Abstract
COPD is prevalent in Western society and its incidence is rising in the developing world. Acute exacerbations of COPD, about 50% of which are unreported, lead to deterioration in quality of life and contribute significantly to disease burden. Quality of life deteriorates with time; thus, most of the health burden occurs in more severe disease. COPD severity and frequent and more severe exacerbations are all related to an increased risk of mortality. Inhaled corticosteroids (ICS) have similar effects on quality of life but ICS/long-acting bronchodilator combinations and the long-acting antimuscarinic tiotropium all improve health status and exacerbation rates and are likely to have an effect on mortality but perhaps only with prolonged use. Erythromycin has been shown to decrease the rate of COPD exacerbations. Pulmonary rehabilitation and regular physical activity are indicated in all severities of COPD and improve quality of life. Noninvasive ventilation is associated with improved quality of life. Long-term oxygen therapy improves mortality but only in hypoxic COPD patients. The choice of an inhaler device is a key component of COPD therapy and this requires more attention from physicians than perhaps we are aware of. Disease management programs, characterized as they are by patient centeredness, improve quality of life and decrease hospitalization rates. Most outcomes in COPD can be modified by interventions and these are well tolerated and have acceptable safety profiles.
Introduction
The term, chronic obstructive pulmonary disease (COPD), is of relatively recent origin but is related to the terms chronic bronchitis and emphysema that refer to some subsets of patients with COPD. These terms have a long history that can be traced as far back as the seventeenth and nineteenth centuries, respectively.Citation1,Citation2 Laennec, in his 1837 treatise, gave the first clinical description of emphysema.Citation3 The respiratory world had to wait another hundred years before a spirometer could be designed to measure time-dependent expiratory volumesCitation4 and allow the physiology of airflow obstruction to be studied. Two phenotypes of COPD emerged, the so-called ‘American Emphysema’ and ‘British Bronchitis.’ Both Fletcher and Burrows showed similarities between these two conditions,Citation5,Citation6 which were later shown to have similar mortality.Citation7 It is worth noting that at this stage the definition of COPD was clinicoradiological.Citation7 William Briscoe is believed to be the first person to use the term COPD in discussions at the 9th Aspen ConferenceCitation8 and Sukumalchantra and Williams were one of the first groups of authors to publish data using the term COPD.Citation9 Following these earlier works, the anatomic location of the primary lesion in COPD was shown to be the small airway and the basis of COPD pathology was suggested to be inflammation.Citation10–Citation12 In 1995 both the American Thoracic Society and the European Respiratory Society published for the first time standards for the diagnosis of COPD.Citation13,Citation14 These standards established the spirometric basis for the definition of COPD.
COPD is now defined by airflow obstruction, which may be associated with chronic bronchitis, bronchiolitis, or emphysema.Citation13 Other synonyms that have historically been associated with COPD include chronic obstructive bronchitis, chronic airflow limitation, chronic airflow obstruction, chronic airways obstruction, nonreversible obstructive airways disease, chronic obstructive airways disease, chronic obstructive lung disease, and some cases of chronic asthma.Citation15
The major manifestation of airflow obstruction in COPD is reduced maximum forced expiratory flow in 1 second (FEV1) and slow forced emptying of the lungs (forced vital capacity, FVC) such that the ratio of FEV1 to FVC is less than 70%. Another characteristic is that these physiologic features do not change markedly over months.Citation14 However, the natural history of this disease is that most of the lung function impairment is progressive.Citation14 Importantly, airway hyperactivity and partial reversibility may also be features of COPD provided that at maximal reversibility, the ratio of FEV1 to FVC is still less than 70%.Citation16
The severity of COPD is classified in terms of FEV1 because FEV1 is reproducible and is considered the most significant predictor of prognosis in COPD as supported by a wide range of studies over time,Citation17–Citation22 Since FEV1 depends on gender, age, and height, the derived variable FEV1% predicted (FEV1%) is used. FEV1% is the ratio of postbronchodilator FEV1 to FEV1 predicted for age and height as determined from epidemiological studies.Citation16
In this review we will look at the prevalence and importance of COPD as a common chronic disease: there is evidence that even from an optimistic view point the prevalence of COPD will rise markedly within the next 20 years. Most of the health burden in COPD arises from pharmacologic intervention whether as in- or outpatients. This is more marked in the later stages of COPD when exacerbations are more frequent. Thus COPD exacerbation has emerged as an important outcome measure in COPD. There are, however, several methodological issues with the concept of an exacerbation from event definition to counting of COPD exacerbations during interventional studies. The relationships of exacerbation to health status and difficulty in modifying physiological measures in COPD have brought health status into focus as an important outcome measure. However the final outcome measure for any intervention is mortality. We may well have treatments that reduce mortality. After a review of each of these concepts we look at how various interventions both pharmacologic and nonpharmacologic modify these interventions. No intervention will be effective unless it is patient friendly and so we also consider patient-focused perspectives on treatment. We will not consider newer drugs where studies have yet to show effects on exacerbations, quality of life and mortality.
The prevalence of COPD
Several studies have examined the prevalence of COPD both in the developed and developing world and concur on the importance of this condition,Citation23–Citation33 In 1990, the total deaths from COPD were estimated at 2.2 million and it was ranked the sixth leading cause of death.Citation25 Even by conservative estimates it is thought that the worldwide number of deaths in 2020 will be 3.5 million and COPD will be ranked third amongst the leading causes of death.Citation25 During 2000, an estimated 10 million US adults reported physician-diagnosed COPD and in the USA alone, COPD was responsible for 8 million physician office and hospital outpatient visits, 1.5 million emergency department visits, 726,000 hospitalizations, and 119,000 deaths.Citation34 The greatest risk factor for disease development in the developed world is tobacco smoke; however in the developing world biomass fuels have been implicated particularly in women.Citation35
Worldwide, COPD affects 9.8% of men and 5.6% of women.Citation36 However a turn-of-the-century report from the UK indicated that the prevalence rates of COPD appear to have peaked in UK men but are continuing to rise in women.Citation37 This reverse in the gender trend in COPD prevalence in the developed world has been confirmed by reports from Austria that have found equal prevalence in both genders for COPD as well as tobacco use.Citation38 Thus the prevalence of COPD in women can be expected to rise worldwide over the next decade.Citation39,Citation40
Exacerbation rates
The importance of COPD exacerbations to the natural history of COPD and disease burden has only been recognized within the past 10 years. In 1975, Tager and Speizer suggested that “once obstructive disease becomes manifest as abnormal FEV1, intercurrent illnesses [exacerbations] have little effect on rates of FEV1 decline” and disease severity and thus the “current concern over rational use of antibiotics…and the economic burden that unnecessary use may represent… [requires] reassessment.”Citation41 Our current conceptualization of COPD exacerbations has carried us well beyond the almost negligible concern expressed in the above statements.
Table shows 9 studies of at least 1 year’s duration in COPD patients conducted within the past 40 years. Many factors affect the ability of such studies to draw definitive conclusions about COPD exacerbations and make it difficult to compare studies. One major factor is the heterogeneous nature of COPD.Citation42 This factor is further accentuated because 3 of the studiesCitation17,Citation43,Citation44 included unselected subjects whereas the other studies included only patients with COPD. Another variable is the method used to detect COPD exacerbation. Relying on the ability of patients to recall exacerbationsCitation17,Citation45 may not be as accurate as active surveillance.Citation43,Citation46,Citation47 Other confounding variables may include geography (eg, weather, climate, air pollution), genetic makeup of the population under study and frequency of follow-up. Data from the London COPD cohort suggest that in a single center study using a selected group of COPD patients, active surveillance may be the most important factor in detecting COPD exacerbations.Citation46
Table 1 Comparison of 9 studies of exacerbations over 30 years
The annual rate of COPD exacerbations has been estimated from several different studies to be as low as 0.5 to a high of 3.5 exacerbations per patient.Citation46,Citation48,Citation49 Hospitalization rates have depended on type of study but range from as low as 0.09 to 2.4 per patient per year.Citation49 Thus, from this brief survey it would appear that the more closely patients are followed up in studies the higher the detection rate for COPD exacerbations. However as far as we are aware, no long term study has found a mean exacerbation rate of greater than about 3 exacerbations/year and so this number may represent an upper limit to the mean rate in all populations and is of significance for interventional studies.
Methodologic issues in exacerbation detection and type of exacerbation
Frequency of monitoring
Of the studies mentioned in Table , the study of Kanner et alCitation44 is the only long-term study of exacerbations with a low detection rate despite close follow up with weekly telephone calls and 3-monthly clinics, but this may have been related to the inclusion of patients without COPD. Their findings are at variance with both the London COPD Study and the INSPIRE Study,Citation46,Citation47 both of which used diary cards to detect exacerbations, and Monto et al who also monitored their patients by weekly telephone calls.Citation43
Event definition
Both Monto and Kanner used a similar definition of exacerbations consisting of increase in or new onset of cough, wheeze and sputum changes but Monto et al also included ‘painful respirations.’ On the other hand the London COPD Study and Anthonisen used a slightly more restrictive definition requiring at least one or more of an increase in or new onset of dyspnea, sputum purulence or sputum volume or one of these and any one of a cold, wheeze, cough, sore throat or fever.Citation45,Citation46 Anthonisen’s lower detection rate compared to the Monto, The London COPD and INSPIRE studies may have been related to his use of patient recall at the 3-monthly follow-up visits. Despite the heterogeneity in the definition of COPD exacerbation these studies all have one feature in common, which is that they used symptom-based definitions of an exacerbation of COPD.
Since the publication of the ISOLDE Study,Citation50 which used a treatment-based definition of exacerbation and the initial results of the London COPD Study, which like Anthonisen, did not, there has been debate about the true incidence of COPD exacerbations. This question has now been answered by the INSPIRE Study which is the first long-term study of COPD exacerbations to compare the two types of exacerbations using the so-called symptom-based definition as well as the treatment-based definition.Citation47,Citation51 The results of the INSPIRE study show that if a symptom-based definition is used exclusively then the exacerbation rate detected is 3 per year but if a treatment-based definition is used then the rate detected is approximately 50% of this or about 1.5 exacerbations per year. Thus about 50% of symptom-defined COPD exacerbations are not treated by physicians and this may represent a different type of exacerbation.
Unreported exacerbations
The above observations lead logically to the hypothesis that about 50% of exacerbations are untreated, or at least not reported to physicians. This feature of COPD exacerbations was first detected by the London COPD Study.Citation48 Initial reports from the London COPD cohort suggested that the size of this reporting gap is about 50% but later reports suggested that it may be as high as 67%Citation52 which is consistent with reports from a Canadian cohort.Citation53 In a study of 625 exacerbations Wilkinson et al showed that patients with a higher reporting rate had a better quality of life.Citation52 Langsetmo et al in a 6-month study of 421 patients with 501 exacerbations have shown that several factors appear to predict the tendency not to report exacerbations and these include older age and more severe disease.Citation53 Further, a small number of symptoms at onset and weekends were associated with a tendency not to report exacerbationsCitation53 or report later.Citation52 These results are supported by those of Vijayasaratha et al who reported on COPD exacerbations in a small number of COPD patients with alpha-1-antitrypsin deficiency and showed that the unreported exacerbations also had fewer symptoms at onset.Citation54
Reported exacerbations
Exacerbations receive the attention of the physician either in general practice or at hospital. COPD carries a greater burden than asthma with respect to general practice consultations and the consultation rate increases with age.Citation55 Data from The Lung Health Study showed that unscheduled general practice consultations may be a valid though indirect measure of exacerbation rates and that this parameter is responsive to therapy.Citation56
A survey of medical admissions found that 7.3% of male and 3.2% of female admissions aged between 56 and 74 years had COPD.Citation57 The number of COPD hospital admissions in a typical UK health district of 250,000 population is about 50% greater than for asthma.Citation57 Similarly in the USA about 50% of discharges classified as chronic airflow disease including asthma (ICD codes 490–496) were due to bronchitis (chronic or unqualified) corresponding to ICD9 codes 490 and 491.Citation34 Also in the UK, inpatient hospital stay exceeds that for asthma by a factor of 500%. Thus COPD carries a significantly greater hospital burden than asthma.
Relationships to exacerbation
Factors associated
Three of the studies quoted in Table attempted to determine factors associated with frequent exacerbations. One of the first long term studies of COPD exacerbations was that of Fletcher et al who studied ‘chest episode’ frequency in a cohort of male postal workers over 10 years. These chest episodes were never rigorously defined by the authors but Fletcher found associations with migraine, hay fever and a history of childhood bronchitis which, apart from migraine, all became nonsignificant when age, phlegm score and airway obstruction were taken into account.Citation17 Fletcher concluded that “chronic mucus hypersecretion is a major cause of chest episodes” which is also supported by the Copenhagen City Heart Study.Citation58 Over the past 10 years several studies have found factors associated with the rate of COPD exacerbations and these include mucus hypersecretion,Citation48,Citation59 lower airway bacterial colonization,Citation60 airway inflammationCitation61 and frequency of virus infection.Citation62 Exacerbations of COPD lead to a deterioration in the quality of life of COPD patients and contribute significantly to disease burden,Citation48,Citation50,Citation63 greater severity of COPDCitation44,Citation64,Citation65 and mortality.Citation66
Prior treatment
One of the first studies to show that withdrawl of inhaled steroids was associated with the occurrence of exacerbations was the Isolde study.Citation67 This has been found in other studies since then but Suissa et al have reanalyzed data from the OPTIMAL Study according to whether patients were initially ICS-exposed or ICS-naive.Citation68 They found that patients with prior ICS treatment who are switched to bronchodilator during randomization are more likely to exacerbate compared to those remaining on ICS. But importantly, they found no difference between treatments in the ICS-naive patients. Thus regardless of definition used, the prior medication history of the patients may be important when exacerbation rates are an outcome measure in COPD studies. This has not been taken into account during analysis of exacerbations in any study of COPD so far.Citation68
Statistical issues
Most of the interventional studies referred to later on used the health care utilization definition of an exacerbation when counting exacerbations as an outcome measure. However, statistical modeling of exacerbation rates has turned out to be rather complex. Cohort studies have shown evidence for a phenotypic frequent exacerbator.Citation48,Citation60 While the occurrence of exacerbations within individual patients may follow a Poisson distribution, exacerbations between patients may not. One suggestion that such exacerbations may follow a gamma distribution has been debated.Citation69,Citation70 Where differences between treatment arms are large such theoretical issues may not be of importance but when differences are small the choice of statistical model will determine the importance of the result.
Health status and health burden
Measurement of health status in COPD has become more frequent since the work of Jones and colleaguesCitation71 and it is a European requirement that all clinical trials of new drugs for COPD should incorporate a symptomatic measure, such as a health status questionnaire, as a coprimary endpoint.Citation72
Quality of life as a concept
The concept of quality of life is a difficult one to measure; it may be defined as the gap between that which is desired and that which is achievable.Citation71 Because of the difficulty of measurement of this parameter and the fact that we in the health services are concerned with more specific targets, clinicians have sought a more restrictive term, health-related quality of life (HRQoL), which is the subjective experience of health on quality of life.
Studies of HRQoL in COPD over a wide range of severity have consistently shown that patients with COPD have significant decrements in their HRQoL.Citation73,Citation74 In COPD patients, quality of life scores have been used in the assessment of effects of medication,Citation75 depression,Citation76 oxygen therapy,Citation73 intermittent positive pressure ventilation,Citation77–Citation79 pulmonary rehabilitation,Citation80–Citation82 hospital readmissionCitation83 and exacerbations.Citation48,Citation84
Health-related quality of life questionnaires
Questionnaires used for assessment of quality of life may be categorized as generic or disease-specific. Generic questionnaires were designed to make standardized comparative assessments between populations of patients. However most questionnaires of this type (for example the Sickness Impact Profile, Short Form 36) may be rather insensitive to changes in health.Citation75 Disease-specific questionnaires include the Chronic Respiratory Questionnaire (CRQ)Citation85 and St. George’s Respiratory Questionnaire (SGRQ).Citation86
The CRQ attempts to overcome the problem of generic questionnaires by customizing the questionnaire to the individual patient and patients are encouraged to compare their current state of health with their previous reports. A self-reported version of the questionnaire existsCitation87 and the minimal clinically important difference between scores using this questionnaire has been reported as 0.5.Citation88
The advantage of the SGRQCitation86 is that it has standardized instructions, which require the same response. There is no evidence that by so doing the SGRQ is less sensitive than other questionnaires to different levels of health.Citation75 However as a standardized questionnaire, the SGRQ has the advantage of allowing direct comparison between different patient populations and treatment groups and has been shown to be responsive when used for these comparisons.Citation71,Citation89 Because of this standardization further analysis of the disturbances to life that occur in airways disease are possible. The SGRQ is scored from 0 (good) to 100 (poor) through a total score. However the SGRQ examines disturbances to quality of life by the symptoms component of the score, which assesses the degree of distress due to frequency and severity of respiratory symptoms, the impacts component, which addresses psychosocial effects and the activities score, which assesses effect on daily activities.Citation86 The SGRQ measures changes in health status and it has been shown that a clinically significant difference is equivalent to a 4-unit change in the SGRQ score.Citation90 The importance of the SGRQ in COPD has been further supported by recent studies showing relationships with systemic biochemical parameters.Citation91–Citation93
Disability and COPD
Disability adjusted life years (DALYs) is a measure of health burden that consists of the sum of years of living lost (YLLs) and years of living with disability (YLDs).Citation25 In 2001 smoking was projected as the major risk factor for 4 million deaths and contributed about 5% of DALYs worldwide.Citation94 Deaths due to smoking are expected to rise to about 8.4 million by 2020.Citation25 By 2020 COPD will be expected to contribute about 4.1% of total DALYs worldwide. One study in Brazil has estimated that COPD accounts for 32% of all DALYsCitation95 and this is consistent with trends already predicted by Murray that increases in DALYs for COPD will be greater in the developing world.Citation25,Citation96 Interventions, which decrease mortality in COPD, will decrease YLLs but there will also be an increase in YLDs. Since no currently known intervention is associated with cure in COPD, DALYs may not be a suitable outcome measure at present in COPD.
Economic burden of COPD
Data from the Office of National Statistics in the United Kingdom showed that there were 203,193 hospital admissions for COPD in 1994.Citation97 The average length of inpatient stay was 9.9 days. The National Health Service Executive published data in 1996 showing that the medical cost of COPD in the United Kingdom was £846 million or £1,154 per person per year.Citation98 The bulk of this cost was due to pharmaceutical treatment.Citation99 Similar data from the United States showed that 72.8 % of yearly expenditure of US$14.7 billion was due to hospitalization and that approximately 50% of the total Medicare payments for those with COPD were incurred by approximately 10% of the Medicare beneficiaries (Division of Epidemiology, NHLBI).
A US study of per patient direct costs (in $US 2005), using recent data yielded attributable cost estimates (costs deemed to be related to COPD) in the range of $2,700 to $5,900/patient annually, and excess cost estimates (total costs incurred by COPD patients minus total costs incurred by non-COPD patients) in the range of $6,100 to $6,600 annually.Citation100 Most of the health burden from COPD comes from hospital visits in late disease.Citation34 In a study of health insurance claims over the period 1999 through 2002, Nurmagambetov et al found that the number of hospital admissions and length of stay in hospitals decreased while the number of outpatient visits increased over this period. During this period the estimated COPD-related total medical costs per patient decreased 22% largely because of a decrease in the cost of hospitalizations for COPD, thus showing that COPD costs can be effectively reduced through decreasing hospitalizationCitation101 and these changes have been related to quality of life scores.Citation102 These data suggest that hospital admission rates are a suitable measure of health burden in COPD and respond to interventions.
Mortality
Mortality rates in COPD
There is substantial variability among and even within countries and between sexes in COPD mortality.Citation103 For example, deaths from COPD are greater in Scotland than the rest of the UK. Though cold temperatures may be a factor in this, it is not entirely clear that this is in fact so, as the death rates from COPD in Canada do not exceed those of the USA, and COPD death rates in Norway are not particularly high (28 per 100,000 males).Citation103 Death rates are lower among women than men in all countries. Surveys of deaths in the US show a substantial increase in death rates from COPD between 1985 and 1995, consistent with an increasing time trend in reporting of COPD.Citation103
Mortality and lung function
In a multicenter prospective study of positive pressure breathing over 33 months, Anthonisen et al found a mortality rate of 23% and showed that mortality in COPD was related to postbronchodilator FEV1 and response to bronchodilators.Citation18 This study involved 985 patients with a mean FEV1 of 36% predicted. When patients were stratified by FEV1, patients in the strata with FEV1 less than 40% predicted had greater mortality even with adjustment for age. Mortality in the group with a FEV1 > 50% predicted was only slightly different from that of the group of healthy smokers. The results of this study are strongly supported by the MIDSPAN study, conducted in the general population in Renfew and Paisley in Scotland starting in 1972 through 1976 and following mortality over 15 years.Citation21 The Scottish study was based on 4439 deaths and the study showed that low FEV1 was related to deaths from all causes but also vascular disease and lung cancer.Citation21 Further support for the relationship of FEV1 to prognosis in COPD comes from the atherosclerosis risk in communities study in which Mannino et al showed that survival in COPD patients is related to GOLD stage.Citation16
Taken together, these studies suggest that impaired lung function is related to mortality in the general population. They also show that in the COPD population FEV1 below 50% of predicted is strongly related to mortality. Thus interventions at this stage of COPD or earlier may be particularly effective.
Mortality and COPD exacerbations
Using a service-based definition of COPD exacerbation defined by need for emergency department treatment, SolerCataluña and colleagues were able to show for the first time that exacerbation frequency influenced mortality.Citation66 This was a study of 304 men with COPD in Spain over 5 years. The study showed that patients with 3 or more exacerbations had a survival rate of 30% at 5 years whereas those without exacerbation had a survival rate of 80%. Survival rate was also influenced by need for readmission, those requiring readmission having a 20% survival at 5 years.Citation66
In-hospital mortality varies between less than 10% and 60%, based on the severity level of the population studied.Citation49 Mannino et al analyzed data from the Atherosclerosis Risk in Communities (ARIC) study and found that the overall rate of death varied from 5.4 per 1000 person years among normal subjects to 42.9 among subjects with GOLD Stage 3 or 4 COPD. Death rates from COPD depend on degree of severity, the highest death rate from COPD occurring in severe COPD.Citation16
Causes of death in COPD
Vascular diseases and lung cancer appear to be major causes of death but more so in the severe COPD category.Citation16 SolerCataluña showed that respiratory causes accounted for 67% of deaths in their cohort.Citation66 The TORCH Study was the first interventional study in COPD with mortality as a primary outcome measure.Citation104 The study was a randomized double blind trial with 4 treatment arms: placebo, salmeterol, fluticasone and salmeterol/fluticasone (SFC). The study recruited 6112 patients in 444 centers in 42 countries and the mortality rate in the 3-year study period was 875 subjects. The study was well designed to study mortality and had an independent adjudication committee for causes of deaths, thus eliminating between-country variations in death certification. Unlike the SolerCataluña study,Citation66 the TORCH study found an almost equal contribution of respiratory (35%) and cardiovascular causes (27%) of deaths in COPD. However the death rates between the two studies were different, the TORCH study having an average annual death rate of 4.7%, about 50% of that of the SolerCataluña study.Citation66 Thus these two studies may have been describing the same events but in two different types of COPD subjects.
Interventions
Inhaled corticosteroids (ICS)
The first long-term randomized controlled trial (RCT) of ICS to show a significant effect in COPD was the ISOLDE study of 751 UK subjects with FEV1 < 85%. The rationale for the ISOLDE study was based on 3 previous studies all having positive effects on FEV1 decline. One, a large, retrospective, open-label study, involved oral corticosteroids;Citation105 another study was conducted in a combined pool of asthma and COPD patients;Citation106,Citation107 and finally, meta-analyses of ICS including (beclomethasone users).Citation108 Though the ISOLDE study aimed for all severities of COPD, the mean FEV1 at recruitment was in fact 50% (SD 14.7%). This was the first prospective study to find an effect of ICS on any outcome measure in COPD. The study found no difference in the primary endpoint that was the annual decline in FEV1 in common with previous studies of budesonide.Citation58,Citation109 However the ISOLDE study found a 25% reduction in exacerbation rates in the fluticasone (500 μg BD) arm compared to the placebo arm.Citation50 Fluticasone propionate significantly reduced the rate of decline in quality of life, delaying the average time for a clinically important reduction in health status from 15 to 24 months compared to placebo. This was a landmark study in COPD therapeutics as it showed for the first time that health status declines at a measurable rate of 3.2 unit/year (in the placebo arm) and further that this outcome may be modified.Citation63,Citation110 From then on health status and exacerbation rates became accepted outcome measures in most interventional studies in COPD.
The earlier long term studies of VestboCitation58 and PauwelsCitation109 either found no effect on, or did not look at, health status or exacerbations. In the Vestbo study, though exacerbations were an outcome measure, there appears to be no precise definition unlike the ISOLDE study where exacerbations were defined by need for treatment. Further, in both the Vestbo and Pauwels studies the patient population was mild COPD (mean FEV1 87% and 77% predicted, respectively) compared to ISOLDE: 50%. The results of the ISOLDE study were supported by the shorter (1 year) TRISTAN study,Citation111 which showed a 19% reduction in exacerbation rates but no significant difference in hospitalization or quality of life scores for fluticasone relative to placebo. Hence the GOLD guidelines (2004) suggest that ICS be used in COPD for severe disease with FEV1 < 50% or where there are frequent exacerbations.
Following the essentially negative Vestbo and Pauwels studies two further studies of budesonide in more severe COPD patients (FEV1 36%) over 1 year with primary outcome measure as the rate of oral steroid-treated exacerbations, also found no effect for the ICS on exacerbation rateCitation112,Citation113 and only one study found improved quality of life in the ICS arm.Citation113 It is likely that the difference between outcomes in the two studies was due to the differing designs. Medications were withdrawn prior to recruitment in the Szafranski studyCitation112 but patients in the Calverley studyCitation113 were optimized with oral steroid therapy prior to randomization as was done in the ISOLDE Study. Though the primary outcome measure of both the Calverley and Szafranski studies was exacerbation rate, it is interesting that both studies found only a trend toward a reduced exacerbation rate in the budesonide arm. Thus it is likely that they were underpowered to detect a difference in effects of ICS and had they been conducted over a longer period (eg, the ISOLDE Study, 3 years) the results might have been different. However we cannot draw definitive conclusions on relative efficacy of these ICS in COPD from these studies as there have been no ‘head to head’ comparisons between fluticasone and budsonide
Long-acting β2-agonists (LABAs)
The latest GOLD guidelines recommend use of a long-acting inhaled beta-adrenergic receptor agonist (β2-agonist) for maintenance treatment of COPD.Citation114 Several studies of LABAs have looked at exacerbations as outcome measures. In a 4-month, randomized, placebo-controlled study of 670 COPD patients, Boyd et al found no difference in exacerbation with salmeterol; however in a similar study which also included an ipratropium arm Mahler et al found similar exacerbation rates in all arms but significantly prolonged time to the first exacerbation for salmeterol compared to placebo (p = 0.005) as well as ipratropium (p = 0.0411).Citation115,Citation116 By the end of the study, 17% of the patients in the salmeterol arm had at least one exacerbation as compared to 27% and 31% in the ipratropium and placebo arms, respectively.Citation116 Dahl et al conducted a placebo-controlled comparative study of formoterol (2 dose strengths: 12 and 24 μg/day), ipratropium and placebo in 780 patients with moderate to severe COPD over 3 months.Citation117 They found no difference in exacerbations requiring treatment but there was a significant decrease in mild exacerbations (ie, those requiring reliever use only) between the formoterol arms and the placebo arm. Larger studies all showed significant falls in exacerbation rates in the salmeterol arm of 20%Citation111 and 15%Citation104 but not formoterolCitation112,Citation113 though there was a significant difference in mild exacerbations requiring just reliever use in the latter two studies.
The first study to describe a significant effect of a LABA on quality of life in COPD was the 4-month placebo-controlled study of salmeterol in 283 COPD patients. The study showed a highly significant improvement in the salmeterol arm of the study.Citation75 This was supported by another study which used the CRQ to measure quality of lifeCitation116 and a later study using formoterol.Citation117 However, the subsequent 3-month study of Rennard et al found only a nonsignificant trend toward better quality of life in the salmeterol arm compared to placebo.Citation118
The early studies found no difference in mortality between LABA and placebo, arguably because of their short durations. Based on many of these small studies, a meta-analysis of 22 trials concluded that β2-agonists are associated with an increased risk of respiratory deaths.Citation119 However this meta-analysis included only seven studies of salmeterol, all of duration 3 to 6 months and even less (five studies) of formoterol of duration 3 to 12 months.Citation119 None of these 12 studies was individually powered to detect differences in death rates. In contrast, a prospective study of 416 patients in five Nordic countries detected an association between treatment with LABAs and decreased death rates following discharge from hospital.Citation120 The only study of COPD patients that was powered to detect a difference in death rates between interventional and placebo arms was the TORCH study. This study showed no statistically significant difference between placebo and salmeterol in death rates but the two survival curves diverged within the first year and the final hazard ratio was compatible with a trend toward improved survival in the salmeterol arm (adjusted hazard ratio: 0.879 [95% CI 0.729–1.061]), compared to placebo.
There are now data that the LABA, salmeterol, may improve survival in COPD. According to Suissa et al, the TORCH study may be analyzed as a 2 × 2 factorial trial (ie, LABA, ICS, both, neither).Citation70 Results by this analysis show that salmeterol is associated with a 17% reduction in mortality over the 3 years of the study.Citation68
ICS–LABA combination therapy
Several studies have examined the therapeutic strategy of combination therapy. Four of these have been comparative placebo-controlled studies with SFCCitation104,Citation111 or formoterol-budesonide.Citation112,Citation113 The formoterolbudesonide studies all excluded any ‘cardiovascular disorder that may have put patients at risk’ but the SFC studies did not exclude such patients. All of these studies recruited patients with moderate to severe COPD and all found similar decrements in exacerbation rates of about 25% compared to placebo and a modest improvement in quality of life relative to placebo. Only the TORCH study was designed with mortality as the primary outcome measure. However the primary analysis found no statistically significant difference in mortality between the SFC and placebo arms (p = 0.052) perhaps because as the authors suggested, an interim analysis was undertaken to look at mortality indices for which the sample size calculation did not allow. The trends in the survival curves of the two arms are certainly suggestive of an effect on mortality that is supported by the prespecified secondary analyses.Citation104
Comparison of ICS with ICS–LABA preparations was undertaken in all four studies. The exacerbation rate with ICS–LABA was no different from ICS in all the four studies. However, in two studies, ICS–LABA was better than ICS alone in total SGRQ scoreCitation104,Citation111 but only in the activities and impacts subscales of the SGRQCitation113 with no difference in the other study.Citation112 In the TORCH study, mortality was significantly better in the SFC arm compared to the fluticasone arm (p = 0.028) but not compared to the salmeterol arm (p = 0.58). Thus it appears that addition of the LABA to fluticasone significantly modified the therapeutic effects of the ICS.Citation104 However, as stated above, when a factorial analysis of the TORCH data is performed, it appears that this may all be due to the LABA.Citation68
Long-acting antimuscarinic agents
Interest in long-acting antimuscarinic antagonists (LAMAs) followed from the ERS and ATS recommendations for the use of the short-acting anticholinergic, ipratropium in COPD.Citation113,Citation121 The pathophysiologic basis for anticholinergic agents in COPD is the increased vagal tone thought to exist in the airways of COPD patientsCitation122,Citation123 and this work has been supported by more recent work on the physiology of tiotropium activity on the airways,Citation124–Citation130 Three major studies, conducted within the last 6 years have established the role of the LAMA, tiotropium, in stable COPD.Citation131–Citation133 The Casaburi study was a 12-month placebo-controlled study of 921 patients with FEV1 of 39% predicted in 50 centers and the Niewoehner study was a 6-month study of 1829 patients with FEV1 36% in 26 Veterans Medical Centers. Both the Casaburi and Niewoehner studies showed about a 14% decrease in patients with exacerbations, a 15%–20% decrease in annual exacerbation rates and in the case of the Casaburi study a 47% decrease in hospitalization rates for exacerbations. In addition, the Casaburi study found that 49% of patients on tiotropium had more than a 4-unit increase in SGRQ total score; these results were supported by the comparative study of Donohue et al.Citation134
None of the above studies was powered to detect differences in mortality. However mortality was a secondary outcome measure of the UPLIFT study (Table ).Citation135 Mortality for all patients in this study was determined on an intent to treat basis and was known at 45 months in 98% subjects (tiotropium) and 97% (placebo).Citation136 At the predetermined endpoint of 4 years and 30 days there was no significant difference between the two arms of the study (odds ratio, 0.89; p = 0.09); however after unblinding, analysis showed that there was a decreased risk of death in the tiotropium arm at 4 years (odds ratio 0.87; 95% CI 0.76–0.99).Citation136
LAMA vs ICS–LABA
There are two studies which allow us to compare these drugs: the INSPIRE and OPTIMAL studies. The largest comparative study so far published is the 2-year INSPIRE studyCitation47,Citation51 which involved 1323 patients randomized to SFC or tiotropium with FEV1 of 39%. The study showed the rate of treated exacerbations, the primary outcome measure, was about 1.30 in both arms and the rate of all exacerbations whether treated or not was about 3.0 in both arms. However there was an unexpected 29% greater risk of dropout from the study in the tiotropium arm. The study found that at the end of 2 years, there was a 2.1 unit (p = 0.038) improvement in quality of life as measured by the SGRQ in the SFC arm compared to the tiotropium arm but this may have been due to a healthy survivor effect, as patients whose wellbeing was deteriorating more rapidly withdrew sooner, more so in the tiotropium arm.Citation47 There was also a 3% mortality rate on treatment in the SFC arm compared to 6% in the tiotropium arm (p = 0.008), which was also unexpected. There is no clear explanation for this difference which the authors attributed to an improvement in survival due to SFC consistent with the results of the TORCH study.
The INSPIRE study also detected a difference in exacerbation phenotype in that antibiotic-treated exacerbations were more frequent in the SFC arm (p = 0.028) but systemic corticosteroid-treated exacerbations were more frequent in the tiotropium arm (p = 0.039) and there was a trend toward fewer hospitalizations for COPD exacerbations in the SFC arm (13% vs 16%; p = 0.085).Citation47 These two results taken together are suggestive of a trend toward less severe exacerbations in the SFC arm. However this must be balanced against an increased risk of pneumonia in the SFC patients (8% vs 4%). One hypothesis that may explain this observation is that in some way SFC decreased the severity of exacerbations, making it less likely that they would be treated.
In the OPTIMAL Study, tiotropium–placebo (N = 156) was compared to tiotropium–salmeterol (N = 148) or a triple therapy combination with tiotropium SFC (N = 145) over 1 year; all comparisons were between the placebo and nonplacebo arms.Citation137 The primary analysis showed no differences in annual exacerbation rates between the three arms of the study though the rate was lowest in the tiotropium SFC arm. However, there was a significant decrease in exacerbations requiring hospitalization in the SFC containing arm (rate ratio 0.53; 95% CI 0.33–086), thus suggesting that addition of SFC reduced the severity of exacerbations which was supported by a significant reduction in all-cause hospitalization. There were also differences in favor of tiotropium SFC in HRQoL. Thus it appears that addition of SFC to tiotropium decreases health burden in COPD. There was no mortality difference on treatment between the two arms. The results of the OPTIMAL study have been supported by two recent studies of triple therapy which showed physiological benefit for the triple therapy combination.Citation138,Citation139 The UPLIFT study design allowed use of other inhaled therapy in both arms and 74% of subjects in this study were on ICS, and thus the UPLIFT study may be seen to extend the results of the OPTIMAL study in that UPLIFT showed that there was a 14% decrease in exacerbation rates on top of usual care (p < 0.001) compared to placebo.Citation136
The data from the UPLIFT study taken together with those of the OPTIMAL study are suggestive of a superior effect for the combination ICS–LABA–LAMA. An economic analysis of this option showed that while the health burden was less (because of better quality of life and fewer hospitalizations) this was achieved at considerable economic cost.Citation140 Thus the option of ICS–LABA–LAMA requires further study.
Macrolide use in COPD
Effects of antibiotics in non-COPD obstructive lung diseases
The evidence that antibiotics, specifically macrolides, may be useful in COPD comes from studies in two other obstructive lung diseases. Studies in diffuse panbronchiolitis, a disease that is prevalent in Far Eastern populations, showed that erythromycin improves survival and symptoms.Citation141–Citation143 This stimulated work in cystic fibrosis a disease common in Caucasian populations where azithromycin has been shown to decrease cystic fibrosis exacerbations and symptoms.Citation144–Citation147
Should macrolides have an effect on COPD? The effectiveness of macrolides in these two diseases led to investigations in the far more globally prevalent COPD. An initial open label study showed a decrease in COPD exacerbations in Japanese subjects.Citation148 These results have been strongly supported by the recently concluded ELECT study.Citation149
The ELECT Study
This randomized, placebo-controlled trial of erythromycin 250 mg twice daily in 109 moderate to severe stable COPD patients already on optimal therapy, was conducted in the long-running London COPD Cohort over 1 year. Patients were excluded if in the opinion of the investigator they had unstable cardiovascular status, for example congestive cardiac failure, cardiac arrhythmias or prolonged corrected QT duration on the electrocardiograph. The primary analysis showed a 35% fall in the exacerbation rate in the interventional arm. Secondary analyses showed that the median time to the first exacerbation was 271 days vs 89 days in the placebo arm (p = 0.020). Exacerbations in the erythromycin arm had a median duration of 9 days vs 13 days in the placebo arm (p = 0.001). The investigators were unable to find any differences in serum C reactive protein or serum interleukin (IL6) between the two arms of the study. There were no differences in sputum markers (IL6, IL8, myeloperoxidase) between the two arms of the study, though this analysis was limited by the variability in spontaneous sputum production by patients in the study. There was one death only in the study: in the placebo arm. Safety analyses showed no trend toward more adverse events or macrolide resistance in the interventional arm over the 1 year of study. Thus the macrolide erythromycin would appear to be well tolerated over 1 year in moderate to severe COPD patients and it reduces COPD exacerbation duration and rates, but the mechanism by which this is achieved is not clear.
Phosphodiesterase inhibitors
These are a class of nonsteroidal anti-inflammatory drugs that may be useful in COPD. Prior comparative studies with LABAs have not shown much promiseCitation150 but in a recent RCT involving 110 COPD patients, the theophylline arm was associated with a lower frequency of COPD exacerbations, fewer days of COPD exacerbations and improved quality of life indices. Mortality data were not available for this study.Citation151 The only large controlled study of the newer phosphodiesterase inhibitors is a 647-patient study of cilomilast, which has reported a 39% reduction in exacerbation rates and an improvement in health status using the SGRQ by 4.1 units compared with placebo (p = 0.001).Citation152 Inhibition of another receptor subtype (PDE7A) has also been suggested to lead to decreased inflammation in COPD.Citation153 Our understanding of prevention of COPD exacerbations using this class of drugs is evolving.
Immunostimulatory agents and vaccines
Vaccines
In a study of elderly patients with chronic lung disease, Nichol et al found that influenza vaccination is associated with significant health benefits with fewer outpatient visits, fewer hospitalizations and a reduced mortality.Citation154 In a Cochrane database review of four studies in COPD, Granger et al found little effect for the injectable antipneumococcal vaccines,Citation155 but Alfageme et al studied the 23 serotype pneumococcal polysaccharide vaccine in 596 COPD patients and showed that the pneumococcal vaccine was effective in the prevention of community acquired pneumonia compared to placebo in patients less than 65 years old or those with severe airflow obstruction (FEV1 < 40%) without a difference in mortality.Citation156 Larger studies are required to examine the effects of the vaccine in those over 65 with COPD.
Immunostimulatory agents
There are inconsistent reports of the effectiveness of these agents in COPD. One study, the detoxified oral immunoactive bacterial extract, OM85, was associated with fewer COPD exacerbations and hospital admissionsCitation157 but a systematic review of 13 trials involving 2066 patients found no consistent evidence of a benefit, though the drug is currently in use.Citation158
Mucolytic agents
A Cochrane database review of 26 trials involving 7335 patients with COPD has shown that mucolytic agents are associated with a 20% reduction in exacerbation rates and decreased days of disability.Citation159 However there were contrasting effects observed in two RCTs. In the BRONCHUS study of 523 patients from 10 European countries, nacetylcysteine had no effect on exacerbation rates but subgroup analyses showed a small decrease in patients who were not on regular inhaled steroids.Citation160 The PEACE study of carbocysteine in 709 Chinese COPD patients found a 25% reduction in exacerbation rates and improved quality of life in the interventional arm.Citation161 The reasons for these differences may depend on the differing populations studied.Citation161 The studies were both of similar sizes and had similar exclusion criteria. However the BRONCHUS study recruited less severe patients with FEV1% predicted 57% vs 43% in the PEACE study. Also in the BRONCHUS study 70% of patients were on ICS vs 16.7% in the PEACE study. The authors in the PEACE study speculated that the difference in the two studies arose because of the differing pharmacologic properties of carbocysteine which may in part depend on inhibition of viral adherence to the airway.Citation161 The authors further speculated that some of the differences between the two study results may also be due to pharmcogenetic differences between Caucasian and Chinese populations as well as the lesser use of ICS in the Chinese population.
Nonpharmacologic therapies pulmonary rehabilitation and exercise
A 1-year RCT of 200 disabled COPD patients with FEV1 39% showed that there were no differences in number of hospitalizations following a 6-week rehabilitation program but fewer days in hospital and fewer primary care home visits in the rehabilitation group.Citation162 This indicates that pulmonary rehabilitation may affect severity of exacerbations but not the rate which has been supported by a study of housebound COPD patients,Citation163 though another study with repeated yearly rehabilitation programs found a decreased rate of hospitalization and exacerbations.Citation164 There were also significant improvements in quality of life scores that remained significant at 1 year after the programCitation162,Citation165 and fewer deaths in the interventional group (3 vs 9).Citation162
The results of the Griffiths study differ somewhat in conclusion from those of a systematic review of 6 random ized controlled trials of pulmonary rehabilitation involving 230 patients (but not including the Griffiths Study) where there was a decreased risk of readmission to hospital (pooled relative risk 0.26 [0.12–0.54]) and decreased mortality (RR 0.45 [0.22–0.91]).Citation166 A Cochrane review of 31 RCTs of pulmonary rehabilitation found significant improvements in quality of life using the CRQ and in two of the three domains of the SGRQ.Citation212 However effects of pulmonary rehabilitation may depend of the initial level of dyspneaCitation82 and repeated pulmonary rehabilitation programs may lose some effectiveness, though they are associated with preserved quality of life indices despite physiologic deterioration.Citation167 Pulmonary rehabilitation is now an accepted intervention in COPD regardless of age or debilityCitation82,Citation163,Citation168 with important benefits for patients on outcomes such as the exercise capacity and quality of life but there is no consensus on its effects on COPD exacerbations and there is weak evidence for at least a short term effect on mortality.
GarciaAymerich et alCitation169 have also shown in a 1-year study that patients with high levels of usual physical activity were at reduced risk of readmission to hospital. These results have been supported by analysis of data from the Copenhagen City Heart Study.Citation170 In this study patients were classified by self-reported physical activity and those who reported some degree of physical activity had a lower risks of hospitalization and all-cause mortality (hazard ratio 0.76, 95% CI 0.65–0.90) and a trend toward decreased respiratory mortality.Citation170
Noninvasive ventilation
The use of noninvasive ventilation (NIV) in acute hypercapnic respiratory failure is an accepted intervention.Citation171 However the evidence for its use in stable COPD is controversial. In one study of a highly selective group of 13 stable COPD patients with frequent admissions for decompensated type two respiratory failure and who tolerated the ventilator, there were significant falls in inpatient length of stay and number of hospital admissions compared to a period prior to intervention.Citation172 In a larger 2-year RCT of NIV with and without long-term oxygen therapy involving 90 patients, there was an improvement in quality of life in the NIV arm but no difference in survival or exacerbations leading to hospitalization.Citation173 However a recent prospective observational study of 99 patients with, and 41 without NIV, showed that survival rates were significantly higher in patients with NIV compared to those without this therapy (hazard ratio; 0.380; 95% CI 0.138–0.606) and this difference persisted after adjustment for baseline differences.Citation174 Interestingly there were beneficial effects in those with high base excess, low pH, low FEV1 or low hemoglobin or excessive hyperinflation.Citation174 In a small Chinese study of 40 patients with hypercapnic respiratory failure, randomized with and without NIV, there was a decrease in exacerbations by 3.9/year (p < 0.01) and a trend toward improved mortality in the interventional group.Citation175 Thus, the observation made 6 years ago is still valid; there is a need for well-designed controlled studies to evaluate the effects of NIV on survival, quality of life and exacerbations.Citation176
Long-term oxygen therapy (LTOT)
The role of LTOT was established about 28 year ago by two major studies.Citation177,Citation178 Guidelines for oxygen use have been generally agreed to by several consensus panels.Citation179 LTOT improves mortality in COPD patients with respiratory failure but there appears to be no effect in the less severe patients.Citation177,Citation178,Citation180 There are also positive effects on quality of lifeCitation73,Citation181 but no proven effect on COPD exacerbations. The relationship with improved quality of life may lead to speculation that this may in part be due to decreased exacerbation rates but most of these studies did not include exacerbation rates as an outcome measure and the MRC study found no relationship with rates of hospitalization.Citation178
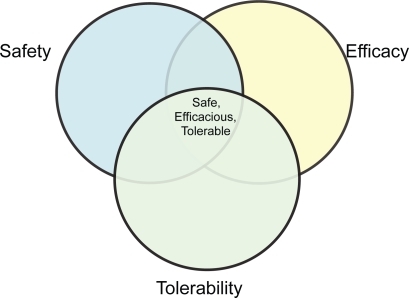
Safety and tolerability
The sections above have highlighted the many therapies that have demonstrated efficacy in reducing exacerbation rate, and thereby impacting favorably on health status and mortality. In this section we will review data on safety and tolerability for the more commonly used pharmacologic interventions where there have been several consistent results. However, the key point remains as to whether such interventions are safe, and tolerable. This is the subject of the following two sections. Only then can we achieve the ‘Holy Grail’ of pharmacotherapy in providing the right drug, at the right dose, to the right patient at the right time.
It is important to make the distinction, having assumed efficacy, between safety and tolerability. A tolerable drug may be unsafe, and likewise a safe drug may be intolerable. There are complex interactions between efficacy, tolerability and safety in individual patients (Figure ). The ideal preparation is efficacious, safe and tolerable.
Useful tolerability data may be obtained from those few key large trials that have now been performed in patients with COPD, and meta-analyses of smaller studies. These safety and tolerability data are summarized in Table .
Tolerability/patient preference
The TORCH trialCitation104 showed that even in highly motivated and monitored clinical trial environments 34% of patients had discontinued a LABA–ICS combination at 3 years, and this rate (and those of the individual components) was significantly better than placebo. Withdrawal from the combination was significantly lower than that with the components alone, suggesting a relationship between efficacy and tolerability. This withdrawal rate matched that for the LABA–ICS combination in the INSPIRE trial,Citation47 a head-to-head comparison with the long-acting anticholinergic drug tiotropium bromide and in which tiotropium was associated with a 42% withdrawal rate. Interpretation of the data from the INSPIRE study has not been supported by that of the UPLIFT study which showed a lower dropout rate in the tiotropium arm, though the designs of these two studies were different in that the INSPIRE study was largely a comparison of two arms each with monotherapy but the UPLIFT study allowed polytherapy in each arm.Citation136 In the OPTIMAL StudyCitation137 premature discontinuation rates were as follows: 47% (tiotropium–placebo), 43% (tiotropium–salmeterol) and 26% (tiotropium–SFC) (p < 0.001). Discontinuation was due to a perceived lack of medication efficacy or physician directed and due to a perceived deterioration in health status. Of these early withdrawals the proportion receiving an open label ICS–LABA combination were 74%, 70% and 54%, respectively.Citation137 These results appear to support those of the INSPIRE study on withdrawals and may indicate a trend in patient preference. However compliance/satisfaction within a trial may not reflect behavior in clinical practice. One retrospective study in the Netherlands found that about 37% of new users of tiotropium continued treatment for 1 year, compared with 14% for ipratropium, 13% for LABA, and 17% for ICS–LABA but compliance was increased in all groups after hospitalization.Citation182 These results are supported by those of a Canadian study of inhaled steroids between 1990 and 1995.Citation183 Differential withdrawal rates also complicate interpretation of data from trials, as a greater number of more severe patients are likely to withdraw from the placebo arm leaving the placebo arm containing a higher proportion of milder patients, potentially biasing against the beneficial effects of an intervention but also making an intent to treat analysis difficult (Table ).
Table 2 Safety and tolerability
Combining the tolerability/patient preference results of the three long term studies discussed above, it would appear that the most tolerable combination is ICS–LABA–LAMA but it is too early to draw a definitive conclusion at this stage.
The smaller studies involving the mucolytic agents or erythromycin found no differences in withdrawal rates compared to placebo.Citation149,Citation160,Citation161
Safety
The TORCH data were the first to suggest that ICS-containing regimens may be associated with an increased risk of a complication termed ‘pneumonia,’ but it should be noted that radiological confirmation of pneumonia was not required and so these pneumonia episodes may not be more precisely characterized.Citation104 The results are however supported by the INSPIRE data which reported pneumonias in 8% in the SFC arm and 4% in the tiotropium arm. Most of these pneumonias required hospitalization and there were three deaths due to pneumonia in INSPIRE, all in the SFC arm. However in spite of this, INSPIRE still reported a mortality reduction on treatment in favor of SFC.Citation47
Reassuringly, TORCH did not demonstrate an increase in the development of cataracts with ICS-containing regimes, or a reduction in bone mineral density or increase in fracture risk. Similarly, LABA-containing regimes were not associated with excess cardiovascular events.Citation104,Citation116,Citation184,Citation185 ICS was associated with an increased incidence of oropharyngeal candidiasis.
The safety of tiotropium has been reported in a meta-analysis of nine randomized trials including data from 8002 patients.Citation186 The data did not support an effect of tiotropium on all-cause (or pulmonary) mortality (OR 0.96, 95% CI 0.63–1.47). Only dry mouth was more common than with placebo, but when compared with other comparator drugs such as ipratropium or salmeterol there was an increased risk of urinary tract infection and, with further allowance for heterogeneity, an observed increased risk of arrythmias (OR 2.33, 95% CI 1.11–4.88). All three of the major tiotropium studiesCitation131–Citation133 excluded patients who had a recent myocardial infarction, heart failure or cardiac arrhythmias. Thus the publication of a recent meta-analysis of seventeen trials involving ipratropium or tiotropium which showed an increased risk of cardiac complications in COPD patients met with some consternationCitation187 and the need for a long term prospective study was felt necessary to answer the question of safety definitively. The UPLIFT study (Table ) was recently published and has shown a decreased risk of cardiac complications in the tiotropium arm vs placebo arm at 4 years, though it is to be noted that patients in both arms of the study were also on other inhaled therapies.Citation136
A systematic review of RCTs of ICS in COPD also showed no overall mortality effect (RR 0.84, 95% CI 0.60–1.18)Citation188 and significant increases in oropharyngeal candidiasis and bruising. No increased risk of fractures or cataracts were observed in the short follow-up periods, and effects on bone mineral density were variable. Similarly, effects on serum cortisol levels varied. This must be interpreted on the background of larger studies demonstrating effects of ICS on cataracts,Citation189,Citation190 osteoporosisCitation191 and adrenal suppression.Citation192
The side effects of LABA are well described. However, the particular concern in COPD has always been of cardiovascular risk, particularly with regard to tachyarrhythmias, following stimulation of cardiac β-adrenoceptors. Small studies of less than 1 year’s duration have assured us of the cardiovascular safety of salmeterol (50 μg twice daily) and formoterol in COPD.Citation115,Citation116,Citation150,Citation184,Citation193–Citation195 The question was also addressed in a meta-analysis of 1410 patients from 7 trials up to 1 year in duration using the LABA salmeterol (50 μg BD) in comparison to 1443 on placebo.Citation184 The relative risk for cardiovascular adverse events was 1.03 (95% CI 0.81.3; p = 0.838). Unlike other drug classes, meta-analyses of efficacy of LABA in COPD have not generally reported safety and tolerability. These results have been confirmed in the 1-year prospective studies for both salmeterolCitation111 and formoterolCitation112,Citation113 and for salmeterol over 4 years.Citation104
With regard to the newer drugs used in COPD no significant cardiovascular side effects were reported for erythromycinCitation149 or cilomilastCitation152 but cilomilast was associated with a significantly greater incidence of gastrointestinal side effects affecting daily life (17% vs 8% placebo). No safety issues have been reported in long-term studies of the mucolytic agents.Citation160,Citation161
Patient-focused perspectives
Metered dose inhalers
Pressurized metered dose inhalers (pMDIs), though easy to use, are critically dependent on inhalational technique to achieve acceptable lung deposition, which can be improved through use of add-on valve holding chambers (spacers), but these are seldom used in the clinical practice possibly because they are cumbersome.Citation196 Inclusion of a dose counter may significantly improve patient satisfaction with metered dose inhalers.Citation196
Breath-actuated devices
These include the dry powder inhalers (DPIs). They are environmentally safe, easily carried by the patient and do not incur patient-centered difficulty with hand inhalation coordination as may occur with pMDI usage. However, in a systematic review of COPD and asthma, Lavorini et al found that there were several problems with their use: (a) failure to exhale before actuation, (b) failure to breathhold after inhalation, (c) incorrect positioning of the inhaler, (d) incorrect rotation sequence, and (e) failure to execute a forceful and deep inhalation.Citation197 In a small study of elderly COPD patients with matched controls, Janssens et al found that 30% of COPD patients did not achieve the recommended flow rateCitation198 and these results are supported by other studies.Citation199,Citation200 Thus significant training may be required for patents to achieve therapeutic benefit form their inhalers.
Nebulizers
Nebulizers are relatively difficult and time consuming to use and need to be maintained. However their use requires less coordination compared to inhalers and one study of 82 patients in the UK found that patients felt overwhelmingly that the benefits of using a nebulizer outweighed potential disadvantages.Citation201
Thus from a patient-centered perspective, the choice of an inhaler device is a key component of COPD therapy and this requires more attention from physicians than perhaps we are aware of.
Disease management programs
The major features of these programs are (1) adherence to guidelines (2) use of a nurse with special training in that disease and (3) central coordination with patientcenteredness.Citation202 During the Dutch Quality of Care Through Patients Eyes (QUOTE) Study of patients with asthma and COPD, the proportion of patients reporting good accessibility improved from 57% to 73% and good coordination of care from 63% to 77% (p < 0.05 in both cases).Citation203,Citation204 Patient satisfaction improved as the study progressed.Citation203 Thus coordination of care and accessibility to health care personnel are two key features of patient satisfaction. An intensive disease-specific self-management programme carried out in Canada has been shown to reduce hospital readmission rateCitation205 or deaths following hospitalization but not readmissions,Citation206 though a systematic review of nurse interventions failed to show a consistent effect on hospitalization.Citation207 Another randomized study of organized care was based on a so-called chronic platform ‘chronic platform,’ which was a web-based application that facilitated phone calls to the case manager, as well as record keeping via phone calls, home visits or home video conferencing, and which again emphasized improved communication and nurse practitioner importance.Citation208 Patient satisfaction was not an outcome measure in this study but patients in the interventional arm had significantly less readmissions to hospital,Citation208 similar to those of a Spanish study of integrated care.Citation209
The results of the above two European and Canadian studies are different from those of a Dutch study which found no effect of a self-management program in 248 COPD patients.Citation210 However the Dutch study involved less severe patients who had not been admitted to hospital. Thus self management programs are associated with significant improvements in several processesCitation211 and may be more useful in the more severe patients who have had at least one hospitalization. Finally we may observe that it appears to be too early to determine whether self-management programs will have an effect on mortality but their fairly uniform effect on exacerbation severity as assessed by hospital admission rates is certainly consistent with this hypothesis and supported by the results of the SolerCataluña study.Citation66
Conclusions
Amongst the common chronic diseases, the term COPD is relatively new but is extremely prevalent in Western society and its incidence is rising in the developing world. COPD is generally underdiagnosed and undertreated. Exacerbations of COPD, about 50% of which are reported to physicians, lead to a deterioration in the quality of life and contribute significantly to disease burden. There is heterogeneity in the definition of COPD exacerbations, and for interventional studies to decrease exacerbation rates in a reproducible manner we must have amore precise definition of a COPD exacerbation. So far an objective marker is lacking but current efforts may soon yield positive results.
Patient quality of life deteriorates with time. Most of the health burden of COPD occurs in the more severe disease and is due to hospitalization which is an outcome measure that can be modified. COPD severity, and frequent and more severe exacerbations are all related to an increased risk of mortality.
Most of these outcome measures can be modified by current therapeutic strategies. Most inhaled steroid medications evaluated in long-term studies have similar effects on quality of life but may appear to differ in their effects on COPD exacerbations. The combination ICS–LABA and the LAMA, tiotropium, are likely to decrease mortality but perhaps only when used over a period of several years. In addition to this, they both improve health status and exacerbation rates. In a single center study the macrolide, erythromycin, has been shown to decrease the rate of COPD exacerbations but effects on mortality and quality of life are unknown. The new phosphodiesterase inhibitor cilomilast has been shown to improve quality of life and exacerbation rates but has significant gastrointestinal side effects.
Nonpharmacologic strategies are also of importance in COPD. Pulmonary rehabilitation is indicated in all severities of COPD and improves quality of life; effects on exacerbation rate and mortality are unclear. An unquantified degree of regular physical activity is associated with decreased hospitalization and mortality and may even prevent or delay onset of COPD. Noninvasive ventilation is associated with improved quality of life but studies have been too small to provide strong evidence for an effect on mortality. Long-term oxygen therapy improves mortality but only in hypoxic COPD patients. Disease management programs have a more holistic approach with emphasis on patient centeredness and communication, and are associated with decreased hospitalization rates and improved quality of life, but so far there are no studies of mortality. Most of these interventions are well tolerated and their safety profile in long-term studies has been shown to be good.
In the past 30 years there have been marked improvements in the treatment of COPD. We now have interventions that are well tolerated and safe and decrease exacerbation rates and improve quality of life. Comparative studies of the nonpharmacologic and pharmacologic approaches are lacking. We must concentrate more efforts on patient education. Recent studies using drug combinations have been unable to achieve more than a 25% fall in exacerbation rates and thus there may be a need for research into new modalities of treatment. Whether LABA or ICS reduce mortality is no longer of relevance, as it is clear that the combination ICS–LABA reduces exacerbation rates and improves quality of life and is thus more efficacious than the individual therapies. Future research will look at combining therapies with different mechanisms of treatment such as ICS–macrolides or LAMA–phosphodiesterase. The goal would be to decrease exacerbation/hospitalization rates by more than 50% either singly or in combination with current modalities of treatment.
Disclosure
The authors declare no conflicts of interest.
References
- BonetTSepultrechretum sive anatomica pructica ex Cadaveribus Morbo denatis, proponens Histoa’s Observations omnium pene humani corporis affectuum, ipsarcomoue Causas recordatis revelansGeneva1679
- PettyTLThe history of COPDInt J Chron Obstruct Pulmon Dis2006131418046898
- LaennecRTHA treatise on the diseases of the chest (English translsation for the French). Preface and notes by Forbes JLondon T and G Underwood1837
- TiffeneauRPinelliAFAire circulant et air captif dans l’exploration de la function ventilatrice pulmonaiteParis Med194713362462818909782
- FletcherCMJonesNLBurrowsBNidenAHAmerican emphysema and British bronchitis: A standardized comparative studyAm Rev Resp Dis19649011314178623
- BurrowsBFletcherCMHeardBEThe emphysematous and bronchial types of chronic airway obstruction: a clinopathological study of patients in London and ChicagoLancet196618308354159957
- JonesNLBurrowsBFletcherCMSerial studies of 100 patients with chronic airway obstruction in London and ChicagoThorax1967223273356035796
- BriscoeWANashESThe slow space in chronic obstructive pulmonary diseasesAnn N Y Acad Sci196512170672214309579
- SukumalchantraYWilliamsMHSerial studies of pulmonary function in patients with chronic obstructive pulmonary diseaseAm J Med1965399419455853045
- HoggJCMacklemPTThurlbeckWMSite and nature of airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med1968278135513605650164
- CosioMGhezzoHHoggJCThe relations between structural changes in small airwayys and pulmonaryfunction testsN Engl J Med197829812771281651978
- PettyTLSilversGWStanfordRESmall airway disease is associated with elastic recoil changes in excised human lungsAm Rev Respir Dis198413042456742609
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD)Am J Respir Crit Care Med1995152S77S1207582322
- ERS Consensus Statement. Optimal assessment and management of chronic obstructive pulmonary disease (COPD)Eur Respir J19958139814207489808
- BTSGuidelinesFor the management of chronic obstructive pulmonary diseaseBMJ199752S2S15
- ManninoDMDohertyDESonia BuistAGlobal Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) studyRespir Med2006100111512215893923
- FletcherCPetoRThe natural history of chronic airflow obstructionBMJ1977116451648871704
- AnthonisenNRWrightECHodgkinJEPrognosis in chronic obstructive pulmonary diseaseAm Rev Respir Dis198613314203510578
- AnthonisenNRConnettJEKileyJPEffects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA19941615391541
- AnthonisenNRSmoking, lung function, and mortalityThorax20005572973410950888
- HoleDJWattGCMDaveySmithGHartCLGillisCRHawthorneVMImpaired lung function and mortality risk in men and women:findings from the Renfew and Paisley prospective population studyBMJ19963137117158819439
- ThomasonMJStrachanDPWhich spirometric indices best predict death from chronic obstructive pulmonary diseaseThorax20005578578810950899
- AïtKhaledNEnarsonDAOttmaniSEl SonyAEltiganiMSepulvedaRChronic airflow limitation in developing countries: burden and prioritiesInt J Chron Obstruct Pulmon Dis2007214115018044686
- BuistASMcBurnieMAVollmerWMBOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet200737074175017765523
- MurrayCJLopezADAlternative projections of mortality and disability by cause 19902020: Global Burden of Disease StudyLancet1997349149815049167458
- SeemungalTHarrinarineRRiosMObstructive Lung Disease in Acute Medical PatientsWest Indian Med J2008577174
- LiuSZhouYWangXBiomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South ChinaThorax2007628389
- DriscollTNelsonDISteenlandKThe global burden of non-malignant respiratory disease due to occupational airborne exposuresAm J Ind Med20054843244516299701
- TakahashiTIchinoseMInoueHShiratoKHattoriTTakishimaTUnderdiagnosis and undertreatment of COPD in primary care settingsRespirology2003850450814629656
- JindalSKAggarwalANGuptaDA review of population studies from India to estimate national burden of chronic obstructive pulmonary disease and its association with smokingIndian J Chest Dis Allied Sci20014313914711529432
- GroenewaldPVosTNormanR2000S Afr Med J20077(8 Pt 2)674681
- ChanYeungMAïtKhaledNWhiteNIpMSTanWCThe burden and impact of COPD in Asia and AfricaInt J Tuberc Lung Dis200482124
- FeenstraTLvan GenugtenMLHoogenveenRTWoutersEFRuttenvan MölkenMPThe impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the NetherlandsAm J Respir Crit Care Med200116459059611520721
- ManninoDMHomaDMAkinbamiLJFordESReddSCChronic Obstructive Pulmonary Disease Surveillance – United States, 19712000MMWR200251S6116
- VarkeyABChronic obstructive pulmonary disease in women: exploring gender differencesCurr Opin Pulm Med2004109810315021178
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and metaanalysisEur Respir J20062852353216611654
- SorianoJBMaierWEggerPRecent trends in physician diagnosed COPD in women and men in the UKThorax20005578979410950900
- SchirnhoferLLamprechtBVollmerWMCOPD prevalence in Salzburg, Austria: results from the Burden of Obstructive Lung Disease (BOLD) studyChest2007131293617218553
- HanMKPostmaDManninoDM2007Gender and chronic obstructive pulmonary disease: why it mattersAm J Respir Crit Care Med2007176121179118417673696
- ManninoDMBuistoASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- TagerISpeizerFERole of infection in chronic bronchitisN Engl J Med197529211563571802896
- JefferyPKStructural and inflammatory changes in COPD: a comparison with AsthmaThorax1998531291369624299
- MontoASHigginsMWRossHWThe Tecumseh study of respiratory illness. Acute infection in chronic respiratory disease and comparison groupsAm Rev Infect Dis1975112736
- KannerRERenzettiADJrKlauberMRSmithCBGoldenCAVariables associated with changes in spirometry in patients with obstructive lung diseasesAm J Med1979674450313706
- AnthonisenNRManfredaJWarrenCPHeshfordEWHardingGKMNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med19871061962043492164
- SeemungalTARDonaldsonGCBhowmikAJeffriesDJWedzichaJATime course and recovery of exacerbations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001611608161810806163
- WedzichaJACalverleyPMSeemungalTAHaganGAnsariZStockleyRAINSPIRE InvestigatorsThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med2008177192617916806
- SeemungalTARDonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1998157141814229603117
- JohnstonAKManninoDMEpidemiology of COPD exacerbationsWedzichaJAMartinezFExacerbations Of Chronic Obstructive Pulmonary Disease (COPD)New YorkInforma Healthcare2008
- BurgePSCalverleyPMAJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ20003201297130310807619
- SeemungalTStockleyRCalverleyPHaganGWedzichaJAInvestigating New Standards for Prophylaxis In Reduction of Exacerbations The INSPIRE Study MethodologyCOPD2007417718317729060
- WilkinsonTMADonaldsonGCHurstJRSeemungalTARWedzichaJAEarly therapy improves outcomes of exacerbations of Chronic Obstructive Pulmonary DiseaseAm J Respir Crit Care Med20041691298130314990395
- LangsetmoLPlattRWErnstPBourbeauJUnderreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohortAm J Respir Crit Care Med200817739640118048806
- VijaysarathaKStockleyRAReported and unreported exacerbations of COPD: analysis by diary cardsChest2008133344117989153
- McCormickAFlemingDCharltonJMorbidity statistics from general practice: Fourth national study 19912: Office of Population Census and LondonSurveys; 1995
- NiewoehnerDEErblandMLDeupreeRHEffect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study GroupN Engl J Med19993401941194710379017
- GoldacreMJFergusonJAInpatient workload in medical specialities: demographic studies and time trends from linked statisticsQ J Med199588649660
- VestboJSorensenTLangePBrixATorrePViskumKLongterm effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease:a randomised controlled trialLancet19993531819182310359405
- ForemanMGDeMeoDLHershCPReillyJJSilvermanEKClinical determinants of exacerbations in severe, earlyonset COPDEur Respir J20073061124113017715170
- PatelISSeemungalTARWilksMLloyd OwenSDonaldsonGCWedzichaJARelationship between bacterial colonisation and the frequency, character and severity of COPD exacerbationsThorax20025775976412200518
- BhowmikASeemungalTASapsfordRJWedzichaJARelation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbationsThorax20005511412010639527
- SeemungalTARHarperOwenRBhowmikARespiratory viruses and symptoms, inflammatory markers in acute exacerbations of COPDAm J Respir Crit Care Med20011618162311719299
- SpencerSCalverleyPMABurgePSJonesPWon behalf of the Isolde Study GroupHealth status deterioration in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116312212811208636
- KannerREAnthonisenNRConnettJfor the Lung Health StudyLower respiratory illnesses promote FEV1 decline in smokers but not exsmokers with mild chronic obstructive pulmonary disease: results from the Lung Health StudyAm J Respir Crit Care Med200116435836411500333
- DonaldsonGCSeemungalTARBhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax20025784785212324669
- SolerCatalunaJJMartínezGarcíaMARomán SánchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax20056092593116055622
- JaradNAWedzichaJABurgePSCalverleyPMAn observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study GroupRespir Med19999316116610464871
- SuissaSErnstPVandemheenKLAaronSDMethodological issues in therapeutic trials of COPDEur Respir J20083192793318216056
- KeeneONCalverleyPMAJonesPWVestboJAndersonJAStatistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisitedEur Respir J200832172418591336
- SuissaSExacerbations and intent-to-treat analyses in randomised trialsEur Respir J2008321117111818827158
- JonesPWQuirkFHBaveystockCMThe St. Georges Respiratory QuestionnaireRespir Med199185Suppl B25311759018
- JonesPWHealth status measurement in chronic obstructive pulmonary diseaseThorax20015688088711641515
- OkubadejoAAJonesPWWedzichaJAQuality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemiaThorax19965144478658368
- SchrierADekkerFWKapteinAADijkmanJHQuality of life in elderly patients with chronic nonspecific lung disease seen in family practiceChest1990988948992209145
- JonesPWBoshTKQuality of life changes in COPD patients with SalmeterolAm J Respir Crit Care Med1997155128312899105068
- BorsonSMcDonaldGJGayleTDeffebachMLakshminarayanSVanTuinenCImprovement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary diseasePsychosomatics1992331902011557484
- Intermittent Positive Pressure Breathing Trial Group (IPPB Trial Group). Intermittent positive pressure breathing of COPDAnn Intern Med1983996126206357018
- MeechamJonesDJPaulEAJonesPWWedzichaJANasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnoeic COPDAm J Respir Crit Care Med19951525385447633704
- GarrodRMikelsonsCPaulEAWedzichaJARandomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2000162(4 Pt 1)133541
- WijkstraPJAltenaRVKraanJOttenVPostmeDSKoeterGHQuality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at homeEur Resp J19947269273
- GoldsteinRSGortEDHStubbingDAvendanoMAGuyattGHRandomised controlled trial of respiratory rehabilitationLancet1994344139413977968075
- WedzichaJABestallJCGarrodRGarnhamRPaulEAJonesPWRandomised controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scaleEur Respir J1998123633699727786
- OsmanLMGoddenDJFriendJARLeggeJSDouglasJGQuality of life and hospital readmission in patients with chronic obstructive pulmonary diseaseThorax19975267719039248
- SpencerSJonesPWfor the GLOBE Study GroupTime course of recovery of health status following an infective exacerbation of chronic bronchitisThorax20035858959312832673
- GuyattGHBermanLBTowensendMPugsleySOChambersLWA measure of quality of life for clinical trials in chronic lung diseaseThorax1987427737783321537
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure for chronic airflow limitation the St. George’s Respiratory QuestionnaireAm Rev Respir Dis1992145132113271595997
- WilliamsJESinghSJSewellLGuyattGHMorganMDDevelopment of a self-reported Chronic Respiratory Questionnaire (CRQSR)Thorax2001561295495911713359
- JaeschkeRSingerJGuyattGHMeasurement of health status. Ascertaining the minimal clinically important differenceControl Clin Trials19891044074152691207
- JonesPWLassersonDRelationship between change in the St. Georges Respiratory Questionnaire score and the patient’s perception of treatment efficacy after one years therapy with nedocromil sodiumAm J Respir Crit Care Med1994149A211
- JonesPLInterpreting thresholds for a clinically significant change in health status in asthma and COPDEur Respir J20021939840411936514
- BroekhuizenRWoutersEFCreutzbergECScholsAMRaised CRP levels mark metabolic and functional impairment in advanced COPDThorax200661172216055618
- GarrodRMarshallJBarleyEFredericksSHaganGThe relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD)Prim Care Respir J20071623624017665081
- SeemungalTARCho Fook LunJDavisGPlasma homocysteine is elevated in COPD patients and is related to COPD severityInt J Chron Obstruct Pulmon Dis20072331332118229569
- LopezADMathersCDEzzatiMJamisonDTMurrayCJGlobal and regional burden of disease and risk factors, 2001: systematic analysis of population health dataLancet2006367174757
- OliveiraAFValenteJGLeiteICThe disease burden attributable to smoking in the state of Rio de Janeiro, Brazil in 2000Clinics20086321522218438576
- SahaRNathASharmaNBadhanSKIngleGKChanging profile of disease contributing to mortality in a resettlement colony of DelhiNatl Med J India20072012512717867616
- The Office of National Statistics of the United Kingdom Available at: www.statistics.gov.uk/
- The National Health Executive of the United Kingdom Available at: www.nationalhealthexecutive.com
- ManninoDMBramanSThe epidemiology and economics of chronic obstructive pulmonary diseaseProc Am Thorac Soc2007450252617878461
- FosterTSMillerJDMartonJPCaloyerasJPRussellMWMenzinJAssessment of the Economic Burden of COPD in the USA Review and Synthesis of the LiteratureCOPD2006321121817361502
- NurmagambetovTAtherlyAWilliamsSHolguinFManninoDMReddSCWhat is the cost to employers of direct medical care for chronic obstructive pulmonary diseaseCOPD200632039
- TraverGAMeasures of symptoms and life quality to predict emergent use of institutional health care resources in chronic obstructive airways diseaseHeart Lung1998176896973192415
- HurdSThe impact of COPD on lung health worldwide: epidemiology and incidenceChest20001171S5S10673465
- CalverleyPMAndersonJACelliBTORCH investigatorsSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med200735677578917314337
- PostmaDSPetersISteenhuisEJSluiterHJModerately severe chronic airflow obstruction. Can corticosteroids slow down obstructionEur Respir J1988122263366234
- DompelingEvan SchayckCPvan GrunsvenPMvan HerwaardenCLAkkermansRMolemaJSlowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. A 4 year prospective studyAnn Intern Med19931187707788470851
- CallahanCMDittusRSKatzBPOral corticosteroid therapy for patients with stable chronic obstructive pulmonary disease. A metaanalysisAnn Intern Med19911142162231824614
- Jan GrunsvenPMvan SchayckCPDerenneJPKerstjensHARenkemaTEPostmaDSLong term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a metaanalysisThorax19995471410343624
- PauwelsRALofdahlCGLaitinenLASchoutenJPPostmaDSPrideNBLongterm treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smokingN Engl J Med19993401948195310379018
- SpencerSCalverleyPMABurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J2004231518431851
- CalverleyPPauwelsRVestboJTRial of Inhaled STeroids ANd longacting beta2 agonists study groupCombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet200336144945612583942
- SzafranskiWCukierARamirezAEfficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J200321748751
- CalverleyPMBoonsawatWCsekeZZhongNPetersonSOlssonHMaintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J20032291291914680078
- Global Initiative for Obstructive Lung Disease Update Available from http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=989.2007
- BoydGMoriceAHPounsfordJCSiebertMPeslisNCrawfordCAn evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD)Eur Respir J1997108158219150318
- MahlerDADonohueJFBarbeeRAEfficacy of salmeterol xinafoate in the treatment of COPDChest199911595796510208192
- DahlRGreefhorstLANowakDFormoterol in Chronic Obstructive Pulmonary Disease I Study GroupInhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116477878411549532
- RennardSIAndersonWZuWallackRUse of a longacting inhaled beta2adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011631087109211316640
- SalpeterSRBuckleyNSSalpeterEEMetaanalysis: anticholinergics, but not βagonists, reduce severe exacerbations and respiratory mortality in COPDJ Gen Intern Med2006211011101916970553
- GudmundssonGGislasonTLindbergEMortality in COPD patients discharged from hospital: the role of treatment and comorbidityRespir Res2006710916914029
- SiafakasNMVermeirePPrideNBOptimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J19958139814207489808
- GrossNJSkorodinMSRole of the parasympathetic system in airway obstruction due to emphysemaN Engl J Med19843114214256749189
- GrossNJCoESkorodinMSCholinergic bronchomotor tone in COPD. Estimates of its amount in comparison with that in normal subjectsChest1989969847
- IncorvaiaCRiarioSforzaGGPravettoniCYacoubMRFratiFImpairment of small airways in COPD patients with frequent exacerbations and effects of treatment with tiotropiumInt J Chron Obstruct Pulmon Dis2008312312618488435
- SinghDTiotropium more knowledge leads to more questionsInt J Chron Obstruct Pulmon Dis20083iii
- KawayamaTHoshinoTIchikiMKurume COPD Study Group Effect of addon therapy of tiotropium in COPD treated with theophyllineInt J Chron Obstruct Pulmon Dis2008313714718488437
- KestenSCassaburiRKukafkaDCooperCBImprovement in selfreported exercise participation with the combination of tiotropium and rehabilitative training in COPD patientsInt J Chron Obstruct Pulmon Dis2008312713618488436
- JohanssonGLindbergARombergKNordströmLGerkenFRoquetABronchodilator efficacy of tiotropium in patients with mild to moderate COPDPrim Care Respir J20081716917518536860
- AndòFRuggeriPGirbinoGCazzolaMTiotropium and salmeterol/fluticasone combination do not cause oxygen desaturation in COPDRespir Med200810281581818343646
- HaagSMatthiesenSJuergensURRackéKMuscarinic receptors mediate stimulation of collagen synthesis in human lung fibroblastsEur Respir J20083255556218480105
- CasaburiRMahlerDAJonesPWWannerASanPGZuWallackRLA longterm evaluation of oncedaily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- VinckenWvan NoordJAGreefhorstAPDutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J20021920921611871363
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once daily inhaled anticholinergic bronchodilatorAnn Intern Med200514331732616144890
- DonohueJFvan NoordJABatemanEDA 6 month, placebocontrolled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- DecramerMCelliBTashkinDPClinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trialCOPD2004130331217136995
- TashkinDPCelliBSennSUPLIFT Study InvestigatorsA 4 year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- AaronSDVandemheenKLFergussonDCanadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasonesalmeterol for treatment of chronic obstructive pulmonary disease: a randomized trialAnn Intern Med200714654555517310045
- SinghDBrooksJHaganGCahnAO’ConnorBJSuperiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPDThorax20086359259818245142
- RabeKFTimmerWSagkriotisAVielKComparison of a combination of tiotropium and formoterol to salmeterol and fluticasone in moderate COPDChest200813425526218403672
- NajafzadehMMarraCASadatsafaviMCostEffectiveness of Therapy with Combinations of LongActing Bronchodilators and Inhaled Steroids for Treatment of COPDThorax20086396296718621985
- KudohSAzumaAYamamotoMIzumiTAndoMImprovement of survival in patients with diffuse panbronchiolitis treated with low dose erythromycinAm J Respir Cri Care Med199815718291832
- TakedaHMiuraHKawahiraMLongterm administration study on TE031 (A56268) in the treatment of diffuse panbronchiolitisKansenshogaku Zasshi19896371782526188
- YamamotoMKondoATamuraMIzumiTInaYNodaMLongterm therapeutic effects of erythromycin and new quinolone antibacterial agents on diffuse panbronchiolitisNihon Kyobu Shikkan Gakkai Zasshi199028130513132273658
- WolterJSeeneySBellSEffect of long term treatment with azithromycin on disease parameters in cystic fibrosis:a randomized trialThorax2002572126
- EquiABalfourLynnIMBushARosenthalMLong term azithromycin in children with cystic fibrosis:a randomised, placebocontrolled crossover trialLancet200236097898412383667
- SaimanLMarshallBCMayerHamblettNMacrolide Study GroupAzithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trialJAMA20032901749175614519709
- ClementATamaletALerouxERavillySFaurouxBJaisJPLong term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled TrialThorax20066189590216809416
- SuzukiTYanaiMYamayaMErythromycin and Common Cold in COPDChest 1202001730733
- SeemungalTWilkinsonTMAHurstJRPereraWSapsfordRJWedzichaJALong term macrolide therapy is associated with decreased COPD exacerbation frequencyAm J Respir Crit Care Med20081781139114718723437
- RossiAKristufekPLevineBEFormoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study GroupComparison of the efficacy, tolerability and safety of formoterol dry powder and oral slow-release theophylline in treatment of COPDChest20021211058106911948033
- ZhouYWangXZengXPositive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 yearRespirology20061160361016916334
- RennardSISchachterNStrekMRickardKAmitOCilomilast for COPD: results of a 6 month, placebocontrolled study of a potent, selective inhibitor of phosphodiesterase 4Chest2006129566616424413
- Fan ChungKPhosphodiesterase inhibitors in airways diseaseEur J Pharmacol200653311011716458289
- NicholKLBakenLNelsonARelation between influenza vaccination and out patient visits, hospitalisation and mortality in elderly patients with chronic lung diseaseAnn Intern Med199913039740310068413
- GrangerRWaltersJPoolePJInjectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary diseaseCochrane Database Syst Rev20064CD00139017054135
- AlfagemeIVazquezRReyesNClinical efficacy of antipneumococcal vaccination in patients with COPDThorax20066118919516227328
- ColletJPDucruetTHaiderSEffect of an immunostimulating agent on acute exacerbations and hospitalization in COPD patientsAm J Respir Crit Care Med1997156171917249412546
- SprenkleMDNiewoehnerDEMacDonaldRRutksIWiltTJClinical efficacy of OM85 BV in COPD and chronic bronchitis:a systematic reviewCOPD2005216717517136978
- PoolePJBlackPNOral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic reviewBMJ2001322127174 [Updated at Cochrane Database Syst Rev 2006 Jul 19;3:CD001287]
- DecramerMRuttenvan MölkenMDekhuijzenPNEffects of Nacetyl cysteine on outcomes in COPD (BRONCHUS trial)Lancet2005365155260
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebocontrolled studyLancet20083712013201818555912
- GriffithsTLBurrMLCampbellIAResults at 1 year of outpatient multidisciplinary pulmonary rehabilitationLancet20003553626810665556
- BoxallAMBarclayLSayersACaplanGAManaging chronic obstructive pulmonary disease in the community. A randomized controlled trial of homebased pulmonary rehabilitation for elderly housebound patientsJ Cardiopulm Rehabil20052537838516327534
- FoglioKBianchiLAmbrosinoNIs it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2 year controlled studyChest20011191696170411399693
- RingbaekTJBrøndumEBoltonSMartinezGLangeP[Rehabilitation of patients with chronic obstructive pulmonary disease. Effect of a 7 week programme after 12 months]Ugeskr Laeger20071691572157617484828
- PuhanMAScharplatzMTroostersTSteurerJRespiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality a systematic reviewRespir Res200565415943867
- FoglioKBianchiLBrulettiGSeven year time course of lung function, symptoms, healthrelated quality of life, and exercise tolerance in COPD patients undergoing pulmonary rehabilitation programsRespir Med20071011961197017531455
- Di MeoFPedoneCLubichSPizzoliCTraballesiMIncalziRAAge does not hamper the response to pulmonary rehabilitation of COPD patientsAge Ageing20083753053518565981
- GarciaAymerichJFarreroEFélezMAIzquierdoJMarradesRMAntóJMfor the Estudi del Factors de Risc d’Agudització de la MPOC investigatorsRisk factors of readmission to hospital for a COPD exacerbation: a prospective studyThorax20035810010512554887
- GarciaAymerichJLangePBenetMSchnohrPAntóJMRegular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort studyThorax20066177277816738033
- LightowlerJVWedzichaJAElliottMWRamFSNoninvasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease:Cochrane systematic review and metaanalysisBMJ200332618512543832
- TuggeyJMPlantPKElliottMWDomiciliary noninvasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysisThorax20035886787114514940
- CliniESturaniCRossiAThe Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patientsEur Respir J20022052953812358325
- BudweiserSHitzlAPJörresRAImpact of noninvasive home ventilation on longterm survival in chronic hypercapnic COPD: a prospective observational studyInt J Clin Pract2007611516152217686094
- XiangPCZhangXYangJNThe efficacy and safety of long term home noninvasive positive pressure ventilation in patients with stable severe chronic obstructive pulmonary diseaseZhonghua Jie He He Hu Xi Za Zhi20073074675018218204
- WedzichaJAOutcome of longterm noninvasive positivepressure ventilationRespir Care Clin N Am2002855957312602415
- Nocturnal Oxygen Therapy Trial Group (NOTT)Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease:a clinical trialAnn Intern Med1980933913986776858
- 178Medical Research Council Working Party (MRC)Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working PartyLancet198116816866162061
- KimVBendittJOWiseRASharafkhanehAOxygen therapy in chronic obstructive pulmonary diseaseProc Am Thorac Soc2008551351818453364
- ChaouatAWeitzenblumEKesslerRA randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patientsEur Respir J1999141002100810596681
- TanniSEValeSALopesPSGuiotokoMMGodoyIGodoyIInfluence of the oxygen delivery system on the quality of life of patients with chronic hypoxemiaJ Bras Pneumol20073316116717724535
- Breekveldt-PostmaNSKoerselmanJErkensJALammersJWHeringsRMEnhanced persistence with tiotropium compared with other respiratory drugs in COPDRespir Med20071011398140517368011
- BlaisLBourbeauJSheehyOLeLorierJInhaled corticosteroids in COPD: determinants of use and trends in patient persistence with treatmentCan Respir J200411273215010729
- FergusonGTFunckBrentanoCFischerTDarkenPReisnerCCardiovascular safety of salmeterol in COPDChest20031231817182412796155
- RodrigoGJNanniniLJRodríguezRoisinRSafety of longacting betaagonists in stable COPD: a systematic reviewChest2008133107987
- BarrRGBourbeauJCamargoCARamFSTiotropium for stable chronic obstructive pulmonary disease: A meta-analysisThorax200661854862 Erratum in: Thorax. 2006;62:19116844726
- SinghSLokeYKFurbergCDInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and metaanalysisJAMA20083001439145018812535
- AlsaeediASinDDMcAlisterFAThe effects of inhaled corticosteroids in chronic obstructive pulmonary disease:a systematic review of randomized placebocontrolled trialsAm J Med2002113596512106623
- GarbeESuissaSLeLorierAssociation of inhaled corticosteroid use with cataract extraction in elderly patentsJAMA19982805395439707144
- CummingRGMitchellPLeederSRUse of inhaled corticosteroids and the risk of cataractsN Engl J Med19973378149203425
- WongCAWalshLJSmithCJInhaled corticosteroid use and bonemineral density in patients with asthmaLancet20003551399140310791523
- LipworthBJSystemic adverse effects of inhaled corticosteroid therapy: A systematic review and metaanalysisArch Intern Med199915994195510326936
- GoldkornADiottoPBurgessCThe pulmonary and extrapulmonary effects of highdose formoterol in COPD: a comparison with salbutamolRespirology2004910210814982610
- RosenkranzBRouzierRKruseMSafety and tolerability of highdose formoterol (via Aerolizer) and salbutamol in patients with chronic obstructive pulmonary diseaseRespir Med200610066667216303295
- CampbellSCCrinerGJLevineBECardiac safety of formoterol 12 microg twice daily in patients with chronic obstructive pulmonary diseasePulm Pharmacol Ther20072057157916911869
- ShethKWassermanRLLincourtWRLocantoreNWCarranzaRosenzweigJCrimCFluticasone propionate/salmeterol hydrofluoroalkane via metereddose inhaler with integrated dose counter: Performance and patient satisfactionInt J Clin Pract2006601218122416981966
- LavoriniFMagnanADubusJCEffect of incorrect use of dry powder inhalers on management of patients with asthma and COPDRespir Med200810259360418083019
- JanssensWVandenBrandePHardemanEInspiratory flow rates at different levels of resistance in elderly COPD patientsEur Respir J200831788317898020
- JarvisSIndPWShinerRJInhaled therapy in elderly COPD patients; time for reevaluation?Age Ageing20073622138
- AlShowairRATarsinWYAssiKHPearsonSBChrystynHCan all patients with COPD use the correct inhalation flow with all inhalers and does training helpRespir Med20071012395240117629471
- BartaSKCrawfordARobertsCMSurvey of patients’ views of domiciliary nebuliser treatment for chronic lung diseaseRespir Med20029637538112117035
- SeemungalTARWedzichaJAIntegrated care: a new model for COPD management?Eur Respir J20062813
- van CampenCSixmaHJKerssensJJPetersLAssessing noninstitutionalized asthma and COPD patients’ priorities and perceptions of quality of health careJ Asthma1997345315389428299
- SixmaHJvan CampenCKerssensJJPetersLQuality of care from the perspective of elderly people: the QUOTEelderly instrumentAge Ageing200029173810690690
- BourbeauJJulienMMaltaisFChronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec. 2003. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific selfmanagement interventionArch Intern Med200316358559112622605
- SridharMTaylorRDawsonSRobertsNJPartridgeMRA nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary diseaseThorax20086319420017901162
- TaylorSJCandyBBryarRM2005Effectiveness of nursing and nurse led chronic disease management innovations for patients with chronic obstructive pulmonary disease: systematic review of evidenceBMJ200533148516093253
- CasasATroostersTGarciaAymerichJmembers of the CHRONIC Project. Integrated care prevents hospitalisations for exacerbations in COPD patientsEur Respir J20062812313016611656
- GarciaAymerichJHernandezCAlonsoAEffects of an integrated care intervention on risk factors of COPD readmissionRespir Med20071011462146917339106
- MonninkhofEvan der ValkPSchermerTvan der PalenJvan HerwaardenCZielhuisGEconomic evaluation of a comprehensive selfmanagement programme in patients with moderate to severe chronic obstructive diseaseChron Respir Dis2004171616281663
- SteutenLVrijhoefBVan MerodeFWesselingGJSpreeuwenbergCEvaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary diseaseInt J Qual Health Care20061842943617032687
- LacasseYGoldsteinRLassersonTJMartinSPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20064CD00379317054186