Abstract
Background
Many of the systemic manifestations of chronic obstructive pulmonary disease (COPD) are mediated through increased systemic levels of inflammatory proteins. We assessed the long term repeatability of Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) over one year and examined the relationships between these systemic markers in COPD.
Methods
Fifty-eight stable COPD patients completed a baseline and one-year visit. Serum IL-6, plasma CRP, and plasma TNF-α were measured. Repeatability was expressed by intraclass correlation coefficient (Ri) and the Bland–Altman method. Pearson correlations were used to determine the relationships between the systemic markers at both visits.
Results
There was moderate repeatability with a very high degree of statistical significance (p ≤ 0.001) between the two visits for all the systemic biomarkers (IL-6, CRP, and TNF-α). CRP was significantly associated with IL-6 at both visits (r = 0.55, p = 0.0001, r = 0.51, p = 0.0002, respectively). There were no other significant associations between the systemic markers at either of the visits.
Conclusions
Systemic inflammatory biomarkers IL-6, CRP, and TNF-α were moderately repeatable over a twelve month period in COPD patients. We have also shown that a robust and repeatable association between IL-6 and CRP exists.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition characterized by progressive airway inflammation.Citation1 It is well recognized that systemic manifestations are also a key component of COPD. These include increased levels of inflammation in the blood,Citation2,Citation3 muscle inflammation and wasting,Citation4,Citation5 cardiovascular disease,Citation6 and osteoporosis.Citation7 There is much interest in the use of biomarkers of systemic inflammation in COPD, as these may have possible applications in disease phenotyping, monitoring of disease progression or exacerbations, and measuring the effects of therapeutic interventions.
It is believed that many of the systemic manifestations of COPD are mediated through increased systemic levels of inflammatory proteins such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP).Citation2–Citation4,Citation8–Citation12 CRP is a commonly used biomarker of systemic inflammation in patients with COPD. Plasma CRP levels are increased in COPD patientsCitation2,Citation3 and are associated with increased mortality.Citation13,Citation14 CRP levels are predictive of cardiovascular risk,Citation15,Citation16 and in patients with COPD also independently predict death due to COPD.Citation13 CRP is also related to other important clinical outcomes including exercise tolerance,Citation2,Citation17 health status,Citation2 and muscle strength.Citation3 CRP is regulated by IL-6, as this cytokine can initiate an acute phase response through the induction of acute phase proteins including CRP in hepatocytes.Citation18 Consequently, it has been shown that IL-6 and CRP levels correlate in COPD patients.Citation2,Citation3,Citation19,Citation20
TNF-α has been shown to play a central role in the muscle wasting and weight loss seen in COPD patientsCitation9,Citation21,Citation22 and several studies have demonstrated increased levels of TNF-α and its receptors in the circulation of COPD patients.Citation4,Citation8,Citation11,Citation12,Citation21 There is some evidence that TNF-α levels are related to levels of IL-6Citation3,Citation19,Citation23 and CRP,Citation3 although this has not been reported in all studies.Citation2
CRP has previously been shown in a study by Pinto and colleaguesCitation17 to be repeatable over a 17 month period and even more recently both IL-6 and CRP have been shown to be repeatable over a six month period.Citation24 However longer term studies of the repeatability of IL-6 and TNF-α are lacking. The repeatability of systemic biomarkers is an important issue, as those with increased variability will have less utility in clinical practice or research. Studies analyzing longer term repeatability are needed if systemic biomarkers are going to be used in long term observational studies or clinical trials of therapeutic interventions.
The main aims of this study were to 1) assess the long term repeatability of systemic biomarkers of inflammation (CRP, TNF-α, IL-6) in COPD patients over one year, 2) to examine the relationships between these systemic markers and confirm the results of previous studies in this area.
Methods
Subjects
Ninety-four stable patients were initially recruited into this single centre study from primary care by media advertising to the study at baseline (2004–2005), however only 58 patients returned for their follow up assessment (2005–2006). The 36 patients were lost to follow up as they either had withdrawn their consent or had not returned for their visit within the one-year timeframe. COPD was diagnosed according to current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,Citation1 based on a smoking history of at least 10 pack-years with typical symptoms (one or more of productive cough, breathlessness, and wheeze) and evidence of airflow obstruction. Patients with a clinical history of asthma, alpha-1-antitrypsin deficiency, an exacerbation or any change in their COPD therapy within four weeks of the study at the baseline and the one-year visit, or a history of lung cancer were excluded. Written informed consent was obtained and the local ethics committee approved the study.
Study design
Spirometry, sputum induction, and peripheral blood sampling were performed at the baseline visit and then repeated one year later. Sputum differential cell counts were performed and IL-8 was measured in the sputum supernatant to characterize airway inflammatory biomarkers. Serum IL-6, plasma CRP, and plasma TNF-α were measured at both visits as biomarkers of systemic inflammation. Height was measured to the nearest cm and weight to the nearest 100 gm. Body mass index (BMI) was calculated as weight in kg divided by squared height in meters.
Spirometry
Maximum expiratory flow volume measurements were performed using the spirometry system on the MasterScreen (Jaeger GmbH, Würzburg, Germany). Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) readings were performed in triplicate. Spirometry during sputum induction was performed with a Vitalograph spirometer (Vitalograph, Buckinghamshire, UK). The equipment was calibrated daily according to the manufacturer’s instructions.
Plasma and serum assays
Plasma and serum were obtained from peripheral blood samples by centrifugation for 15 minutes, at 1500 g and at 4 °C or ambient temperature, respectively. Plasma and serum samples were stored at −80 °C until analysis. Plasma TNF-α and serum IL-6 was measured by high sensitivity ELISA (Quantikine, R&D Systems Europe, Oxon, UK) with a lower limit of detection of 0.5 pg/ml and 0.156 pg/ml respectively. Serum was used for the IL-6 assay as there was insufficient plasma available from all patients. Plasma CRP was measured by high sensitivity particle-enhanced immunonephelometry (Cardiophase; BN systems, Dade Behring, Newark, NJ, USA) with a lower limit of detection of 0.175 mg/L.
Statistical analysis
The Kolmogorov – Smirnov test determined normality of the data and nonparametric data were natural log transformed. Naturally log-transformed data was therefore used for IL-6, CRP, and TNF-α for our anlaysis.
Paired t test was performed to determine the mean differences between the baseline and one-year visit. Repeatability was expressed as an intraclass correlation coefficient (ICC) [Ri = between-subject variance/(within- + between- subject variance)]. P < 0.05 was considered statistically significant. Ri values of 0 to 0.2 are usually interpreted to indicate slight repeatability, 0.21 to 0.40 to indicate fair repeatability, 0.41 to 0.60 to indicate moderate repeatability, 0.61 to 0.8 to indicate good repeatability, and 0.81 to 1.00 to indicate very good repeatability. Graphical representation of repeatability between the two visits were reported using the Bland – Altman method.Citation30 The limits of agreement are expressed as ± 2 standard deviations (SD) of the mean of differences betweeen the two measurements within which 95% of the repeated measures are expected to lie and for our naturally log-transformed data the limits of agreement were antilogged to express the fold changes expected over a one-year period.
Pearson correlations were used to determine the relationships between the systemic markers at both the baseline and one-year visit. Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA) and GraphPad (GraphPad Software, Inc., La Jolla, CA, USA) software.
Previous publications studying the repeatability of sputum biomarkers,Citation25–Citation29 plasma IL-6,Citation24 and CRPCitation17 used varying sample sizes from 10–88 COPD patients. Thus, we enrolled 94 subjects, and considered that the matched data from the returning 58 COPD patients who had both baseline and one-year measurements was an adequate sample size for this study.
Results
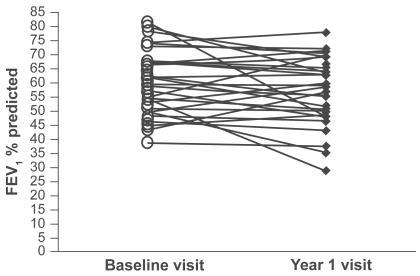
The study population was composed of 58 subjects, with 40 patients categorized as GOLD stage 2, 15 patients as stage 3, and 3 patients at stage 4 (see for demography). FEV1 percent predicted did not change over the one-year period (mean 56.7 vs 57.5, p = 0.1) () and neither did BMI (mean 27.45 vs 27.31, p = 0.4). shows the baseline and one-year values for other pulmonary function tests. Peripheral blood samples were obtained from all 58 patients at both visits.
Table 1 Baseline characteristics of the study patients
Table 2 Pulmonary function at the baseline visit and one-year visit
Repeatability of systemic biomarkers
summarizes the measurements of the systemic inflammatory biomarkers and BMI at the baseline and one-year visit. Using paired T tests there was no statistically significant change in these measurements between both visits (p > 0.05 for all comparisons).
Table 3 Repeatability of systemic measurements over the one year interval
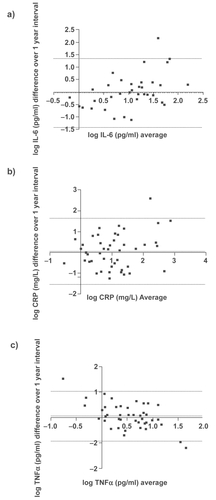
Using ICC analysis, there was moderate agreement with a very high degree of statistical significance (p ≤ 0.001) between the two visits for all the systemic biomarkers (IL-6, CRP, and TNF-α). Graphical representations of the repeatability of systemic biomarkers using the Bland – Altman methods are shown in . These plots indicate moderate reproducibility, as the mean differences of the repeated measurements lie close to zero and all values are randomly scattered around the mean difference with the majority of values lying within the limits of agreement (±2 SD). Antilogging the limits for IL-6, CRP, and TNF-α show that 95% of cases for the one-year measurement will have at least a 3.8-, 5.2-, and 2.8-fold change from the baseline measurement, respectively.
Relationships between systemic biomarkers
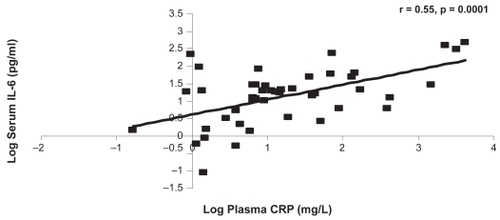
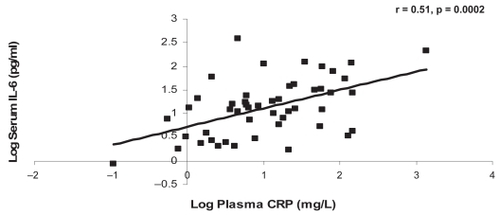
CRP was significantly associated with IL-6 at the baseline and one-year visits (r = 0.55, p = 0.0001, r = 0.51, p = 0.0002, respectively) (). There were no other significant associations between the systemic markers at either of the visits ().
Table 4a Relationships between systemic inflammatory biomarkers and BMI at the baseline visit
Table 4b Relationships between systemic inflammatory biomarkers and BMI at the one-year visit
Discussion
The main novelty of this study is the repeatability of systemic biomarkers in a moderate to severe COPD population over one-year. IL-6, CRP, and TNF-α showed moderate repeatability over one year. We also found a moderate association between CRP and IL-6, confirming the results of previous studies,Citation2,Citation3,Citation19,Citation20 but no association between these proteins and TNF-α, in contrast to previous reports.Citation3,Citation19,Citation23
The repeatability of systemic inflammatory biomarkers over 12 months in COPD patients was analyzed by three statistical methods; first, we observed no significant changes in group mean values for any of the measurements. Second, using ICC, which is a recognized method for assessing the repeatability of measurements, we observed moderate levels of agreement for systemic inflammatory markers, as the Ri values were ≥0.5. Third, we also present Bland – Altman plots to view the repeatability of data from individual subjects. To our knowledge, this is the first study to compare the long term reproducibility of IL-6 and TNF-α in COPD patients, and we demonstrate moderate repeatability for these systemic biomarkers over a 12 month period.
It has previously been shown that CRP did not change over a 17 month interval,Citation17 but we are not aware of any similar information concerning IL-6 and TNF-α. The assessments of repeatability in our study can be used in the following ways; the within-subject SDs can be used to aid the statistical powering of clinical trials with pharmacological interventions and the Bland–Altman plots show the variation that could be expected for individual measurements in clinical practice.
The systemic biomarkers that we investigated all appear to be related to pathophysiological processes in COPD; CRP is related to cardiovascular riskCitation15,Citation16 and has been shown to be associated with many other important clinical outcomes of COPD. For example previous studies have been shown CRP to be related to the symptom domain of St George’s Respiratory Questionnaire (SGRQ), impaired energy metabolism, and muscle strength.Citation2,Citation3,Citation17 IL-6 is known as a powerful promoter of CRP production in the liverCitation18 and is associated with CRP levels in COPD patients,Citation2,Citation3,Citation6,Citation19 whilst TNF-α and its receptors are increased in the muscles of COPD patients thus implicating it in conditions such as cachexia in COPD.Citation4,Citation31,Citation32
The present study confirms that the link between IL-6 and CRP exists in our group of moderate to severe COPD patients and that it is a robust relationship that does not change over one year. The moderate association (r = 0.51–0.55) on two occasions adds confidence to our interpretation that IL-6 promotes CRP production in COPD patients.
The finding that neither IL-6 nor CRP was related to the TNF-α is in contrast to two previous studies that showed a relationship, albeit a weak correlation, between TNF-α and IL-6Citation3,Citation19 and TNF-α and CRP existedCitation3 in COPD patients. One methodological explanation for the lack of correlation in the current study could be due to the short half life of TNF-α compared to IL-6 or CRP. TNF-α has a short half-life of a few minutes and is also difficult to detect because it forms complexes with soluble TNF-α receptors (sTNF-R) and has high renal clearance.Citation33,Citation34 Circulating sTNF-R may be a more sensitive marker of TNF-α activation as they have slower renal clearance.Citation33,Citation34 Increased levels of these soluble receptors have been detected in COPD patients.Citation11,Citation21 In addition the current study population consisted mainly of moderate COPD patients and a different relationship to TNF-α may have been observed in a population consisting of a range of disease severities.
BMI is recognized as an important tool in defining phenotypes of COPD patients and a reduced BMI has been shown to be an independent risk factor for mortality in COPD and to be associated with disease severity.Citation35,Citation36 Increased skeletal muscle apoptosis have also been shown to be associated with a lower BMI and reduced exercise tolerance.Citation5 In our study BMI was not related to circulating levels of IL-6, CRP, and TNF-α. This suggests that body mass loss may not be caused through systemic inflammation. However, there are studies that have shown CRP and BMI to be associated to each other.Citation2,Citation4,Citation17 These findings were found in severe COPD patients with FEV1 < 40% in comparison to the current study population comprised mainly of moderate disease. Although, in one recent study by Karadag and colleaguesCitation37 moderate patients were grouped into low BMI and normal-to-high BMI and they showed that the low BMI patients had higher levels of CRP and TNF-α. Conversely, in our population of patients we did not observe significant weight loss in individual patients over one-year, with our mean values of BMI being >25 kg/mCitation2 at both visits. This perhaps could also account for the lack of correlation observed between change in BMI and inflammatory biomarkers.
TNF-α and IL-6 levels have been shown to be increased in weight losing COPD patientsCitation4,Citation9 However the present study does not support this finding. In the study by Eid and colleagues,Citation4 weight loss characterized by reduced fat free mass (FFM) and skeletal muscle mass was associated with increased systemic inflammatory response. They showed that TNF-α, sTNF-R, and IL-6 were inversely related to FFM in patients with normal BMI. Another study also showed that CRP was inversely related to FFM in hypermetabolic patients.Citation8 FFM appears to be a better marker of weight loss compared to BMI, particularly since FFM has been shown to be a predictor of mortality in the presence of normal BMI.Citation38 BMI has the advantage of being easy to measure but is a limited value as it does not represent changes in body composition or reflect skeletal muscle depletion.
The limitations of our study are: (a) we did not measure many other systemic biomarkers that may be of interest in COPD due to limited resources and therefore we decided to measure IL-6, CRP, and TNF-α because of their potential role in the pathopyhysiology of COPD; (b) we measured our samples only once at enrollment and at 12 months. It would have been of interest to have performed “serial sampling”; a recent studyCitation39 using 11 visits performed over a one-month interval to assess inflammatory biomarkers suggests that intra-patient variability is reduced with the use of a “rolling mean” of serial samples; (c) our population was composed of mainly moderate-to-severe disease, and the inclusion of more very severe patients would have been of interest especially to examine the repeatability by different GOLD severities; (d) we did not capture any clinical changes such as exacerbation within in the one-year interval excluding the four-week interval leading up to their study visit which may have influenced our results; (e) we were unable to perform analysis related to changes in therapeutic regime as we were limited by numbers of patients that had actually had a change in their medications such as stopping/starting inhaled corticosteroids (ICS) by the one-year visit. Although, it has recently been confirmed by Sin and colleaguesCitation40 and Kuniaski and colleaguesCitation20 that ICS use does not affect IL-6 and CRP levels measured; and (f) we did not relate our findings to other clinical parameters of COPD such as partial pressure of oxygen in arterial blood, exercise tolerance, and health status over an one-year interval.
In conclusion, the systemic inflammatory biomarkers IL-6, CRP, and TNF-α were moderately repeatable over a 12 month period in COPD patients. We have also shown that a robust and repeatable association between IL-6 and CRP exists. However, there was a lack of correlation of TNF-α to IL-6 or CRP, possibly due to the instability of TNF-α, or perhaps because TNF-α genuinely plays a different role to IL-6 and CRP in the pathophysiology of COPD.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)2007Accessed March 28, 2007 Available from http://www.goldcopd.com
- BroekhuizenRWoutersEFCreutzbergECScholsAMRaised CRP levels mark metabolic and functional impairment in advanced COPDThorax2006611172216055618
- YendeSWatererGWTolleyEAInflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjectsThorax2006611101616284220
- EidAAIonescuAANixonLSInflammatory response and body composition in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011648 Pt 11414141811704588
- AgustiAGSauledaJMirallesCSkeletal muscle apoptosis and weight loss in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2002166448548912186825
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- JorgensenNRSchwarzPHolmeIHenriksenBMPetersenLJBackerVThe prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional studyRespir Med2007101117718516677808
- ScholsAMBuurmanWAStaal van den BrekelAJDentenerMAWoutersEFEvidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary diseaseThorax19965188198248795671
- de GodoyIDonahoeMCalhounWJMancinoJRogersRMElevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patientsAm J Respir Crit Care Med199615326336378564110
- GanWQManSFSenthilselvanASinDDAssociation between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysisThorax200459757458015223864
- TakabatakeNNakamuraHAbeSCirculating leptin in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19991594 Pt 11215121910194168
- HigashimotoYYamagataYTayaSSystemic inflammation in chronic obstructive pulmonary disease and asthma: Similarities and differencesRespirology200813112813318197923
- DahlMVestboJLangePBojesenSETybjaerg-HansenANordestgaardBGC-reactive protein as a predictor of prognosis in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007175325025517053205
- ManSFConnettJEAnthonisenNRWiseRATashkinDPSinDDC-reactive protein and mortality in mild to moderate chronic obstructive pulmonary diseaseThorax2006611084985316738034
- PearsonTAMensahGAAlexanderRWMarkers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart AssociationCirculation2003107349951112551878
- RidkerPMRifaiNRoseLBuringJECookNRComparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular eventsN Engl J Med2002347201557156512432042
- Pinto-PlataVMMullerovaHTosoJFC-reactive protein in patients with COPD, control smokers and non-smokersThorax2006611232816143583
- KishimotoTThe biology of interleukin-6Blood19897411102473791
- GarrodRMarshallJBarleyEFredericksSHaganGThe relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD)Prim Care Respir J200716423624017665081
- KunisakiKMRiceKLJanoffENRectorTSNiewoehnerDEExhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: a prospective studyTher Adv Respir Dis200822556419124359
- TakabatakeNNakamuraHAbeSThe relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001614 Pt 11179118410764309
- Di FranciaMBarbierDMegeJLOrehekJTumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19941505 Pt 1145314557952575
- KaradagFKarulABCildagOYilmazMOzcanHBiomarkers of systemic inflammation in stable and exacerbation phases of COPDLung2008186640340918807087
- LeeTMLinMSChangNCUsefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatinAm J Cardiol2008101453053518312772
- BeehKMBeierJKornmannOManderABuhlRLong-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPDChest2003123377878312628878
- BalzanoGStefanelliFIorioCEosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway functionAm J Respir Crit Care Med19991605 Pt 11486149210556110
- BrightlingCEMonterioWGreenRHInduced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatabilityRespir Med20019512999100211778799
- BoorsmaMLutterRvan de PolMAOutTAJansenHMJonkersRERepeatability of inflammatory parameters in induced sputum of COPD patientsCOPD20074432132918027159
- PelemanRARytilaPHKipsJCJoosGFPauwelsRAThe cellular composition of induced sputum in chronic obstructive pulmonary diseaseEur Respir J199913483984310362050
- BlandJMAltmanDGStatistical methods for assessing agreement between two methods of clinical measurementLancet1986184763073102868172
- SevenoaksMJStockleyRAChronic Obstructive Pulmonary Disease, inflammation and co-morbidity – a common inflammatory phenotype?Respir Res200677016669999
- ReidMBLiYPTumor necrosis factor-alpha and muscle wasting: a cellular perspectiveRespir Res20012526927211686894
- BemelmansMHvan TitsLJBuurmanWATumor necrosis factor: function, release and clearanceCrit Rev Immunol19961611118809470
- Diez-RuizATilzGPZangerleRBaier-BitterlichGWachterHFuchsDSoluble receptors for tumour necrosis factor in clinical laboratory diagnosisEur J Haematol1995541187859870
- LandboCPrescottELangePVestboJAlmdalTPPrognostic value of nutritional status in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199916061856186110588597
- ScholsAMSlangenJVolovicsLWoutersEFWeight loss is a reversible factor in the prognosis of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981576 Pt 1179117979620907
- KaradagFKirdarSKarulABCeylanEThe value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary diseaseEur J Intern Med200819210410818249305
- VestboJPrescottEAlmdalTBody mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart StudyAm J Respir Crit Care Med20061731798316368793
- SapeyEBayleyDAhmadANewboldPSnellNStockleyRAInter-relationships between inflammatory markers in patients with stable COPD with bronchitis: intra-patient and inter-patient variabilityThorax200863649349918057097
- SinDDManSFMarciniukDDThe effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008177111207121418310480