Abstract
Objective
To determine the feasibility and efficacy of a six-month, cell phone-based exercise persistence intervention for patients with chronic obstructive pulmonary disease (COPD) following pulmonary rehabilitation.
Methods
Participants who completed a two-week run-in were randomly assigned to either MOBILE-Coached (n = 9) or MOBILE-Self-Monitored (n = 8). All participants met with a nurse to develop an individualized exercise plan, were issued a pedometer and exercise booklet, and instructed to continue to log their daily exercise and symptoms. MOBILE-Coached also received weekly reinforcement text messages on their cell phones; reports of worsening symptoms were automatically flagged for follow-up. Usability and satisfaction were assessed. Participants completed incremental cycle and six minute walk (6MW) tests, wore an activity monitor for 14 days, and reported their health-related quality of life (HRQL) at baseline, three, and six months.
Results
The sample had a mean age of 68 ±11 and forced expiratory volume in one second 18% predicted. Participants reported that logging their exercise and symptoms (FEV1) of 40 ± was easy and that keeping track of their exercise helped them remain active. There were no differences between groups over time in maximal workload, 6MW distance, or HRQL (p > 0.05); however, MOBILE-Self-Monitored increased total steps/day whereas MOBILE-Coached logged fewer steps over six months (p =0.04).
Conclusions
We showed that it is feasible to deliver a cell phone-based exercise persistence intervention to patients with COPD post-rehabilitation and that the addition of coaching appeared to be no better than self-monitoring. The latter finding needs to be interpreted with caution since this was a purely exploratory study.
Trial registration
ClinicalTrials.gov (NCT0373932).
Background
Exercise is a well established intervention to improve physical functioning, symptoms, and quality of life in patients with chronic obstructive pulmonary disease (COPD).Citation1 However, patients face multiple barriers to exercise persistence including episodic exacerbations of their illness, limited ongoing support, and low self-regulatory capacity.Citation2–Citation4 Supervised exercise training is a cornerstone of pulmonary rehabilitation (PR), a recommended multicomponent short-term intervention for patients with moderate to severe disease.Citation5 While notable improvements in functional outcomes are associated with rehabilitation, maintenance of independent exercise in the face of a progressive illness such as COPD is challenging. Numerous approaches of varying intensity and duration have been tested to help patients maintain functional gains achieved with PR including, extending the duration of rehabilitation,Citation6 booster rehabilitation,Citation7 or a combination of regular telephone follow-up contacts with clinic- or home-based supervised exercise sessions,Citation8,Citation9 support groups,Citation2 or a pedometer-based home walking program.Citation10 As a whole, these resource intensive models have yielded only modest long term effects compared to usual care. Despite these negative findings, the epidemiological evidence continues to argue for further refinement and testing of novel, targeted, cost-effective interventions to help patients at various stages of COPD maintain physically active lives.Citation11
The pervasive integration of information and communication technologies in everyday life has ushered in a new age of anytime–anywhere information access and exchange. As an example, nearly 90% of the US adult population used a cell phone for their communication needs in 2008.Citation12 Such widespread adoption of a simple innovation has opened up opportunities to cost-effectively scale up evidence-based interactive health promotion and disease management interventions.Citation13 Cell phones are personal to the individual; they are always on and always connected thus enabling new types of health and social interactions otherwise not previously possible. Several recently published studies showed that cell phones can, in the short term, deliver effective behavior change interventions in young to middle age healthy adultsCitation14–Citation17 as well as improve glucose and blood pressure control in patients with diabetes.Citation18–Citation20 Two studies, including our work on use of the Internet and cell phones to support dyspnea self-management, show that older adults with COPD are able and willing to use mobile devices for collaborative monitoring and communication to support self-care.Citation21,Citation22 Therefore, the purpose of this exploratory study was to determine the feasibility and efficacy of a first generation, cell phone-based exercise persistence intervention for patients with COPD following completion of PR.
Methods
Study design
We conducted a randomized, repeated measures (0, 3, and 6 months) exploratory study to determine the feasibility and efficacy of a six-month cell phone-mediated cognitive-behavioral exercise persistence intervention, MOBILE (Mobilizing Support for Long-term Exercise), for patients with COPD. Patient graduates from four PR programs who successfully completed a two-week run-in period were randomized to receive either the MOBILE-Coached (MOBILE-C) or the active control, MOBILE-Self-Monitored (MOBILE-SM) intervention. Since this was a feasibility study, we did not conduct an a priori power calculation;Citation23 however, we had a target sample of 20 participants based on a realistic projection of the available PR graduate pool who would be willing to participate in the study over the limited recruitment time frame. The study was approved by the institutional review board at the University of Washington and was registered with ClinicalTrials.gov (NCT0373932).
Recruitment
Due to privacy regulations, participants were initially approached by PR coordinators for their interest in the research study. The PR coordinators provided the study with names and contact information of patients who expressed an interest in learning more about the study. The study RA either contacted the prospective participant via phone or arranged to meet him or her at the PR site to explain the study and obtain consent. The goal was to approach interested participants during the final 2–3 weeks of their PR program in order to allow a two-week run-in period; however, due to scheduling challenges, some participants did not start their run-in until they completed PR.
Sample
Inclusion criteria were: a) stable COPD; b) pulmonary function results show moderate to severe disease according to GOLD criteria (forced expiratory volume in one second <70% and [FEV1]/forced vital capacity [FVC] FEV1 <80%); c) no plans to participate in a maintenance program; d) patients receiving supplemental oxygen were acceptable saturation was maintained at >88% on <6 L/min if their O2 of nasal oxygen during the six minute walk (6MW) test; e) age ≥ 40 years; f) ability to speak, read, and write English; and g) permission from health provider. Exclusion criteria were: a) active symptomatic illness (eg, cancer, heart failure, ischemic heart disease, neuromuscular disease, psychiatric illness); b) unable (eg, severe arthritis) or unwilling to use the study issued cell phone; and c) reside outside of the wireless coverage area.
Study procedures
Participants completed a two-week run-in period in order to determine their ability to adhere to the exercise and symptom self-monitoring protocol. They were trained on entering data via the cell phone (Treo 650 or 700™, Palm Inc., Sunnyvale, CA, USA), asked to provide a return demonstration, and were given a step-by-step help booklet with screenshots of the cell phone displays. The Treo™ model was chosen because at the time of software development in 2005, it was the most affordable device with the largest screen size and touch screen interface, features that patients from another study felt were important.Citation22 The study paid for the monthly data service plan. Participants who submitted at least 80% of exercise and symptom data during the run-in and chose to proceed with the study were scheduled for baseline testing (spirometry, incremental cycle, and 6MW tests, as well as questionnaires) and randomly allocated to MOBILE-C or MOBILE-SM. A biostatistician who was not involved in the day-to-day study operations generated the randomization sequence and placed the randomization in separate sealed opaque envelopes. The randomization scheme was stratified by gender to ensure balanced allocation. The interventionist was not blind to group assignment; however, the outcome assessments were performed by a research assistant who was blinded to this information. All participants met individually with the study interventionist (HQN) for a 30 to 45-minute baseline consultation as described below. Participants returned for testing three and six months later.
MOBILE interventions
Participants from both treatment groups met with HQN at baseline to collaboratively design a safe, simple, and effective independent exercise program that they were likely to sustain over time. The exercise program was individualized according to participants’ performance on the exercise tests, dyspnea at end of exercise, access to community-based exercise facilities, and preferred exercise mode. They were encouraged to accumulate up to a total of 150 minutes of moderate-intensity endurance exercise per week (3–5 sessions per week) per national physical activity guidelines and to continue with upper and lower body resistance exercises initiated during PR. The nurse also discussed signs and symptoms participants typically experienced with the onset of a COPD exacerbation, strategies for self-care, and how to adjust exercise as needed during these episodes. Participants were given a copy of a generic exacerbation action plan with their specific signs and symptoms listed and were encouraged to discuss and modify the action plan with their health provider. They were provided a booklet with exercise tips, local resources, and pictures of stretching and strengthening exercise as well as an Omron HJ-112 digital pedometer (Omron Healthcare, Bannockburn, IL, USA); pedometers are simple self-monitoring tools that provide immediate and objective feedback and, in turn, may provide behavioral reinforcement.Citation24 Both programs were grounded in behavior change theories and operationalized through the behavioral components of self-monitoring (MOBILE-SM) and motivational feedback and assistance with problem solving to develop self-regulatory capacity for exercise persistence (MOBILE-C).Citation25–Citation27
MOBILE-Coached (MOBILE-C)
There were two components to MOBILE-C: collaborative monitoring of symptoms and exercise and ongoing reinforcement feedback.
Collaborative monitoring of symptoms and exercise
Participants submitted daily information about their symptoms and exercise. An audio alarm was set on the cell phone calendar tool to remind participants to complete their entries at a time that was acceptable to them. The data were transmitted in real-time to a central server and the nurse was able to review these data for each participant or in aggregate form.
Participants used Likert scales to rate their overall health (excellent to poor) and respiratory symptoms (difficulty breathing [0: none to 4: severe], cough [0: none to 4: almost constant], and trouble with sputum [0: none to 4: severe]). Automatic alerts were sent to the nurse’s cell phone if participants responded having “marked” symptoms for two consecutive days. The nurse followed up via text messaging or telephone as necessary. Participants were encouraged to contact their health provider regarding the increasing symptoms, to follow their treatment plan, and to continue to log their data during the exacerbation episode.
Participants entered the following information about their exercise: mode (walking, biking, other endurance exercises, and upper and lower body strengthening exercises), duration (minutes and/or total daily steps), and worst dyspnea during the endurance exercise using a 0–10 modified Borg scale. If participants indicated they were not able to exercise, they were asked to select reasons from a list of common barriers (COPD flare-up, difficulty breathing, too tired, not motivated, depressed, too busy, sick, bad weather, bored with exercise, family responsibilities, on vacation, and pain/discomfort). Once the data were submitted and sent to the central server, participants received an instant text feedback summarizing the exercises they completed for that week.
Reinforcement feedback
Ongoing reinforcement feedback was provided via weekly short text messages to the participant’s cell phone, by the nurse, based on submitted exercise and symptom information, eg, “You’ve got it, getting more ‘bang’ for your buck is what the conditioning is all about. What a great feeling it must be to know you can do more!”, “I saw that you were quite busy this week and not able to get much walking as usual. Perhaps you could do three 10-minute walks until things slow down?” Participants confirmed receipt of these messages by replying with short text responses or less frequently, with several follow up text messages. Examples of participant messages include: “I think I mentioned I do more exercise when nagged so do nag!!”, “This program helps me a lot. I am able to stay focused. Again thanks”, and “Thank you for that, atta boy!”
Because of the small QWERTY keyboard and 160 character restriction for each message, participants were telephoned for situations where more extensive interactions was appropriate, eg, coaching on problem solving strategies to overcome reported barriers to exercise, assessing whether participants were experiencing an exacerbation and encouraging follow up with their health provider, or assistance with adjustments to exercise goals in response to changes in health status. Template text messages were also pre-programmed on the cell phone in an attempt to make it easier for participants to communicate via text messaging, eg, “I need to talk to you. Can you call me at home?”, “I think I’m having a COPD flare-up. I’ve called my doc”, “Will increase the duration of my walks this week by x”, “Just wanted to let you know I’m planning to take a vacation from exercise for a week”.
MOBILE-Self-Monitored (MOBILE-SM)
Participants continued to use the cell phone to enter information about their symptoms and exercise on a daily basis and were encouraged to call the research office if they had questions about their exercise or COPD over the course of the study. They were informed that self-monitoring helped others stay committed to their exercise program. A standard text message was sent to participants each week to thank and encourage them to continue to submit their data, eg, “Thank you for your continued efforts with the entries.”, “Thank you for your regular entries. Your effort is greatly appreciated.” Aside from the daily automatic calendar reminder to log their information, MOBILE-SM participants did not receive any other prompting or personalized feedback; the symptom alert was also disabled.
Measurements
Technical issues and usability
Participants were asked several open-ended questions by telephone, regarding technical difficulties within the first week of the run-in period. Technical difficulties such as missing or compromised data, battery failure, damage, malfunctioning or lost cell phones were tracked. Usability and acceptability were assessed at three and six months with a survey using a Likert type scale and a semi-structured exit interview at six months.
Beliefs and attitudes towards exercise and self-care
At baseline, participants completed one-item questions assessing their: 1) satisfaction with rehabilitation’s effects on their overall health, “Given the effort you’ve put into your pulmonary rehabilitation program, how satisfied are you with your overall health?” (0: not at all satisfied to 5: very satisfied); 2) expectations that persisting with exercise would help maintain their health, “If you were to continue to exercise on your own after the rehab program, how much do you expect that the exercise will help with your overall health?” (0: not at helpful to 5: very helpful); and 3) perceived importance, motivation, and confidence to continue their exercise, “How [important is it to you-, motivated are you-, confident are you-] to continue to exercise on your own as a way to manage your breathing?” (0: not at all to 10: totally). They also completed two validated questionnaires that measured the degree of autonomous self-regulation for exercise (15-items, Self-Regulatory Questionnaire-Exercise [SRQ-E])Citation28,Citation29 and activation for self-care (13-items, Patient Activation Measure [PAM]).Citation30,Citation31 Self-efficacy for overcoming barriers to exercise was measured using a 15-item Exercise Barriers Efficacy Scale;Citation32,Citation33 this scale was re-administered at three and six months.
Support for exercise
Support for exercise was measured with a 13-item Social Support and Exercise Survey.Citation34 This instrument has been used extensively in both healthy and clinical populations to assess enacted support from family and friends specific to exercise (eg, exercised with me, encouraged me to exercise).Citation35 Participants were also asked to rate their overall perceived support for exercise from people closest to them using a 1 to 5 point Likert scale.
Exercise performance
Exercise performance was assessed using the six-minute walk (6MW) and incremental cycle ergometer tests. Participants performed two 6MW tests according to ATS guidelines and the longer of the two tests was used for analysis.Citation36 The incremental cycle ergometer test protocol was similar to that used in the National Emphysema Treatment Trial.Citation37 Duration and peak workload measured in watts was recorded. Cardiorespiratory status was not measured since previous studies showed minimal to no changes in physiological parameters with moderate-intensity, independent exercise programs.Citation38,Citation39
Free-Living Ambulatory Physical Activity
Free-living ambulatory physical activity was measured using a pager-sized, lightweight, Stepwatch® 3 Activity Monitor (SAM; OrthoCare Innovations, Washington DC, USA) fastened above the right ankle. The SAM is a dual-axis accelerometer linked to a microprocessor sensor that directly and continuously records gait cycles (strides) based on acceleration, position, and timing information. Stride counts are doubled to represent steps. The SAM has been validated for use in healthy and chronically ill older adults in laboratory and community settings and has an accuracy of 98%–99%.Citation40,Citation41 Participants wore the SAM during waking hours for 14 days at baseline, three, and six months. The SAM was programmed to record in one-minute epochs; a valid day was defined as having 10 or more hours (600 min) of monitor wear. The device software was used to calculate total step counts, percent of time during waking hours without any steps (inactive), percent active time at moderate (31–80 steps/min) and high intensity activity (>80 steps/min), and peak performance (average steps/min of the best 30 minutes of the day).
Health related quality of life (HRQL)
Health-related quality of life (HRQL) was measured with two validated generic and disease-specific instruments, the Medical Outcomes Study Short-Form 36 (MOS-SF36)Citation42 and St. George’s Respiratory Questionnaire (SGRQ).Citation43,Citation44 The SF-36 produces two composite scales of physical and mental functioning with higher scores indicating better HRQL. The 76-item SGRQ measured the three domains of respiratory symptoms, activities, and impact, providing a total SGRQ score. Lower scores indicate better HRQL.
Statistical analysis
Independent Student’s t-tests or chi-squared tests were used to compare baseline characteristics between groups. General linear mixed models, a well-established class of linear models particularly suited for longitudinal repeated measures data that does not exclude individuals with missing data, were used to estimate the effect of treatment, adjusting for individual variation in the outcomes of exercise performance, physical activity, and HRQL. Significance was determined using Wald tests (p < 0.05). Since this was an exploratory study and not designed to be adequately powered for detecting differences between groups, we did not adjust the alpha levels for testing multiple outcome variables.
Results
Sample flow and baseline characteristics
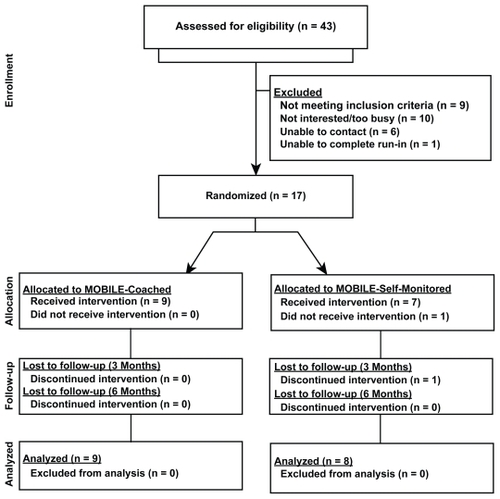
A total of 43 patients with COPD were referred to the research study from October 2006 to April 2008. Nine patients were deemed ineligible for the following reasons: not completing PR due to death or other reasons, other pulmonary diagnoses, non-English speaking, major surgery, medical complications immediately post-PR, or severe hearing impairment. Other patients expressed no interest in the study (n = 8), were too busy (n = 2), not reachable (n = 6), or withdrew after the first run-in week (n = 1), leaving a total of 17 participants who completed the two-week run-in for randomization to MOBILE-SM (n = 9) or MOBILE-C (n = 8) ().
The sample characteristics are described in . Age and disease severity were not comparable between the two groups, suggesting that randomization in this small sample was only marginally successful. Both groups were similarly satisfied with the positive effects of PR on their overall health and had high expectations that continued participation in exercise would maintain their health. Motivation and confidence to persist with exercise tended to be lower in MOBILE-SM. Overall activation for self-care and self-regulation for exercise were relatively high for both groups. While enacted support for exercise from family and friends were similarly low across all participants, the MOBILE-C group reported slightly higher overall perceived support for their exercise.
Table 1 Baseline sample characteristics
Process and mediating measures
Technical issues and usability
There were three separate instances where the cell phone fell out of the wireless network and participants were instructed via telephone to perform either a soft or hard reset with removal of the batteries without difficulties. Two devices had to be exchanged due to persistent unreliable connections to the network. Since 90% of the participants already had a cell phone for personal use, the study-issued cell phone was seldom carried by the participant. Forgetting to charge the device and not getting the audio reminder were primary reasons participants cited for forgetting to log their daily information. Participants in both groups found that it was easy to submit their data and that the time (approximately 1–2 min) to complete the task was acceptable (). They agreed that keeping track of their exercise helped them stay committed. Text messaging was not perceived favorably by all MOBILE-C participants. Three participants who found it difficult to navigate to the texting tool and use the small keyboard expressed a strong preference for communication via the telephone. One participant who text messaged the nurse most frequently stated that she developed her texting skills because she wanted to communicate with her grandchildren and actually came to appreciate this form of communication.
Table 2 Usability of study-issued cell phones and daily log entries
Exercise and symptom entries
Participants submitted a total of 2338 exercise and 2400 symptom entries (); there was a wide range in the number of entries across both groups. Overall, MOBILE-C participants were more “adherent” in submitting their exercise and symptom data compared to MOBILE-SM, 87% vs 66% over six months.
Validation of self-reported exercise
Weighted correlation between self-report of exercise (n =448 days) and total SAM step counts for the particular monitored day was modest (r =0.36, p =0.15) but trended in the right direction. The percentage of time engaged in moderate to high intensity physical activity was greater on days that participants reported exercising compared to days when they did not exercise.
Barriers to exercise and adverse events
Participants in both groups made a total of 811 entries reporting their barriers to exercise. The most frequent exercise barriers were being too busy (n = 300) or having family responsibilities (n = 119), followed by being too tired (n = 106). Four participants experienced a COPD exacerbation that required treatment with antibiotics or oral prednisone; two had a fall unrelated to exercise, that required surgery or immobilization; and one participant was listed for lung transplant and received her transplant three months into the study.
Self-efficacy for overcoming barriers to exercise
Self-efficacy was lower for the MOBILE-SM group compared to MOBILE-C at baseline (4.8 ± 1.6 vs. 7.1 ± 0.6) but increased at six months (6.0 ± 0.9) whereas the MOBILE-C group showed a decline (6.2 ± 0.6) (group × time interaction, p = 0.08)
Exploratory analysis of outcomes
Exercise performance
The MOBILE-SM group showed modest increases whereas the MOBILE-C group had declines in maximal workload on the incremental cycle ergometer and 6MW distance; these differences were not statistically significant (see ; p =0.29 and 0.12).
Table 3 Changes in exercise performance and physical activity at three and six months post-pulmonary rehabilitation
Free-living ambulatory physical activity
Trends in the four physical activity parameters (total steps/day, percentage time being inactive, percentage time active at moderate or high intensity, and peak performance) also tracked changes observed in the laboratory exercise performance tests (); MOBILE-SM participants increased their physical activity over six months whereas MOBILE-C showed a decline. There was a significant interaction of treatment group and time in three of the four physical activity outcomes. MOBILE-SM participants accrued more steps per day (p =0.04), presumably through greater participation in moderate to high intensity exercise (p =0.003), and had higher peak performance (p =0.002). The proportion of waking time that all participants were inactive did not significantly change over time.
Health-related quality of life (HRQL)
There were no between group differences for the SGRQ total score (p =0.15) or the SF-36 physical and mental composite scales (p =0.51 and p =0.38) ().
Table 4 Changes in health-related quality of life (HRQL) at three and six months post-pulmonary rehabilitation
Qualitative interviews
Irrespective of the treatment groups, two major themes emerged from the semi-structured exit interviews: 1) Participants’ desire to stay accountable; and 2) Participants’ motivation to establish an independent exercise routine after PR.
“I wanted to stay accountable since I knew that if I commit to something, I’d be more likely to do it. The cell phone kept me honest, I knew I had to fess up if I didn’t do my exercises–that kind of motivation really helps. The summary feedback of how much I’ve done for the week also helps me know if I’m slacking off and how much I still need to do.” “The study has made me more motivated than before to keep exercising even though I don’t like it. I know it makes me feel good so I have to keep going. Having to report what I do and getting feedback on how I’m doing makes me pay attention to what I’m doing”
“Participating in this program for an extra six months after rehab was really helpful because it gave me time to establish a routine for exercise. I now do the exercises for myself because I know it makes me feel good.”
Intervention time and costs
All participants had an initial 30–45 minute face-to-face meeting with a nurse (~$25, assuming an annual salary of $80K). The nurse spent up to five minutes per MOBILE-C participant per week reviewing submitted data and providing text reinforcement (5 mins × 24 weeks; two hours, ~$80). Most MOBILE-C participants received at least one or two 10-minute telephone calls over the course of six months (~$15). Approximately 1–2 minutes were spent on text messages per week to MOBILE-SM participants (1 min × 24 weeks; ~$20). We purchased a total of 10 Treo 650/700™ cell phones at $300/device; each phone was used by at least two participants; wireless data and text message service was $35/month per device. Software development cost was only $3,000 (30% FTE for an experienced programmer for two months) since we were able to modify an existing platform. The approximate total intervention costs were $655 and $580 for a MOBILE-C and MOBILE-SM participant, respectively. Nearly 90% of the intervention cost was due to the software, hardware, and wireless service; however, per person costs are expected to be far lower in the future since patients are likely to use their own cell phones, pooled data service negotiations are now possible with wireless providers, and incremental costs for additional users will be negligible.
Discussion
We showed that it is feasible to deliver a cell phone-based exercise persistence intervention to patients with COPD and that the addition of coaching by a nurse (MOBILE-C) did not appear to be better than ongoing self-monitoring (MOBILE-SM) for maintenance of functional outcomes six-months post-PR. In fact, MOBILE-SM showed significant improvements in physical activity over time compared to declines in MOBILE-C. To our knowledge, this is the first study to test the use of a first generation, cell phone-based intervention to encourage exercise persistence for patients with COPD after PR.
The primary assumption of this study was that exercise persistence is critical to maintenance of gains in or at least attenuation of expected decline in physical functioning for a chronic, progressive illness such as COPD. Short-term PR remains the recommended standard of care for patients with moderate to severe COPD. Unfortunately, the limited nature of PR as currently structured and reimbursed provides insufficient support for ongoing self-regulatory demands associated with long-term behavior change and efforts at developing durable exercise habits. The MOBILE interventions were designed as two competing models to extend the effects of PR by using what has now become a pervasive personal communication tool, the cell phone. MOBILE-SM was modeled as a low intensity, affordable active control intervention (individualized exercise plan, pedometer, and daily self-monitoring of symptoms and exercise) that could easily be scaled up. MOBILE-C included the same elements in addition to immediate feedback of exercise progress and more real-time, ongoing reinforcement and problem-solving assistance from a nurse, mirroring the intensity of previous exercise maintenance approaches.Citation8,Citation10
Based on behavior change theory and findings from previous studies, we hypothesized that MOBILE-C participants who received more intensive follow up and support would show a trend in better maintenance of functional outcomes; however, this was not the case. The statistically significant differences between groups in physical activity may have been spurious since this study was not sufficiently powered to detect group differences. Nonetheless, the consistent trend in the activity data suggests two possible explanations for the unexpected finding that MOBILE-SM was more effective compared to MOBILE-C. The differences in baseline characteristics, greater number of adverse events in MOBILE-C (two falls and three COPD exacerbations), and relatively small sample size may have created a situation where outliers adversely influenced the mean estimates. Also, MOBILE-C participants scored higher on all measured outcomes at baseline, thus regression to the mean offers another possible explanation.
Alternatively, the MOBILE-SM active control intervention was rather robust. Use of pedometers alone has been shown to increase total step counts on non-PR days for patients participating in PR.Citation45 Moreover, the act of recording pedometer steps which presumably increases the relevance and processing of this information resulted in higher step counts than if participants wore the pedometers without recording their steps as found in a recent study.Citation46 Since we did not measure ambulatory physical activity pre-PR, we could not determine if levels at six months for both groups were still above baseline.
Step counts vary substantially depending on disease severity. A recent cross-sectional study showed that patients with GOLD Stage IV disease accrued on average <3,000 steps/day compared to ~8,000 steps/day for GOLD I–II patients.Citation47,Citation48 deBlock and colleaguesCitation45 reported that patients with moderate to very severe COPD averaged 2,200 steps/day pre-PR and increased their step counts by +1,200 steps/day post-PR. In another study of a 12-week pedometer-based cognitive behavioral intervention in GOLD Stage I–III COPD patients, Hospes and colleaguesCitation49 found a difference of 2,152 steps/day between intervention (+785 steps/day) and control patients (−1,367 steps/day). Participants in this study registered on average 5,700 steps/day six months after PR which translates to approximately 2.5 miles of ambulatory activity per day. While lower than what is typically recommended for healthy adults (10,000 steps/day), this level of activity is likely well above pre-PR activity levels though this will need to be measured in future studies. A recent study of a six-month PR program found that significant increases in walking time above baseline (10 min/day; +20%) was observed only after six months of training, not earlier at three months. This increase was actually not due to the exchange of shorter periods of walking for longer periods; 83% of patients substituted lying time for more short bursts of walking time.Citation50 Additional work will need to examine whether extended supervised training or a program like MOBILE or a combination thereof is more cost-effective in helping patients with COPD sustain a physically active lifestyle.
The automated audio reminders, originally intended to prompt participants to submit information about their daily symptoms and exercise actually served as a potent prompt for exercise as evidenced by the qualitative findings. Participants in both groups often alluded to the sense of accountability as a key factor that sustained their commitment to persist with exercise, a common observation of other studies as well.Citation51 Similarly, the act of reporting their barriers to exercise in real-time may have activated certain internal cognitive or motivational processes and should be explored further as a possibly effective strategy to motivate certain subgroups of patients. In contrast to a previous study that identified chest infections (COPD exacerbations) as the most common barrier to exercise based on retrospective self report,Citation2 our study found that being “too busy” was the number one reason participants cited for not exercising. This difference in findings may be due to disease severity and seasonality; however, the stark differences between retrospective recall and real-time reporting merits additional study to truly understand the barriers to exercise in this population. Finally, the usability data and qualitative feedback suggested that text messaging had only modest utility for one third of the MOBILE-C participants; thus, the relative advantage of instant personalized communication with the nurse was not fully realized.
The small sample size and self-selected nature of the sample represent important limitations of this exploratory study. Participants who are referred by PR coordinators, are able to complete PR, and are able to complete the run-in period are already a highly selected, motivated group; it was however encouraging that we excluded prospective participants for reasons other than successful completion of the run-in. Recruitment of participants from four PR programs could have introduced heterogeneity in baseline characteristics; however, there is also within-center variability in response to PR. The quality of life instruments used in this study may not been responsive to measuring change. We were not able to maximize the full potential of the cell phone as an always on, connected device for real-time support since most participants did not carry the device on them due to the bulky form factor but also, because 90% of the participants already had their own cell phone. An important technical consideration for future studies of cell phone-based health interventions is the need to develop platform-independent applications that can easily load on devices that patients own. Aside from the costs of purchasing devices that quickly become obsolete, patients are far more likely to use health applications that reside on their own personal device. Finally, the most optimal model of integrated care combined with active elements of PR remains unclear and continues to be an ongoing area of research; thus the approach we took of “back loading” a technology mediated intervention post-PR is only one of many possibilities for integrating communication tools to support patients.
Conclusions
We found that patients with COPD were willing and able participate in a first generation cell phone-based exercise persistence intervention despite several technological challenges. To our surprise, the self-monitored group showed greater improvements in free-living physical activity compared to the coached group that received personalized reinforcement and support over six months but this finding should be interpreted with caution. Additional research is warranted on the use of mobile devices and other interactive communication technologies to support patients with COPD in their self-management efforts across the illness trajectory, not just in relation to maintaining functional gains immediately post-PR. Cell phones will only continue to evolve and are expected to be robust ubiquitous computing devices in the future. Second generation devices already integrate motion and physiological sensors, location-based tracking, persuasive audiovisual media, and applications that support the formation of organic social networks; these tools will no doubt transform and perhaps, enhance the construction of more effective and personalized behavioral interventions for health promotion and disease management in the near future. However, the primary methodological challenge for future research on any technology-mediated clinical or behavioral intervention is the rapid changes in the technologies themselves which could undermine both internal and external study validity.
Author contributions
Nguyen, Steele, and Benditt contributed to the concept and design of the study. Nguyen, Gill, and Wolpin acquired data and performed analysis and interpretation of data. Nguyen, Gill, Wolpin, Steele, and Benditt drafted the article and revised it critically for important intellectual content. Final approval of the version to be submitted was given by Nguyen, Gill, Wolpin, Steele, and Benditt. No author has any financial or personal relationships with other people or organizations that could inappropriately bias this work. The study sponsor played no role in the study design, data collection, analysis and interpretation of data, and writing of the manuscript. This study was supported in part by: R03 NR009361 and 1KL2RR025015-01; Omron Healthcare donated the pedometers.
References
- RiesALBauldoffGSCarlinBWPulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice GuidelinesChest20071315 Suppl4S42S17494825
- BrooksDKripBMangovski-AlzamoraSGoldsteinRSThe effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary diseaseEur Respir J2002201202912166571
- Donesky-CuencoDJansonSNeuhausJNeilandsTBCarrieri-KohlmanVAdherence to a home-walking prescription in patients with chronic obstructive pulmonary diseaseHeart Lung200736534836317845881
- HeppnerPSMorganCKaplanRMRiesALRegular walking and long-term maintenance of outcomes after pulmonary rehabilitationJ Cardiopulm Rehabil2006261445316617228
- Rodriguez-RoisinRGlobal Initiative for Chronic Obstructive Lung Disease (GOLD) writing groupGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2008Accessed July 30, 2009 Available from http://www.goldcopd.com/
- TroostersTGosselinkRDecramerMShort- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trialAm J Med2000109320721210974183
- FoglioKBianchiLAmbrosinoNIs it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled studyChest200111961696170411399693
- RiesALKaplanRMMyersRPrewittLMMaintenance after pulmonary rehabilitation in chronic lung disease: A randomized trialAm J Respir Crit Care Med2003167688088812505859
- GrosboisJMLamblinCLemaireBLong-term benefits of exercise maintenance after outpatient rehabilitation program in patients with chronic obstructive pulmonary diseaseJ Cardiopulm Rehabil199919421622510453428
- SteeleBGBelzaBCainKCA randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary diseaseArch Phys Med Rehabil200889340441218295615
- Garcia-AymerichJLangePBenetMSchnohrPAntóJMRegular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort studyThorax200661977277816738033
- Harris InteractiveCell Phone Usage Continues to Increase April 42008 Available from: http://www.harrisinteractive.com/harris_poll/index.asp?PID=890Accessed April 26, 2009
- PatrickKGriswoldWGRaabFIntilleSSHealth and the mobile phoneAm J Prev Med200835217718118550322
- HurlingRCattMBoniMDUsing internet and mobile phone technology to deliver an automated physical activity program: randomized controlled trialJ Med Internet Res200792e717478409
- PatrickKRaabFAdamsMAA text message-based intervention for weight loss: randomized controlled trialJ Med Internet Res2009111e119141433
- BrendryenHDrozdFKraftPA digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trialJ Med Internet Res2008105e51
- KingACAhnDKOliveiraBMAtienzaAACastroCMGardnerCDPromoting physical activity through hand-held computer technologyAm J Prev Med200834213814218201644
- KimHSSongMSTechnological intervention for obese patients with type 2 diabetesAppl Nurs Res2008212848918457747
- LoganAGMcIsaacWJTislerAMobile phone-based remote patient monitoring system for management of hypertension in diabetic patientsAm J Hypertens200720994294817765133
- KrishnaSBorenSABalasEAHealthcare via cell phones: a systematic reviewTelemed J E Health200915323124019382860
- LiuWTWangCHLinHCEfficacy of a cell phone-based exercise programme for COPDEur Respir J200832365165918508824
- NguyenHQDonesky-CuencoDWolpinSA randomized trial of an Internet-based versus a face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: A pilot studyJ Med Internet Res2008102e918417444
- KraemerHCMintzJNodaATinklenbergJYesavageJACaution regarding the use of pilot studies to guide power calculations for study proposalsArch Gen Psychiatry200663548448916651505
- BravataDMSmith-SpanglerCSundaramVUsing pedometers to increase physical activity and improve health: a systematic reviewJAMA2007298192296230418029834
- BanduraASocial foundations of thought and action A social cognitive theoryEnglewood Cliffs, NJPrentice Hall1986
- BanduraASelf-efficacy: the exercise of controlNew York, NYW.H. Freeman1997
- RothmanAJBaldwinASHertelAWVohsKBaumeisterLSelf-regulation and behavior change: Disentagling behavioral initaition and behavioral maintenanceHandbook of Self-RegulationNew York, NYGuildford Press2004
- RyanRMConnellJPPerceived locus of causality and internalization: examining reasons for acting in two domainsJ Pers Soc Psychol19895757497612810024
- LevesqueCSWilliamsGCElliotDPickeringMABodenhamerBFinleyPJValidating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviorsHealth Educ Res200722569170217138613
- HibbardJHMahoneyERStockardJTuslerMDevelopment and testing of a short form of the patient activation measureHealth Serv Res2005406 Pt 11918193016336556
- HibbardJHStockardJMahoneyERTuslerMDevelopment of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumersHealth Serv Res2004394 Pt 11005102615230939
- LarsonJCoveyMKapellaMBarriers to efficacy: COPD version [abstract]Am J Respir Crit Care Med2003167A673
- DuncanTEDuncanSCMcAuleyEThe role of domain and gender-specific provisions of social relations in adherence to a prescribed exercise regimenJ Sport Exerc Psychol1993152220231
- SallisJFGrossmanRMPinskiRBPattersonTLNaderPRThe development of scales to measure social support for diet and exercise behaviorsPrev Med1987168258363432232
- LittMDKleppingerAJudgeJOInitiation and maintenance of exercise behavior in older women: predictors from the social learning modelJ Behav Med2002251839711845560
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med200216611111712091180
- FishmanAMartinezFNaunheimKNational Emphysema Treatment Trial Research GroupA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- NormandinEAMcCuskerCConnorsMValeFGerardiDZuWallackRLAn evaluation of two approaches to exercise conditioning in pulmonary rehabilitationChest200212141085109111948036
- StulbargMSCarrieri-KohlmanVDemir-DevirenSExercise training improves outcomes of a dyspnea self-management programJ Cardiopulm Rehabil200222210912111984209
- CavanaughJTColemanKLGainesJMLaingLMoreyMCUsing step activity monitoring to characterize ambulatory activity in community-dwelling older adultsJ Am Geriatr Soc200755112012417233695
- GardnerAWMontgomeryPSScottKJAfaqABlevinsSMPatterns of ambulatory activity in subjects with and without intermittent claudicationJ Vasc Surg20074661208121417919876
- WareJEJrSherbourneCDThe MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selectionMed Care19923064734831593914
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory QuestionnaireAm Rev Respir Dis19921456132113271595997
- JonesPWQuirkFHBaveystockCMThe St George’s Respiratory QuestionnaireRespir Med199185Suppl B2531 discussion 33–371759018
- de BlokBMde GreefMHten HackenNHSprengerSRPostemaKWempeJBThe effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot studyPatient Educ Couns2006611485516455222
- ClemesSAParkerRAIncreasing our understanding of reactivity to pedometers in adultsMed Sci Sports Exerc200941367468019204581
- WatzHWaschkiBBoehmeCClaussenMMeyerTMagnussenHExtrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional studyAm J Respir Crit Care Med2008177774375118048807
- WatzHWaschkiBMeyerTMagnussenHPhysical activity in patients with COPDEur Respir J200933226227219010994
- HospesGBossenbroekLTen HackenNHvan HengelPde GreefMHEnhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling programPatient Educ Couns200975227427819036552
- PittaFTroostersTProbstVSLangerDDecramerMGosselinkRAre patients with COPD more active after pulmonary rehabilitation?Chest2008134227328018403667
- KingACFriedmanRMarcusBOngoing physical activity advice by humans versus computers: the Community Health Advice by Telephone (CHAT) trialHealth Psychol200726671872718020844