructive lung disease. To examine this, we screened 9141 individuals for the common F508del allele, and we compared lung function and prevalences of COPD and asthma in F508del heterozygous individuals versus noncarriers.Citation23,Citation24
Two hundred and fifty individuals were heterozygous and none homozygous for the cystic fibrosis F508del allele.Citation23,Citation25 Among heterozygotes, none had previously been admitted to a hospital due to cystic fibrosis or carried an additional 394delTT or N1303K allele in the CFTR gene.Citation24 Second to the F508del allele, the 394delTT and N1303K alleles are the two most common cystic fibrosis alleles in Denmark with allele frequencies of about 1% or more in the Danish population.Citation26
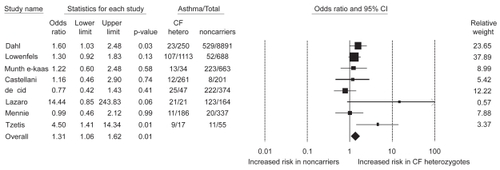
Based on triplicate measurements of pulmonary function, we found that heterozygous individuals had lower FEV1 and FVC than noncarriers ().Citation24 Consistent with this, heterozygous individuals also reported that they had asthma more often than noncarriers, whereas the prevalence of COPD did not differ between the two groups ().Citation23,Citation24 These findings were independent of influence from the common but less severe polythymidine tract variants in intron-8 of the CFTR gene.Citation27 The shorter this polythymidine tract is, the more often exon-9 is skipped from CFTR mRNAs leading to a CFTR protein without chloride activity.Citation28 Studies of asthma risk in cystic fibrosis heterozygotes, however, have not convincingly shown increased asthma among heterozygotes.Citation23,Citation24,Citation27,Citation29–Citation39 Combining all the available data from the literature in a meta-analysis,Citation23,Citation29,Citation31,Citation34–Citation37,Citation39 the summary odds ratio for asthma in cystic fibrosis/F508del heterozygotes is 1.3 (1.1–1.6; p = 0.01) ().
Figure 3 Levels of FEV1 and FVC by cystic fibrosis F508del carrier status. Values are means ±SEM, based on 10-year age groups. Number of measurements: F508del, n = 270; and noncarriers, n = 10,002. P-values are by general linear repeated-measures analysis comparing F508del heterozygotes versus noncarriers. Adapted with permission from Dahl M, Nordestgaard BG, Lange P, Tybjaerg-Hansen A. J Allergy Clin Immunol. 2001;107:818–823.Citation24 Copyright © Elsevier.
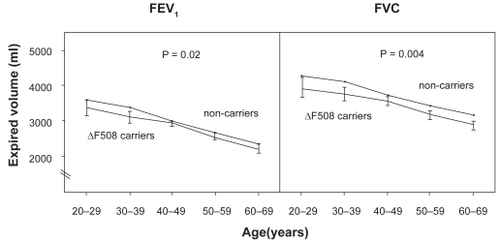
Figure 4 Odds ratios for asthma and COPD in cystic fibrosis F508del heterozygotes versus noncarriers in the 1976 to 1978, 1981 to 1983, and 1991 to 1994 examinations of the Copenhagen City Heart Study. Multiple logistic regression analyses allowed for age, sex, tobacco consumption, passive smoking history, and familial asthma. “Total” refers to disease diagnosed at at least one examination. Adapted with permission from Dahl M, Nordestgaard BG, Lange P, Tybjaerg-Hansen A. J Allergy Clin Immunol. 2001;107:818–823.Citation24 Copyright © Elsevier.
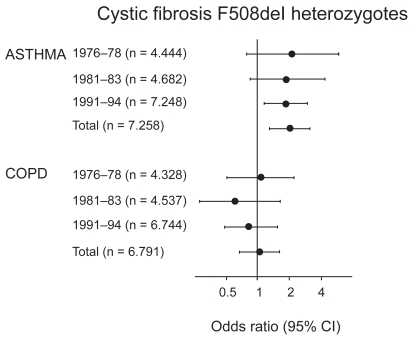
Figure 5 Cross-sectional and case-control studies of asthma risk in cystic fibrosis/F508del heterozygotes. Box sizes are proportional to inverse-variance weights (random effects model). Lines represent 95% confidence intervals.
In conclusion, cystic fibrosis F508del heterozygotes are not at greater risk of COPD, but may be overrepresented among people with asthma and have poorer lung function than non-carriers. Combining recent reports from the literature, the risk for asthma in cystic fibrosis heterozygotes is roughly 30% elevated. Thus, F508del cannot be used as a biomarker for COPD or asthma in clinical settings. It is possible that F508del can be used as a clinical marker for asthma in certain subgroups of patients in the future, when other susceptibility markers for asthma are available.
Mannose-binding lectin and COPD
Mannose-binding lectin (MBL) is an acute phase protein secreted from the liver.Citation40,Citation41 It has antibody-like function in the innate immune system, where it binds sugarmoieties on microorganisms and on apoptotic cells. Bound MBL can clear its targets through promoting opsonophagocytosis or by activating the complement system through MBL-associated protease 1 and 2. Deficiency of MBL levels/activity in plasma due to polymorphisms in the MBL-2 gene is thought to weaken normal innate immune functions against microorganisms and apoptotic cells in the body.Citation42 Genetically reduced MBL in plasma raises the susceptibility for disease in COPD and asthma.Citation13,Citation14,Citation43 Thus, we determined the risk of COPD and asthma in MBL deficient individuals versus controls in our study of the population at large.Citation44 We did not find an increased risk of COPD or asthma with MBL deficiency (). The risk of death due to respiratory disease was unaffected by MBL deficiency status.
Figure 6 Hazard ratios for asthma and COPD by mannose-binding lectin deficiency. Adapted with permission from Dahl M, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. J Exp Med. 2004;199:1391–1399.Citation44 Copyright © 2004 Rockefeller University Press.
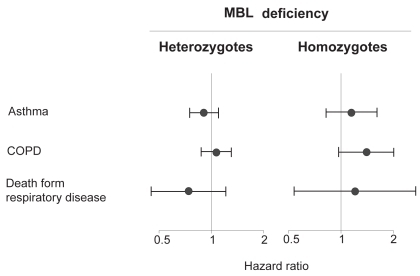
Only few other studies have examined the potential relation between MBL deficiency and obstructive lung disease.Citation13,Citation14,Citation43 Our study had ample statistical power to test the hypothesis that MBL deficiency is associated with risk of obstructive lung disease. In conclusion, genetic MBL deficiency is not a major risk factor for COPD in the Danish general population. At present, genetic variants in the MBL-2 gene cannot be used as biomarkers of obstructive lung disease.
In children, MBL could have an important function in pulmonary host defence during the vulnerable period when the adaptive immune system is immature.Citation45 We tested whether adult MBL deficient individuals more often than controls were hospitalized or died due to pulmonary infection, but found no increased frequency of pulmonary infection with MBL deficiency.Citation44 These results suggest that MBL does not have a major role in pulmonary defence against microorganisms in adult Danish individuals.
Other genetic markers of COPD
We have used a candidate gene approach to evaluate genetic markers in the α1-antitrypsin, CFTR, and MBL genes, and their relation to COPD. Other approaches include linkage studies, transcript-expression analyses, and animal model genetics, all of which can be used to identify certain genetic candidates for COPD. To translate results from such studies into clinical medicine, an estimation of risk of disease is needed. This can be achieved through association studies of the population at large such as ours.
In COPD recent focus has been on the enzyme serine proteinase inhibitor E2 (SERPINE2), as this enzyme is a family relative of α1-antitrypsin (SERPINA1), the most important risk factor of inherited COPD. Supporting that SERPINE2 is of importance to COPD susceptibility, polymorphisms in the SERPINE2 gene have been shown to associate with features of COPD.Citation46,Citation47 The enzymes epoxide hydrolase 1 (EPHX1) and superoxide dismutase 3 (SOD3) are also of potential interest as markers in COPD,Citation48–Citation51 as these enzymes may inhibit increased oxidative stress in the lungs due to cigarette smoke inhalations. Comprehensive reviews on other candidate genes in COPD are readily available.Citation52,Citation53
Plasma fibrinogen and COPD
Pulmonary inflammation in COPD is associated with increased levels of acute phase reactants in plasma, and these reactants could potentially be used to predict risk of future COPD events. To test whether the acute phase reactant fibrinogen predicts future COPD, we determined lung function and relative risk of COPD hospitalization in individuals with increased levels of plasma fibrinogen. We found that individuals with baseline plasma fibrinogen of more than 3.3 g/L versus less than 2.7 g/L had reduced lung function, increased cumulative incidence of COPD hospitalization (), and an increased relative risk of 1.7 (1.1–2.6) for COPD hospitalization during 6 years of follow-up.Citation54 These findings suggest that plasma fibrinogen is a significant predictor of future COPD in the population at large.
Figure 7 Kaplan-Meier curves showing rate of COPD hospitalizations during follow-up. Number at risk at the beginning of each year is shown below the horizontal axis. P < 0.001 for plasma fibrinogen >3.3 g/L versus <2.7 g/L, P = 0.003 for >3.3 g/L versus 2.7–3.3 g/L, and p = 0.31 for 2.7-3.3 g/L versus <2.7 g/L on log-rank test. Adapted with permission from Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Am J Respir Crit Care Med. 2001;164:1008–1011.Citation54 Copyright © 2001 American Thoracic Society.
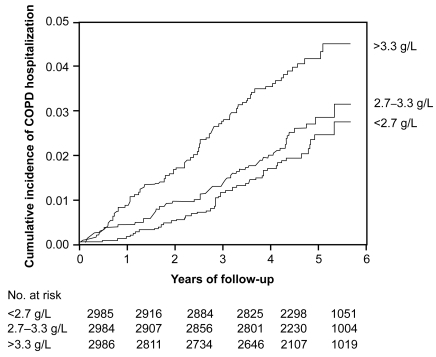
A later study confirms plasma fibrinogen as a marker of COPD prognosis: Gan et al estimated a 0.37 g/L difference in plasma fibrinogen between COPD patients and controls.Citation15 The clinical utility of plasma fibrinogen as a biomarker of COPD seems limited based on its low predictive value for COPD.Citation54 IL-6 regulates fibrinogen production from the liver. Thus, the results point to an important function for fibrinogen, IL-6, or factors upstream of IL-6 in the pathogenesis of COPD. Further studies are required to elucidate which part, if any, fibrinogen or IL-6 has in the progression of COPD.
Plasma C-reactive protein and COPD
COPD is associated with elevated C-reactive protein (CRP) concentration in serum which equals concentrations in plasma,Citation15 and it is possible that CRP as a marker of pulmonary inflammation could be used to predict risk of future COPD events. To test whether plasma CRP predicts future COPD events, we measured CRP in a subgroup of individuals with high risk of clinical COPD, that is, individuals with spirometry-defined airway obstruction.Citation55 During 8 years of follow-up, we recorded admissions and deaths due to COPD, and relative risks for COPD hospitalization or deaths were determined. We found that individuals with serum CRP of more than 3 mg/L versus ≤3 mg/L at baseline had increased cumulative incidence of COPD hospitalization and death (), an increased hazard ratio of 1.4 (1.0–2.0) for COPD hospitalization, and an increased hazard ratio of 2.2 (1.2–3.9) for COPD death.Citation55 Absolute risks for COPD hospitalization and death increased with baseline serum CRP levels (). The highest absolute 10-year risks for COPD hospitalization and death were 54% and 57% among individuals with baseline CRP above 3 mg/L, above 70 years of age, with a tobacco consumption of more than 15 g/day, and an FEV1% predicted less than 50%.
Figure 8 Cumulative incidence of COPD events according to baseline serum CRP levels. Cumulative incidences of COPD hospitalization and death were increased in individuals with baseline CRP > 3 mg/L versus ≤3 mg/L. Adapted with permission from Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Am J Respir Crit Care Med. 2007;175:250–255.Citation55 Copyright © 2007 American Thoracic Society.
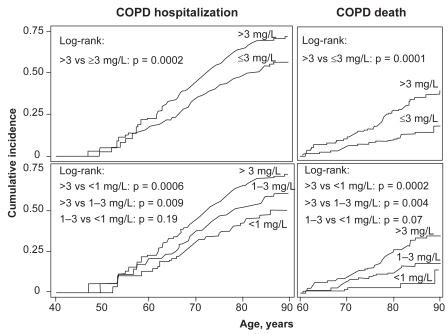
Figure 9 Absolute 10-year risks of COPD hospitalization and COPD death according to FEV1% predicted, tobacco consumption, age, and serum CRP. The dependent variables for the Poisson regressions are number of COPD hospitalizations or COPD deaths during the subsequent 10 years. The highest absolute 10-year risks for COPD hospitalization and death – 54% and 57% – were found among individuals with CRP > 3 mg/L, above 70 years of age, with tobacco consumption > 15 g/day, and FEV1%predicted <50%. Adapted with permission from Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Am J Respir Crit Care Med. 2007;175:250–255.Citation55 Copyright © 2007 American Thoracic Society.
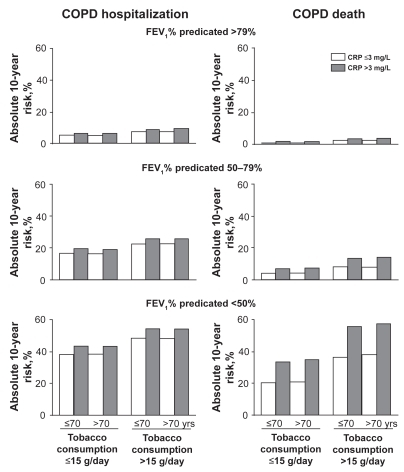
The data suggests an important role for CRP, or its main regulatory cytokine IL-6, in COPD progression and development. Supporting a role for IL-6 in COPD pathogenesis, IL-6 may stimulate inflammatory cell recruitment to the lung, and lead to emphysema-like airspace enlargement and respiratory muscle wasting in animal models of lung disease.Citation56–Citation65 Thus, our own and previous data seem to suggest that IL-6 or factors upstream of IL-6 are involved in the pathogenesis of COPD. In the future, CRP levels could be used clinically to assess prognosis in patients with airway obstruction. High absolute risk as depicted in may motivate patients at the highest risk to quit smoking or to receive medication.
Other biochemical markers of COPD
In COPD, biochemical biomarkers represent a new field that we have just begun to explore. The potential is great. COPD is a multicomponent disease for which airway inflammation plays an imperative role. This component, among several others, needs descriptive measures to provide a more comprehensive and clinically accurate assessment of COPD.
Biochemical COPD markers can be measured in exhaled breath, induced sputum, bronchoalveolar lavage fluid, lung biopsies, and plasma. Compared to biomarkers measured in other sample material, biochemical markers determined in plasma are reliably measured using equipment that is cost-effective and readily available in clinical settings. However, biochemical markers, and in particular those measured in plasma, may be modulated by morbidities other than COPD, and thus may in some circumstances represent epiphenomena unrelated to the COPD phenotype.
The validation of a biomarker has been proposed to involve the following three phases:Citation8 1) demonstration that the marker frequency/magnitude is associated with a clinical outcome. 2) Phase I/II trials demonstrating effects on the marker with therapeutic intervention. Are there dose-dependent effects of treatment on the marker? 3) Demonstration that treatment-related changes in the marker are associated with positive changes in clinical outcomes. Is the marker applicable to all disease stages and all interventions?
In COPD the biochemical markers are many, but these have been less well investigated than biochemical markers in asthma. When evaluating therapies for COPD, markers that could surrogate for critical pathological processes in COPD would be highly warranted. For example desmosine as part of mature elastin indicates elevated turnover of elastic fibres in COPD.Citation66 Novel lung-specific markers also appear promising,Citation67,Citation68 in particular surfactant protein-A, which is elevated in lung tissue and sputum in COPD patients when compared with controls in a proteomics study.Citation69 The exploration and validation of potential markers for COPD is currently ongoing and no specific marker has so far been implemented for clinical use.Citation7–Citation9,Citation70–Citation72
Conclusions
Using data from up to 9245 adult participants from the Copenhagen City Heart Study and information from the national Danish Hospital Discharge Registry and the national Danish Causes of Death Registry, we explored new potential markers of COPD.
The risk of COPD is marginally elevated for the genetic biomarkers, MS and MZ, in the α1-antitrypsin gene, whereas the SZ biomarker is an important risk factor of COPD. The ZZ genotype is already in clinical use as a genetic marker for early-onset COPD. We have set-up an analysis for diagnosing the SZ and ZZ genotypes together with measurement of plasma α1-antitrypsin levels to be used broadly, including in COPD patients. This will help clinicians to detect α1-antitrypsin-deficient individuals with a high risk for COPD, and opens up for early smoking prevention counseling and potential therapy for individuals identified with α1-antitrypsin deficiency through this new analysis.
The genetic biomarker, F508del, in the cystic fibrosis gene was not associated with COPD, but with increased asthma risk. Aggregating results from previous reports, the risk of asthma is only marginally elevated. Thus, F508del cannot at present be used as a marker of COPD or asthma in clinical settings.
Genetic deficiency of MBL is not a major risk factor for COPD or asthma, and it appears that genetic variants in the MBL-2 gene cannot at present be used as biomarkers for obstructive lung disease in the Danish population.
Elevated plasma fibrinogen and CRP levels were associated with increased COPD risk and may be good for prognostication once a diagnosis of COPD has been established. In the future after further validation, it is possible that these markers, particularly CRP, can be used to categorise individuals with low, medium, or high risk of COPD, and thus point out patients in need of intensified prevention and treatment for COPD. Furthermore, the observed association between COPD and elevated fibrinogen and CRP levels suggests that either factor or upstream factors regulating fibrinogen and CRP expression are important players in the pathogenesis of COPD. To determine whether fibrinogen or CRP are causally related to COPD, we are currently investigating if genetically increased levels of fibrinogen and CRP are associated with increased risk of future COPD. We anticipate that fibrinogen and CRP-regulating factors (IL-6, TNF-α, IL-1) rather than fibrinogen and CRP themselves are involved in COPD pathogenesis, as IL-6, TNF-α, and IL-1 are already factors with established functions in COPD. IL-6 is involved in inflammation, vascular permeability, and cell proliferation, while TNF-α, and IL-1 initiates and maintains inflammation, and activates endothelial and epithelial cells.Citation6
The best known early disease markers for COPD remain genetic variations in alpha-1-antitrypsin. They cause lifelong reduced levels of alpha-1-antitrypsin in plasma and increase the risk for COPD. As such, future studies on markers in COPD should ideally demonstrate association between a marker and COPD, as well as change in the COPD risk with changes in the marker concentration or function (genetically or medically induced). Randomized pharmacological trials or mendelian randomization designs are useful in this regard and will enable us to assess the importance of early markers for COPD much better. The study of disease markers in COPD can improve the phenotyping of different types of COPD. Linking the markers to critical pathologic events in COPD can provide novel insights into the pathogenesis of COPD. With the recent focus on heterogeneity in COPD and the mounting efforts in identifying novel markers for COPD this research field seems in progress.
Acknowledgments
Supported by the Danish Lung Association, Danish Heart Foundation, and Copenhagen County Foundation.
Disclosures
Neither author has conflicts of interest to disclose.
References
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med20011631256127611316667
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med20063e44217132052
- DahlMBauerAKArredouaniMProtection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/IIJ Clin Invest200711775776417332894
- OwenCARoles for proteinases in the pathogenesis of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2008325326818686734
- Taraseviciene-StewartLVoelkelNFMolecular pathogenesis of emphysemaJ Clin Invest200811839440218246188
- YoshidaTTuderRMPathobiology of cigarette smoke-induced chronic obstructive pulmonary diseasePhysiol Rev2007871047108217615396
- CazzolaMMacNeeWMartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J20083141646918238951
- JonesPWAgustiAGOutcomes and markers in the assessment of chronic obstructive pulmonary diseaseEur Respir J20062782283216585091
- VestboJAndersonWCoxsonHOEvaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE)Eur Respir J20083186987318216052
- Epidemiology of chest pain and angina pectoris, with special reference to treatment needsActa Med Scand Suppl198468211206587759
- The Copenhagen City Heart StudyOsterbroundersogelsen. A book of tables with data from the first examination (1976–78) and a five year follow-up (1981–83). The Copenhagen City Heart Study GroupScand J Soc Med Suppl19894111602711133
- SchnohrPJensenGLangePScharlingHAppleyardMThe Copenhagen City Heart Study – Østerbrounders⊘gelsen. Tables with data from the third examination 1991–1994Eur Heart J Suppl20013H1H83
- Garcia-LaordenMISole-ViolanJRodriguezDCMannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adultsJ Allergy Clin Immunol20081223687437418582923
- YangIASeeneySLWolterJMMannose-binding lectin gene polymorphism predicts hospital admissions for COPD infectionsGenes Immun2003426927412761563
- GanWQManSFSenthilselvanASinDDAssociation between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysisThorax20045957458015223864
- OlafsdottirISGislasonTThjodleifssonBGender differences in the association between C-reactive protein, lung function impairment, and COPDInt J Chron Obstruct Pulmon Dis2007263564218268938
- GooptuBLomasDAPolymers and inflammation: disease mechanisms of the serpinopathiesJ Exp Med20082051529153418591408
- DahlMTybjaerg-HansenALangePVestboJNordestgaardBGChange in lung function and morbidity from chronic obstructive pulmonary disease in alpha1-antitrypsin MZ heterozygotes: A longitudinal study of the general populationAnn Intern Med200213627027911848724
- DahlMNordestgaardBGLangePVestboJTybjaerg-HansenAMolecular diagnosis of intermediate and severe alpha(1)-antitrypsin deficiency: MZ individuals with chronic obstructive pulmonary disease may have lower lung function than MM individualsClin Chem200147566211148177
- DahlMHershCPLyNPBerkeyCSSilvermanEKNordestgaardBGThe protease inhibitor PI*S allele and COPD: a meta-analysisEur Respir J200526677615994391
- HershCPDahlMLyNPBerkeyCSNordestgaardBGSilvermanEKChronic obstructive pulmonary disease in alpha1-antitrypsin PI MZ heterozygotes: a meta-analysisThorax20045984384915454649
- DahlMNordestgaardBGTybjærg-HansenABrunsDELoYMDWittwerCTMolecular diagnosis of intermediate and severe alpha1-antitrypsin deficiency by use of multiplex polymerase chain reactionMolecular testing in laboratory medicineWashington DCAACC Press2002250258
- DahlMTybjaerg-HansenALangePNordestgaardBGDeltaF508 heterozygosity in cystic fibrosis and susceptibility to asthmaLancet1998351191119139654257
- DahlMNordestgaardBGLangePTybjaerg-HansenAFifteen-year follow-up of pulmonary function in individuals heterozygous for the cystic fibrosis phenylalanine-508 deletionJ Allergy Clin Immunol200110781882311344348
- DahlMTybjaerg-HansenAWittrupHHLangePNordestgaardBGCystic fibrosis Delta F508 heterozygotes, smoking, and reproduction: studies of 9141 individuals from a general population sampleGenomics19985089969628826
- EstivillXBancellsCRamosCGeographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. The Biomed CF Mutation Analysis ConsortiumHum Mutat1997101351549259197
- DahlMTybjaerg-HansenALangePNordestgaardBGAsthma and COPD in cystic fibrosis intron-8 5T carriers. A population-based studyRespir Res2005611316212675
- DelaneySJRichDPThomsonSACystic fibrosis transmembrane conductance regulator splice variants are not conserved and fail to produce chloride channelsNat Genet199344264317691356
- CastellaniCQuinziiCAltieriSMastellaGAssaelBMA pilot survey of cystic fibrosis clinical manifestations in CFTR mutation heterozygotesGenet Test2001524925411788092
- ConneallyPMMerrittADYuPLCystic fibrosis: population geneticsTex Rep Biol Med1973316396504595586
- de CidRChomelJCLazaroCCFTR and asthma in the French EGEA studyEur J Hum Genet20019676911175304
- GriesenbachUGeddesDMAltonEWThe pathogenic consequences of a single mutated CFTR geneThorax199954Suppl 2S19S2310451687
- HoffjanSNicolaeDOberCAssociation studies for asthma and atopic diseases: a comprehensive review of the literatureRespir Res200341414748924
- LazaroCde CidRSunyerJMissense mutations in the cystic fibrosis gene in adult patients with asthmaHum Mutat19991451051910571949
- LowenfelsABMaisonneuvePPalysBSchoniMHRedemannBDeltaF508 heterozygosity and asthmaLancet19983529859879752839
- MennieMGilfillanABrockDJListonWAHeterozygotes for the delta F508 cystic fibrosis allele are not protected against bronchial asthmaNat Med199519789797489375
- Munthe-KaasMCLodrup CarlsenKCCarlsenKHCFTR gene mutations and asthma in the Norwegian Environment and Childhood Asthma studyRespir Med20061002121212816678395
- SchroederSAGaughanDMSwiftMProtection against bronchial asthma by CFTR delta F508 mutation: a heterozygote advantage in cystic fibrosisNat Med199517037057585155
- TzetisMEfthymiadouAStrofalisSCFTR genemutations–including three novel nucleotide substitutions – and haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary diseaseHum Genet200110821622111354633
- HolmskovUThielSJenseniusJCCollections and ficolins: humoral lectins of the innate immune defenseAnnu Rev Immunol20032154757812524383
- TurnerMWThe role of mannose-binding lectin in health and diseaseMol Immunol20034042342914568388
- EisenDPMinchintonRMImpact of mannose-binding lectin on susceptibility to infectious diseasesClin Infect Dis2003371496150514614673
- NagyAKozmaGTKeszeiMTreszlAFalusASzalaiCThe development of asthma in children infected with Chlamydia pneumoniae is dependent on the modifying effect of mannose-binding lectinJ Allergy Clin Immunol200311272973414564351
- DahlMTybjaerg-HansenASchnohrPNordestgaardBGA population-based study of morbidity and mortality in mannose-binding lectin deficiencyJ Exp Med20041991391139915148337
- KochAMelbyeMSorensenPAcute respiratory tract infections and mannose-binding lectin insufficiency during early childhoodJAMA20012851316132111255386
- DemeoDLMarianiTJLangeCThe SERPINE2 Gene Is Associated with Chronic Obstructive Pulmonary DiseaseAm J Hum Genet20067825326416358219
- ZhuGWarrenLAponteJThe SERPINE2 gene is associated with chronic obstructive pulmonary disease in two large populationsAm J Respir Crit Care Med200717616717317446335
- DahlMBowlerRPJuulKCrapoJDLevySNordestgaardBGSuperoxide dismutase 3 polymorphism associated with reduced lung function in two large populationsAm J Respir Crit Care Med200817890691218703790
- DemeoDLHershCPHoffmanEAGenetic determinants of emphysema distribution in the national emphysema treatment trialAm J Respir Crit Care Med2007176424817363767
- JuulKTybjaerg-HansenAMarklundSLangePNordestgaardBGGenetically increased antioxidative protection and decreased chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200617385886416399992
- SmithCAHarrisonDJAssociation between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysemaLancet19973506306339288046
- SeifartCPlagensAGenetics of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2007254155018268927
- SilvermanEKProgress in chronic obstructive pulmonary disease geneticsProc Am Thorac Soc2006340540816799082
- DahlMTybjaerg-HansenAVestboJLangePNordestgaardBGElevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011641008101111587987
- DahlMVestboJLangePBojesenSETybjaerg-HansenANordestgaardBGC-reactive protein as a predictor of prognosis in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200717525025517053205
- DiCosmoBFGebaGPPicarellaDAirway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivityJ Clin Invest199494202820357962549
- HierholzerCKalffJCOmertLInterleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injuryAm J Physiol1998275L611L6219728057
- JohnstonRASchwartzmanINFlyntLShoreSARole of interleukin-6 in murine airway responses to ozoneAm J Physiol Lung Cell Mol Physiol2005288L390L39715516495
- KuhnCIIIHomerRJZhuZAirway hyperresponsiveness and airway obstruction in transgenic mice. Morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lungAm J Respir Cell Mol Biol20002228929510696065
- ParkCSChungSWKiSYIncreased levels of interleukin-6 are associated with lymphocytosis in bronchoalveolar lavage fluids of idiopathic nonspecific interstitial pneumoniaAm J Respir Crit Care Med20001621162116810988147
- RomaniLMencacciACenciEImpaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicansJ Exp Med1996183134513558666893
- RomanoMSironiMToniattiCRole of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitmentImmunity199763153259075932
- SuwaTHoggJCKlutMEHardsJvan EedenSFInterleukin-6 changes deformability of neutrophils and induces their sequestration in the lungAm J Respir Crit Care Med200116397097611282775
- XingZBraciakTJordanaMCroitoruKGrahamFLGauldieJAdenovirus-mediated cytokine gene transfer at tissue sites. Overexpression of IL-6 induces lymphocytic hyperplasia in the lungJ Immunol1994153405940697930613
- YoshidaMSakumaJHayashiSA histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B geneProc Natl Acad Sci U S A199592957095747568174
- LuisettiMMaSIadarolaPDesmosine as a biomarker of elastin degradation in COPD: current status and future directionsEur Respir J2008321146115718978133
- KobayashiHKanohSMotoyoshiKSerum surfactant protein-A, but not surfactant protein-D or KL-6, can predict preclinical lung damage induced by smokingBiomarkers20081338539218595202
- SinDDManSFMarciniukDDThe effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20081771207121418310480
- OhlmeierSVuolantoMToljamoTProteomics of human lung tissue identifies surfactant protein a as a marker of chronic obstructive pulmonary diseaseJ Proteome Res2008 (in press, available in on-line version)
- BhattacharyaSSrisumaSDemeoDLMolecular Biomarkers for quantitative and discrete COPD phenotypesAm J Respir Cell Mol Biol2008 (in press, available in on-line version)
- LarssonKInflammatory markers in COPDClin Respir J200828487
- SnellNNewboldPThe clinical utility of biomarkers in asthma and COPDCurr Opin Pharmacol2008822223518468485