Abstract
Chronic obstructive pulmonary disease (COPD) is a leading cause of death and disability worldwide. The Global Burden of Disease study has concluded that COPD will become the third leading cause of death worldwide by 2020, and will increase its ranking of disability-adjusted life years lost from 12th to 5th. Acute exacerbations of COPD (AECOPD) are associated with impaired quality of life and pulmonary function. More frequent or severe AECOPDs have been associated with especially markedly impaired quality of life and a greater longitudinal loss of pulmonary function. COPD and AECOPDs are characterized by an augmented inflammatory response. Macrolide antibiotics are macrocyclical lactones that provide adequate coverage for the most frequently identified pathogens in AECOPD and have been generally included in published guidelines for AECOPD management. In addition, they exert broad-ranging, immunomodulatory effects both in vitro and in vivo, as well as diverse actions that suppress microbial virulence factors. Macrolide antibiotics have been used to successfully treat a number of chronic, inflammatory lung disorders including diffuse panbronchiolitis, asthma, noncystic fibrosis associated bronchiectasis, and cystic fibrosis. Data in COPD patients have been limited and contradictory but the majority hint to a potential clinical and biological effect. Additional, prospective, controlled data are required to define any potential treatment effect, the nature of this effect, and the role of bronchiectasis, baseline colonization, and other cormorbidities.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) affects between 12 and 24 million people in the United States, where it is the fourth leading cause of death, accounting for over 106,000 deaths in 1996. Worldwide, COPD is the sixth leading cause of death (CitationPetty 2000; CitationWard et al 2000; CitationHalbert et al 2003; CitationHalpern et al 2003; CitationWouters 2003) and is the only condition in the top 10 causes of death with an increasing prevalence and mortality (CitationPauwels et al 2001; CitationMannino et al 2002; CitationPetty 2000; CitationStoller 2002). The Global Burden of Disease study undertaken by the World Bank and the World Health Organization concluded that COPD will become the third leading cause of death worldwide by 2020, and its ranking relative for number of disability-adjusted life-years lost will increase from 12th to 5th (CitationGulsvik 2001). Currently, oxygen therapy for hypoxemic patients and cigarette-smoking cessation are the only interventions known to alter the natural history of COPD.
Although an exact definition of acute exacerbations of COPD (AECOPD) remains controversial (CitationPauwels et al 2004), a generally accepted definition is that of ‘a sustained worsening of the patient’s condition, from the stable state and beyond normal day-today variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD’ (CitationRodrigues-Roisin 2000). Acute exacerbations of COPD (AECOPD) account for about 13 million office visits each year in the US (CitationNiederman et al 1999; CitationSethi 1999; CitationGonzales et al 2001), and account for 31%–68% of the total cost of COPD in the US and Europe (CitationWard et al 2000; CitationMcGuire et al 2001; CitationStrassels et al 2001; CitationAndersson et al 2002; CitationMiravitlles et al 2002). In 1996, acute exacerbations of COPD resulted in the sixth highest use of hospital bed days/yr in the US (176 million) and the sixth highest number of days lost from work (57.5 million) (CitationDruss et al 2002). As such, AECOPDs are a major source of health-care expenditure (CitationHalpern et al 2003). This cost is particularly evident in those AECOPDs that require hospitalization (CitationMiravitlles et al 2002; CitationOostenbrink and Ruttenvan Molken 2004).
The negative implications of AECOPDs have been highlighted by numerous investigators. A review of eighteen studies confirmed that AECOPD worsen health-related quality of life (HRQL) (CitationSchmier et al 2005). A two-year longitudinal study noted that more frequent exacerbations had a deleterious effect on health status in patients with moderate disease (forced expiratory volume in one second [FEV1] 35%–50% predicted) (CitationMiravitlles et al 2004). The greatest improvement in HRQL occurs during the first four weeks after a single episode, although continued improvement occurs over 26 weeks. Conversely, recurrence of AECOPD markedly attenuates improvement (CitationSpencer et al 2003).
AECOPDs also result in measurable, albeit modest, acute effects on pulmonary function (CitationSeemungal et al 2000). A modest improvement in pulmonary function, particularly in lung volume, has been reported over the first several weeks after therapy is initiated (CitationParker et al 2005; CitationStevenson et al 2005) Repeated AECOPDs are associated with loss of pulmonary function; the decrement in FEV1 has ranged from 7–8 mL/yr for patients with more frequent episodes (CitationDonaldson et al 2002; CitationKanner et al 2001).
Thus, AECOPD are associated with very significant healthcare expenditures, deterioration in HRQL that may be sustained, and appreciable deterioration in pulmonary function. Accordingly, preventing AECOPDs, or reducing their severity, should have favorable clinical, physiological and economical effects in COPD patients.
COPD is an inflammatory disease
Over the past several years, numerous studies have confirmed the important role of inflammation in the airways and lung parenchyma of COPD (CitationHill et al 1999; CitationSethi 2000; CitationAaron et al 2001; CitationGompertz et al 2001; CitationWedzicha 2002; CitationPietila and Thomas 2003; CitationWhite et al 2003). Many have advocated a central pathogenic role of this inflammatory response (CitationHogg et al 2004; CitationShapiro and Ingenito 2005; CitationTraves and Donnelly 2005; CitationWouters 2005). This inflammatory response contributes to the increased oxidative stress noted during an AECOPD (CitationTsoumakidou et al 2005). Both neutrophilic and eosinophilic inflammation have been described, with a multitude of inflammatory mediators implicated including interleukin-8 (IL-8), leukotriene B4 (LTB4), tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony stimulating factor (GMCSF), regulated upon activation: normal T cell expressed/secreted (RANTES), and endothelin-1 (ET-1) (CitationHill et al 1999; CitationSethi 2000; CitationGompertz et al 2001; CitationRoland et al 2001; CitationWhite et al 2003). One group followed 68 patients with COPD and an emphysematous phenotype for 2–3 years, with 30 developing an AECOPD associated with expectorated sputum adequate for analysis (CitationFujimoto et al 2005). During an AECOPD, total sputum cells, lymphocytes, neutrophils, eosinophils, IL-8, neutrophil elastase, eosinophilic cationic protein (ECP), and RANTES increased compared to the stable state. Prospective studies using bronchoscopic techniques have confirmed increased expression of RANTES in both the surface epithelium and subepithelial lymphomononuclear cells, increased numbers of neutrophils, as well as increases in CXCL5 (ENA-87), CXCL8 (IL-8), and CXCR2 during an AECOPD (CitationZhu et al 2001; CitationQiu et al 2003; CitationDrost et al 2005). Collectively, these data confirm a local, inflammatory process and increased oxidant stress status during exacerbations. Novel pathways promulgating this inflammatory response have recently been defined (CitationIto et al 2005).
As COPD progresses, the lungs are infiltrated by activated macrophages (CitationRutgers et al 2000; CitationAmin et al 2003; CitationCaramori and Adcock 2003) and lymphocytes (CitationSaetta et al 1993; CitationO’Shaughnessy et al 1997; CitationHogg et al 2004). Macrophages and lymphocytes, are not only increased in the airways and alveoli in COPD (CitationDi Stefano et al 2004; CitationShapiro and Ingenito 2005), but the prevalence of these two cell types, rather than of neutrophils as previously suspected, correlates with the severity of airflow obstruction (CitationDi Stefano et al 1996, Citation2001; CitationO’Shaughnessy et al 1997; CitationKemeny et al 1999; CitationHill et al 2000; CitationTurato et al 2002; CitationHogg et al 2004) and emphysema (CitationFinkelstein et al 1995; CitationRussell, Culpitt, et al 2002; CitationRussell, Thorley, et al 2002).
Macrophages are key cells of the innate immune system, secreting cytokines and chemokines when stimulated by pathogen-associated molecular patterns (PAMPs). Many of the lung lymphocytes are type 1 cytokine-producing CD8 T cells (CitationSaetta et al 1998, Citation1999, Citation2002; CitationMajo et al 2001; CitationGrumelli et al 2004). The CD8+ T cells predominance has been most evident in studies that used chronic bronchitis as an entry criteria (CitationSaetta et al 1997), and is less evident when study design selected against chronic bronchitis (CitationHogg et al 2004). Although macrophages and CD8+ T cells could synergistically contribute to progressive lung destruction in COPD in several ways (CitationBarnes et al 2003), the exact mechanism remains unproven. The systemic nature of COPD and AECOPDs have been recently documented (CitationAgusti 2005). Plasma fibrinogen, IL-6, C-reactive protein, and endothelin-1 concentrations increase during AECOPDs (CitationDev et al 1998; CitationWedzicha 2000; CitationRoland et al 2001; CitationHurst et al 2006).
Lung infections increase the inflammation of COPD
Various infectious and noninfectious stimuli can stimulate the inflammatory response associated with an AECOPD. Environmental pollutants, including particulate matter and nonparticulate gases can provoke an inflammatory response in-vitro and in in-vivo (CitationDevalia et al 1994; CitationOhtoshi et al 1998; CitationRudell et al 1999). Epidemiological studies have suggested increased respiratory symptoms and mortality during times of increased air pollution (CitationSunyer et al 1993; CitationGarcia-Aymerich et al 2000; CitationSunyer et al 2000). Despite these data, a large proportion of AECOPDs are felt to reflect infection, whether bacterial or viral (CitationSethi 2004). Viral infections are a well recognized cause of AECOPD (CitationWilkinson et al 2004; CitationJohnston 2005). The most frequently reported agents include rhinovirus, coronavirus, influenza, parainfluenza, adenovirus, respiratory syncytial virus (RSV), and human metapneumovirus (CitationStott et al 1968; CitationGump et al 1976; CitationSeemungal et al 2001; CitationGreenberg 2002; CitationRhode et al 2003; CitationTan et al 2003; CitationPletz et al 2004; CitationWedzicha 2004; CitationHamelin et al 2005).
Atypical infectious agents, including Chalmydia pneumoniae, Mycoplasma pneumoniae, and Legionella species, have been reported to cause AECOPD, although the majority of the data involve C. pneumoniae (CitationBeaty et al 1991; CitationBlasi et al 1993; CitationMogulkoc et al 1999; CitationKarnak et al 2001; CitationLieberman et al 2001; CitationSeemungal et al 2002). The etiologic role of bacteria in individual AECOPD episodes has been a subject of much controversy (CitationHirschmann 2000; CitationMurphy et al 2000). Recent comprehensive reviews have highlighted the evolution of these concepts (CitationSethi and Murphy 2001; CitationSethi 2004). Sputum cultures have been the classic methodological approach to identifying potentially pathogenic bacteria in AECOPD, with the most frequently isolated organisms being nontypeable Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Other, less frequently identified organisms include Haemophilus parainfluenzae, Staphylococcus aureus, Pseudomonas aeruginosa and other Gram-negative rods. Pseudomonas spp. or other enteric Gram-negative rods have been identified in the sputum of patients with greater airflow obstruction (CitationEller et al 1998; CitationMiravitlles et al 1999) and prior use of antimicrobial agents (CitationMonso et al 2003).
Bronchoscopic studies using bronchoalveolar lavage or protected specimen brush have confirmed that potentially pathogenic bacteria are identified in many COPD patients at baseline and during AECOPDs (CitationFagon et al 1990; CitationMonso et al 1995; CitationPela et al 1998; CitationSoler et al 1998). An analysis of pooled data from six published studies confirmed a high frequency of colonization in stable COPD patients in contrast with healthy subjects; a clear shift to higher potentially pathogenic organism load was noted during an AECOPD (CitationRosell et al 2005). Importantly, several groups have demonstrated that bacterial colonization, as defined by sputum cultures, is associated with a greater sputum and systemic inflammatory response, more frequent clinical AECOPD episodes and greater symptoms and worse health status (CitationSoler et al 1999; CitationBresser et al 2000; CitationHill et al 2000; CitationPatel et al 2002; CitationBanerjee et al 2004).
Novel methodological approaches have provided even more compelling data implicating bacterial pathogens in AECOPD. The longitudinal use of sputum culture and molecular typing of bacterial pathogens isolated from sputum has demonstrated that acquisition of a bacterial strain with which the patient had not been previously infected is associated with a greater than twofold increase in the risk of an exacerbation (CitationSethi et al 2002). Although the identification of a new strain of nontypeable H. influenzae was not associated with a symptomatic exacerbation in the majority of patients, collaborative work has highlighted inherent differences in new H. influenzae strains associated with symptomatic exacerbations which lead to greater neutrophil recruitment, adherence to epithelial cells and greater IL-8 release from epithelial cell cultures compared to those not associated with such a clinical response (CitationChin et al 2005). A similar approach has confirmed the same increased risk of AECOPD for M. catarrhalis (CitationMurphy et al 2005).
An immune response to one or more microbial pathogens has been utilized recently to define further the role of bacteria in individual AECOPD episodes. This methodological approach has been most widely reported for H. influenzae and M. catarrhalis (CitationMusher et al 1983; CitationYi et al 1997; CitationBakri et al 2002; CitationSethi 2004; CitationMurphy et al 2005), and to examine the interaction between bacterial and viral infection (CitationBandi et al 2003). In one such study, 16/35 exacerbations (46%) were associated with evidence of acute viral infection, whereas 11 (31%) were associated with the development of new serum IgG to homologous H. influenzae isolates; evidence of a viral infection was identified in 24/35 exacerbations (79%) (CitationBandi et al 2003). These data show that viral and bacterial infections can co-exist during symptomatic AECOPD, although antecedent viral infection is not required for H. influenzae- associated exacerbations.
Macrolides in COPD
Treatment of AECOPD
Given compelling data that bacterial infection is likely etiologic in approximately 50% of AECOPDs, it is not surprising that antimicrobial therapy has been intensively studied in this disease. Numerous placebo-controlled trials of antibiotics in AECOPDs have been published, with systematic reviews suggesting a modest treatment effect favoring antimicrobial therapy (CitationBach et al 2001; CitationMcCrory et al 2001; CitationSaint et al 2001). Saint and colleagues (2005) examined nine placebo controlled trials and concluded that there was a small but statistically significant improvement with antibiotic therapy, which was especially significant in patients with low baseline flow rates. The American College of Chest Physicians, American College of Physicians, and the American Society for Internal Medicine systematically analyzed 11 randomized, placebo-controlled studies of antibiotic treatment and also concluded that antibiotics are beneficial (CitationBach et al 2001; CitationMcCrory et al 2001; CitationSnow et al 2001). Additional studies have been published since these systematic reviews were published. CitationAllegra and colleagues (2001) noted that overall clinical response rate was improved with amoxicillin-clavulanate versus placebo in a multi-center trial in well-defined AECOPD; response clearly varied by the severity of baseline airflow obstruction, with the greatest difference between placebo and antimicrobial agent noted in patients with the lowest FEV1. Nouria and colleagues (2001) demonstrated dramatic improvement with ofloxacin therapy compared with placebo in a group of patients with severe COPD admitted with respiratory failure.
Based on data such as these, most recent international guidelines have incorporated recommendations as to which AECOPD patient is more likely to have bacterial infection likely to benefit from an antimicrobial agent (CitationBlasi et al 2006). Patients with at least two cardinal symptoms of an AECOPD (increase in dyspnea, increase in sputum production, and/or change in sputum color) experience a benefit with antibiotic therapy (CitationAnthonisen et al 1987). An exacerbation associated with sputum purulence may also be more likely to benefit from antibiotic treatment (CitationStockley et al 2000; CitationWilson 2005). Increasing sputum purulence has been clearly associated with bacterial growth in sputum samples (CitationAllegra et al 2005; CitationBlasi et al 2006). Measurement of serum procalcitonin levels have recently been suggested to define AECOPD patients with a higher likelihood of bacterial infection (CitationReichenberger et al 2001; CitationChrist-Crain et al 2004). Hence, the available data suggest that antimicrobial therapy provides benefit in carefully selected patients with more severe disease and more severe exacerbations.
Numerous controlled trials have compared newer agents with established ones. These studies have generally been designed as noninferiority trials for registrational purposes and therefore provide limited comparative information regarding clinical response for various antimicrobial classes (CitationMiravitlles and Torres 2004; CitationSethi 2004; CitationWilson 2005). Not surprisingly, clinical response in these trials is generally similar among different antimicrobial classes. However, more recent trials using novel experimental designs have suggested interesting differences between different antimicrobial classes. For example, in defining ‘bacterial eradication’ by sputum culture, CitationWhite and colleagues (2003) noted that patients with persistent bacteria after a ten day course of antimicrobial therapy exhibited elevated sputum inflammatory markers. In addition, some investigators have suggested that a more rapid resolution of symptoms may be seen with quinolones in contrast to comparator agents (CitationMiravitlles et al 2004; CitationMartinez et al 2005). These data suggest that eradication of bacterial pathogens may be associated with important clinical outcomes.
The most provocative data have revolved around the finding that some agents may lengthen the disease-free interval (DFI), ie, the time between exacerbations (CitationAnzueto et al 1999; CitationSaint et al 2001; CitationChodosh 2005; CitationWilson 2005). Because antimicrobial therapy that completely eradicates bacteria is associated with reduced inflammation (CitationWhite et al 2003), such agents should result in greater symptomatic resolution and better long-term clinical outcomes (CitationAnzueto et al 1999; CitationChodosh 2005; CitationWilson 2005). Based on these considerations, AECOPD clinical trials have increasingly, incorporated an assessment of how different antibiotics affect the DFI (CitationMartinez et al 2005). An early study demonstrated equivalence between antibiotics in short-term clinical response, although post-hoc analysis suggested that failure to clear the organism from the sputum post-therapy was associated with a shorter DFI (CitationChodosh et al 1998). CitationWilson and colleagues (2002) noted fewer recurrences of AECOPD with gemifloxacin (29%) compared to clarithromycin (41.5%, P = 0.016). The MOSAIC study investigators contrasted moxifloxacin with various comparators (cefuroxime, clarithromycin or amoxicillin) (CitationWilson et al 2004). Although all antibiotics resulted in equivalent short-term clinical response, moxifloxacin-treated patients required fewer additional antibiotics and experienced a longer DFI. In contrast, CitationLode and colleagues (2004) did not demonstrate a difference between levofloxacin and clarithromycin with respect to the DFI, although the patient population was a bit less ill. Thus, the effect of antibiotic class on DFI remains a crucial endpoint for future studies of AECOPD.
In general, macrolides have provide adequate coverage for the most frequently identified pathogens in AECOPD (CitationMartinez 2004), although differences among the compounds in H. influenzae activity have been evident. For example, azithromycin appears to have improved bacteriologic and clinical activity relative to clarithromycin (CitationMartinez et al 2005). The role of the macrolides in AECOPD therapy remains controversial although they have been generally included in published guidelines (CitationBalter et al 2003; Pauwels et al; CitationMartinez 2004; CitationWoodhead et al 2005; CitationBlasi et al 2006). These guidelines usually advocate stratification of patients by likelihood of clinical failure, with macrolide therapy suggested for patients with milder underlying disease, a low likelihood of infection with organisms which are not covered with standard antibiotic regimens (eg, P. aeruginosa, drug-resistant bacteria), or host factors that predict treatment failure.
Macrolides as immunomodulatory agents
Macrolides are macrocyclical lactones consisting of greater than 8-membered rings. This very large class (>2000 compounds) comprises both natural substances isolated from fungi and other organisms, as well as synthetic molecules of a similar structure (CitationJain and Danziger 2004). The most commonly, clinically used agents are semi-synthetic 14-, 15-, or 16-membered ring antibiotics related to erythromycin (). These include erythromycin, roxithromycin and clarithromycin as typical members of the 14-member class, and azithromycin as the prototypical 15-member compound (CitationLabro 2004). Macrolide antibiotics bind to 50S ribosomes of both prokaryotes and eukaryotes, inhibiting transpeptidation or translocation of nascent peptides. Macrolides have good bioavailability by the oral route, superb tissue penetration, favorable side-effect profiles, and prolonged tissue persistence. For these reasons, and their broad efficacy against Gram-positive, some Gram-negative, mycobacterial, chlamydial, mycoplasmal, and Legionella species, macrolides are well established in the therapy of respiratory infections.
Figure 1 Molecular structure of 14 member macrolides (erythromycin, A), fifteen member compounds (azithromycin, B) and a 16 member compound (C) (CitationJaffe and Bush 2001).
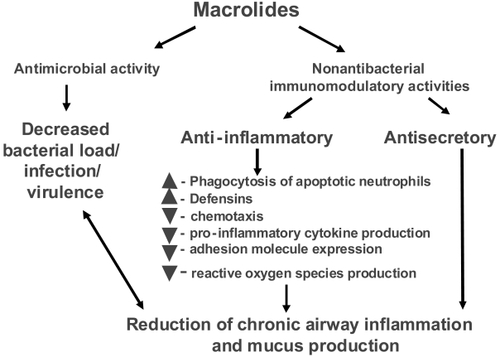
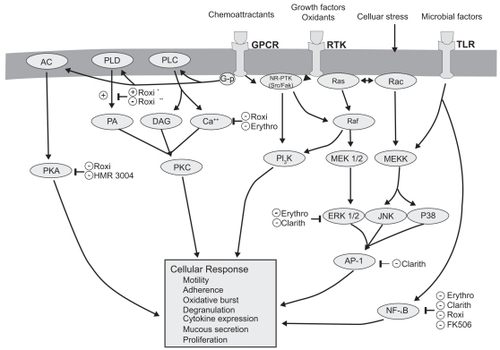
Macrolides exert broad-ranging immunomodulatory effects on mammalian cells in vitro and in vivo, as comprehensively reviewed by others and briefly summarized in Table 1 and illustrated in (CitationZalewska-Kaszubska and Gorska 2001; CitationLabro 2004; CitationTsai and Standiford 2004). Among their many activities, macrolides have been shown to exert effects on a wide range of cells including nasal and bronchial epithelial cells, alveolar macrophages, monocytes, eosinophils, neutrophils and lymphocytes. The effect on signaling pathways including NF-κB and AP-1 (CitationDesaki et al 2000, Citation2004, CitationKikuchi et al 2002) is evident in . Macrolides exert a host of effects that collectively limit tissue damage by neutrophils. In addition to effects on chemoattractants, these include inhibiting their oxidant burst, impairing degranulation, and increasing the rate of neutrophil apoptosis. There is also evidence that macrolides decrease mucus viscosity (CitationTamaoki et al 1995), and suppress angiogenesis (CitationYasunami and Hayashi 2001). Importantly, all these immunomodulatory effects are evident at concentrations attainable clinically by low-dose administration, and are not seen using 16-member macrolides. Most immunomodulatory effects are shared by the 14- and 15-member agents, although in some cases the effects vary among the individual compounds. For example, roxithromycin appears to exhibit consistent effects in vitro and in vivo (CitationAgen et al 1993; CitationScaglione and Rossoni 1998). Similarly, clarithromycin and azithromycin induce apoptosis of peripheral blood lymphocytes to a qualitatively greater extent than josamycin (CitationIshimatsu et al 2004). Hence, macrolides have many properties that could mitigate the neutrophilic inflammation central to airway damage, and might also improve aspects of airflow obstruction.
Figure 2 Molecular targets of macrolides (CitationTsai and Standiford 2004).
Macrolides also exert diverse actions that suppress microbial virulence factors (CitationShryock et al 1998; CitationPechere 2001; CitationWozniak and Keyser 2004). Although P. aeruginosa possesses high innate resistance in vitro (MIC ≥ 128 μg/ml), macrolides accumulate within the microbes over time, and suppress elaboration of elastase, lecithinase, and pyocyanin. Macrolides also alter the structure of LPS and outer membrane proteins, with a net effect of decreasing microbial adhesion to host cells. By in hibiting alginate production, macrolides destroy pseudomonal biofilm, facilitating killing by other antibiotics. Finally, sub-inhibitory concentrations of macrolides impede microbial motility of both P. mirabilis and P. aeruginosa by inhibiting flagellin synthesis. Importantly, these activities are also common to 14- and 15-ring macrolides, and have not seen using rokitamycin, a 16-member ring macrolide that has potent bactericidal activity against susceptible organisms. Furthermore, differences among individual agents have been reported with azithromycin exhibiting the greatest effect on quorum sensing (CitationMolinari et al 1993). Given these immunomodulatory properties and unique effects on bacterial pathogens macrolide antimicrobial agents have the potential to serve a unique role in the management of chronic inflammatory lung disorders, including COPD.
Macrolides in non-COPD chronic pulmonary inflammatory disorders
Macrolides have increasingly been used for their immunomodulatory properties in a wide range of chronic disorders. These will not be discussed in detail but results in chronic, inflammatory pulmonary disorders will be highlighted with a goal of presenting the rationale for their potential use in COPD.
Diffuse panbronchiolitis
Diffuse panbronchiolitis (DPB) is a chronic inflammatory disorder of the respiratory bronchioles and adjacent centrilobular regions. It is seen mainly, but not exclusively, in Japan, and presents with chronic cough, exertional dyspnea, and reticulonodular infiltrates on chest radiography (CitationKoyama and Geddes 1997). Chronic sinusitis is almost always noted. In fact, in geographical locations where this disease is found, the presence of airflow obstruction and chronic sinusitis in a nonsmoker should immediately raise suspicion of DPB. The appearance on high resolution computed tomography of the chest is characteristic (CitationAkira et al 1988; CitationNishimura et al 1992). Early in the disease course, H. influenzae and S. pneumoniae are cultured from respiratory secretions, while in many cases P. aeruginosa is noted in advanced stages (CitationKadota et al 2003).
The majority of investigators have confirmed a marked increase in neutrophils in BAL fluid of DPB patients (CitationIchikawa et al 1993; CitationMukae et al 1995; CitationKadota et al 2001; CitationHiratsuka et al 2003). The percentage of neutrophils appears to be higher in DPB patients with P. aeruginosa infection (CitationOishi et al 1994). Not unexpectedly, neutrophil chemotactic activity is markedly increased in DPB patients compared to normal, healthy volunteers (CitationKadota et al 1993; CitationOda et al 1994). Similarly, DPB patients have high concentration of IL-8 in BAL fluid (CitationSakito et al 1996), particularly in those infected with P. aeruginosa (CitationOishi et al 1994). Importantly, the histologic pattern of DPB includes bronchiolar wall thickening with an infiltration of lymphocytes, plasma cells, and histiocytes (CitationHomma et al 1983). Some investigative groups have noted that DPB patients have a higher number of lymphocytes and a reduced CD4/CD8 ratio compared to patients with idiopathic bronchiectasis and healthy subjects (CitationMukae et al 1995). β-defensins have noted to be elevated in the BAL fluid of DPB patients, with the plasma concentration of β-defensin 2 (HBD-2) correlating with the concentration in BAL fluid (CitationHiratsuka et al 2003). All these findings support a central role for inflammation in the pathogenesis of DPB, and suggest that Pseudomonas involvement is at least associated with more severe involvement, and may contribute to an accelerated deterioration.
Prior to the onset of chronic macrolide therapy, the prognosis of DPB was dismal, with a 10 year survival of approximately 75% in cases without P. aeruginosa infection but less than 22% with P. aeruginosa infection (CitationKoyama and Geddes 1997; CitationKudoh et al 1998). In the mid-1980’s, Kudoh and colleagues first noted that erythromycin therapy improved outcome in DPB (CitationKudoh et al 1984; CitationKudoh et al 1987). Subsequent case series (CitationKoyama and Geddes 1997) and a randomized, placebo-controlled trial confirmed this beneficial effect (CitationYamamoto 1991). Importantly, this favorable effect was independent of the presence of P. aeruginosa infection (CitationSawaki et al 1986; CitationNagai et al 1991) or the presence of chronic respiratory failure (CitationOhno et al 1993). In addition to erythromycin, beneficial results have been confirmed with clarithromycin (CitationTakeda et al 1989), roxithromycin (CitationAshitani et al 1992; CitationNakamura et al 1999), and azithromycin (CitationKobayashi et al 1995).
Increasing mechanistic insight on the clinical effects of macrolide therapy in DPB has become available. Improved symptoms, pulmonary function and arterial blood gases have been noted regardless of the presence of Pseudomonas infection, although there appears to be less marked improvement in pulmonary function in patients in whom bacteria persist after macrolide therapy, compared to those in whom sputum cultures become negative (CitationFujii et al 1995). As such, the effect of macrolides is unlikely to exclusively reflect an antibacterial effect, as clinical improvement may be seen despite lack of change in bacterial isolates or number in at least 50% of treated patients (CitationKadota et al 2003; CitationNakamura et al 1999).
Numerous investigators have demonstrated favorable effects of macrolide therapy on the inflammatory process in DPB. It is unlikely that the effect is purely a result of the antibacterial activity of the macrolide, because the maximal serum and sputum levels of erythromycin have been documented to be below the MICs of the clinically identified isolates (H. influenzae and P. aeruginosa) (CitationNagai et al 1991). Supporting an anti-inflammatory effect, clinical treatment with a macrolide has been shown to decrease neutrophils (CitationIchikawa et al 1993; CitationKadota et al 1993; CitationOda et al 1994; CitationOishi et al 1994; CitationFujii et al 1995; CitationNakamura et al 1999; CitationHiratsuka et al 2003), neutrophil chemotactic activity (CitationKadota et al 1993; CitationOda et al 1994), neutrophil-derived elastolytic-like activity (CitationIchikawa et al 1993), and concentrations of IL-8 and LTB4 (CitationNakamura et al 1999). The latter has been shown to be independent of the ability to ‘eradicate’ organisms from sputum culture. However, patients in whom bacteria persist despite chronic macrolide therapy are less likely to experience improvement in BAL fluid neutrophil percentage (CitationFujii et al 1995). In addition, macrolide therapy decreases the percentage of BAL lymphocytes and BAL concentration of HBD-2, which suggests a primary effect on the underlying pathogenic process (CitationHiratsuka et al 2003). In summary, these data confirm that marked beneficial effects are seen with macrolide therapy in DPB patients, and these effects likely reflect the anti-inflammatory properties of these agents and in addition to potential antimicrobial effects. The similarities to potential effects in COPD are evident.
Macrolides in asthma
Asthma has a number of biological features that are distinct from COPD (CitationDouwes et al 2002; CitationSutherland and Martine 2003). Macrolide antibiotics have been utilized in asthma for more than forty years. For example, troleandomycin (TAO) was extensively studied as a steroid-sparing agent in asthma (CitationNiven and Argyros 2003), with inconclusive results (CitationEvans et al 2003). Other investigators have examined more widely available macrolide agents. The most widely reported outcome has been a modest improvement in bronchial hyperresponsiveness (CitationMiyatake et al 1991; CitationShimizu et al 1994; CitationKamoi et al 1995; CitationAmayasu et al 2000; CitationKostadima et al 2004). Clinical response has been more difficult to judge in uncontrolled studies (CitationGarey et al 2000). Several controlled trials have been published, as has a recent Cochrane systematic review (CitationRicheldi et al 2005). In general, these studies have documented modest symptomatic improvement but little change in standard spirometric indices (CitationShoji et al 1999; CitationAmayasu et al 2000).
Other investigators have provided mechanistic data regarding the effect of macrolide therapy in patients with asthma. CitationKraft and colleagues (2002) noted improved FEV1 in patients with evidence by polymerase chain reaction (PCR) of mycoplasma or chlamydia in upper or lower respiratory tract specimens (38/55 patients). PCR-positive patients treated with clarithromycin experienced a decrease in TNF-α, IL-5 and IL-12 mRNA in BAL fluid and TNF-α mRNA in airway tissue; PCR-negative patients treated with clarithromycin exhibited decreased TNF-α and IL-12 mRNA in BAL and TNF-α mRNA in airway tissue. No significant changes were seen in subjects treated with placebo. CitationBlack and colleagues (2001) conducted a multicenter, multinational study of 232 adults with mild to moderate asthma and serological evidence of C. pneumoniae infection, randomizing patients to roxithromycin 150 mg bid or placebo for six weeks. A modest increase in peak expired flow was noted in the macrolide treated patients after six weeks; daytime and nighttime symptoms showed nonsignificant improvements, as did improved quality of life scores. The differences between these two studies may reflect disparity in patient characteristics at baseline, differing macrolides administered, and differing definition of an atypical infection. These points may be important in interpreting data in COPD patients, in whom chronic infection with Chlamydia has been felt to be important (CitationBlasi et al 2002).
Macrolides in noncystic fibrosis bronchiectasis
Bronchiectasis is a disorder of the bronchi and bronchioles manifesting cough, chronic sputum production, dyspnea, and wheezing (CitationBarker 2003). The cornerstones of therapy include early identification and treatment of acute exacerbations, suppression of the microbial load, treatment of underlying conditions, promotion of bronchial hygiene, control of bronchial hemorrhage, surgical resection of extremely damaged/focal disease and reduction of excessive pulmonary inflammation (CitationBarker 2003).
It is likely that bronchiectasis involves a vicious cycle of infection with subsequent inflammation and mediator release. Investigators have confirmed an intense cellular infiltrate with mononuclear cells (CD4+ T lymphocytes and macrophages), a prominent neutrophilia and increased IL-8 expression (CitationGaga et al 1998). Increased expression of IL-8 and other potent chemoattractants (TNF-α and LTB4) have also been reported (CitationAngrill et al 2001; CitationRichman-Eisenstat et al 1993; CitationTsang et al 2000). In addition, patients colonized with potentially pathogenic organisms experience a greater inflammatory response which includes higher BAL neutrophil count and higher BAL concentrations of elastase, myeloperoxidase, TNF-α and IL-8 (CitationAngrill et al 2001).
Bronchiectasis patients with P. aeruginosa in the sputum have more severely impaired pulmonary function and greater sputum volume (CitationHo et al 1998). These patients also appear to exhibit the highest concentration of TNF-α and LTB4 (CitationTsang et al 2000). It is evident that bronchiectasis is characterized by augmented airway inflammation that appears related to bacterial colonization, organism load and differing bacterial species.
A small number of studies have examined the effect of chronic macrolide therapy in bronchiectasis unrelated to cystic fibrosis. After 12 weeks of roxithromycin (4 mg/kg bid) children with bronchiectasis and increased airway hyperreactivity experienced little change in FEV1 but experienced improvement in sputum purulence and airway responsiveness (CitationKoh et al 1997). In a placebo-controlled trial, CitationTsang and colleagues (1999) administered erythromycin (500 mg bid) for eight weeks to 11 patients with bronchiectasis (CitationTsang et al 1999). P. aeruginosa was identified at baseline in ten of the patients and H. influenzae in the eleventh. Patients treated with macrolides experienced an improvement in spirometry and 24-hour sputum volume, but no parallel improvement in sputum pathogens, sputum leukocytes, or levels of IL-1α, IL-8, TNF-α or LTB4 (CitationTsang et al 1999). CitationTagaya and colleagues (2002) noted improved sputum production in 16 patients with bronchiectasis (n = 11) or chronic bronchitis (n = 5) treated with clarithromycin (400 mg/day) compared to patients treated with amoxicillin or cefaclor. CitationDavies and Wilson (2004) examined thrice weekly (after initial loading) azithromycin therapy in 39 patients with bronchiectasis, greater than four exacerbations in the previous 12 months, and persistent symptoms. Only modest improvements were noted in pulmonary function, although symptoms scores and acute exacerbations decreased; these findings were independent of P. aeruginosa colonization. In a small, cross-over study, CitationCymbala and colleagues (2005) noted a decrease in acute exacerbation rate and mean 24-hour sputum volume, but no change in pulmonary function with twice weekly azithromycin therapy for six months. These data are particularly germane, given the frequent development of bronchiectasis following AECOPD (CitationO’Brien et al 2000).
Macrolides in cystic fibrosis
A robust body of literature has emerged demonstrating that macrolide antibiotics are beneficial in the treatment of cystic fibrosis (CF) lung disease (CitationEqui et al 2002; CitationWolter et al 2002; CitationSaiman et al 2003; CitationSouthern et al 2005). Numerous studies have strengthened the conclusion that there is excessive inflammation in CF airways. CitationKhan and colleagues (1995) noted higher inflammatory cells and mediators in infants with CF, even when no evidence of bacterial infection was identified. This augmented inflammatory response may be intrinsic to CF airway epithelial cells, as CitationDiMango and colleagues (1998) noted that in response to Pseudomonas, epithelial cells having mutations in the cystic fibrosis gene (CFTR) elaborated higher levels of inflammatory mediators than cells with normal CFTR.
As with other inflammatory pulmonary disorders, a high prevalence of chronic airway infection occurs in CF. P. aeruginosa appears to be the most prevalent organism, with 80% of patients infected by 18 years of age (CitationTrulock et al 2003). This infection has major consequences, as the survival of CF patients harboring Pseudomonas aeruginosa is shorter than that of CF patients uninfected by Pseudomonas.
A series of studies have confirmed the benefit of macrolide therapy in CF. CitationJaffe and colleagues (1998) performed an open-label study of daily azithromycin in children with CF in the UK, noting a modest improvement in spirometry with azithromycin therapy. The first well-constructed clinical trial of azithromycin in CF was reported by CitationWolter and colleagues (2002). Sixty CF patients (mean age 27.9 years) were randomly assigned in a double-blind fashion to 250 mg azithromycin daily or placebo for 3 months. A modest improvement favoring the macrolide was noted in spirometry, the requirement for intravenous antibiotic treatment for acute exacerbations, and quality of life. CitationEqui and colleagues (2002) performed a randomized, double-blind, placebo-controlled, crossover study of 41 CF patients (age range 8–18 years). Patients received daily azithromycin adjusted to body weight or placebo for 6 months. After a 2-month washout period, patients were crossed over to the alternative therapy for 6 months. A modest improvement in FEV1 was noted favoring azithromycin; however, there was no difference in FVC, FEF25–75, pulmonary exacerbation rate, antibiotic use, changes in exercise tolerance, quality of well-being, bacterial culture densities, or sputum levels of interleukin-8 and neutrophil elastase.
The largest trial of macrolide therapy in CF was completed by CitationSaiman and colleagues (2003), who performed a multi-centered, randomized, double-blinded, placebo-controlled study of 185 patients ≥6 years of age who were chronically infected with P. aeruginosa and had a percent predicted FEV1 ≥30. Patients were treated with weight-adjusted azithromycin dosage. After 24 weeks treatment, the percent predicted FEV1 improved in the azithromycin group (4.4%), whereas it declined in the placebo group (1.8%, p = 0.001). Furthermore, azithromycin-treated patients were less likely to experience an exacerbation than those treated with a placebo (HR 0.65, 95% CI 0.44–0.95, p = 0.03). Subsequent post-hoc analyses confirmed that the improvement in secondary endpoints was independent of the change in FEV1 (CitationSaiman et al 2005).
The mechanism for this favorable macrolide effect remains controversial and is likely multifactorial (CitationMartinez and Simon 2004). There is growing evidence that macrolides may be beneficial by directly altering P. aeruginosa biology (CitationJaffe and Bush 2001; CitationNguyen et al 2002; CitationWagner et al 2005). Suggested mechanisms include: (1) microbicidal effects on P. aeruginosa that are in stationary as opposed to exponential growth phase; (2) reduction in the elaboration of virulence factors; (3) alteration in biofilm formation; (4) decrease in bacterial adherence to epithelial cells; (5) inhibition of bacterial motility; and (6) providing a synergistic effect to other antibiotics (CitationMartinez and Simon 2004). It is likely that beneficial anti-inflammatory effects also play a role () (CitationJaffe and Bush 2001; CitationNguyen et al 2002; CitationMartinez and Simon 2004).
Table 1 Anti-inflammatory and bacterial virulence effects of macrolide antibiotics
Macrolides in chronic obstructive pulmonary disease
Taken together, the available data confirm that a macrolide can result in clinical improvement in patients with severe, chronic inflammatory lung diseases associated with frequent bacterial colonization and chronic infection. These data provide a rationale for expansion of the study of macrolide therapy to other, more common, inflammatory airway disorders, including COPD. Given the importance of inflammation and bacterial infection in the pathogenesis of COPD and its exacerbations, it is evident that macrolides may offer unique advantages as disease-modifying agents and that such an effect could be multifactorial ().
This possibility is supported by three older published studies. First, a randomized trial contrasting moxifloxacin with clarithromycin in acute exacerbations confirmed clinical response to clarithromycin in 88.4% of patients, despite achieving bacteriologic eradication in only 48.8% (CitationWilson et al 1999). An immunomodulatory effect of the macrolide was suggested to explain this discrepant finding (CitationWilson 2005). Second, CitationSuzuki and colleagues (2001) reported results of an open-label, prospective randomized trial of erythromycin therapy (200–400 mg/day) versus riboflavin (10 mg/day) for twelve months in COPD patients (FEV1 ~ 1.4 liters). During the course of therapy, the risk and frequency of experiencing a common cold using a predefined, standardized definition and subsequent acute exacerbations were prospectively evaluated. enumerates the significant benefits noted with macrolide therapy. Although the results are intriguing, the unblinded data collection limits the strength of the conclusions in this study.
Table 2 Effect of 12 months of erythromycin therapy on common colds and AECOPDs in patients with COPD
Third, CitationGomez and colleagues (2000) identified 54 patients with a long history of chronic bronchitis (>10 years), a frequency more than five episodes per year, and at least two respiratory hospitalizations in the previous year. Patients were treated with azithromycin 500 mg daily for three days every 21 days from September through May and were compared to a similar, retrospective group of untreated COPD patients. Although there were no baseline differences between the treated patients and the control patients, over the course of follow-up macrolide treated patients experienced fewer exacerbations and hospitalizations per year (). Not unexpectedly, during exacerbations the azithromycin treated group experienced infection with S. pneumoniae (n = 21), P. aeruginosa (n = 6), and K. pneumoniae (n = 3). Interestingly, within the first 15 days of prophylactic treatment, 21 of these exacerbations were caused by S. pneumoniae isolates with diminished susceptibility to macrolides and penicillins. In the control group, the predominant isolates during an exacerbations included H. influenzae (n = 29) and S. pneumoniae (n = 9; two exhibiting diminished sensitivity to azithromycin). This study, although using a suboptimal, uncontrolled design, suggests that a beneficial response can be seen with chronic macrolide prophylaxis in high-risk COPD patients. The mechanism of improvement was not addressed, but could be related to the antibacterial activity (particularly with regards to H. influenzae).
Table 3 Effect of azithromycin prophylactic therapy in COPD patients at high risk of AECOPD and treatment failure
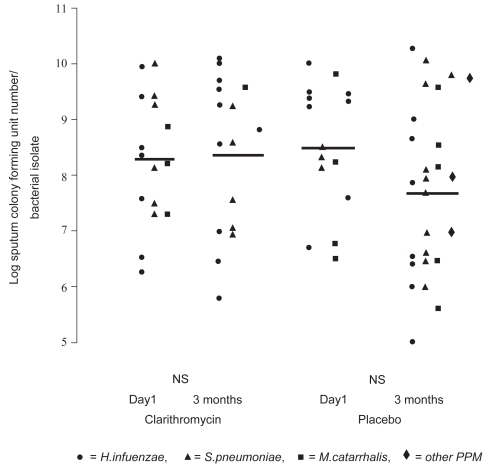
More recently, two groups have reported small controlled studies. CitationBasyigit and colleagues (2004) randomized 30 stable male COPD patients (mean age ~69 yrs, FEV1~ 36% predicted) to clarithromycin (500 mg bid) or placebo for two weeks in addition to standardized bronchodilator therapy. Although no baseline differences were noted between the treatment groups, clarithromycin-treated patients experienced significantly improved sputum inflammatory markers (), whereas physiologic studies did not change. The results of serum inflammatory markers were qualitatively similar in the two groups. In a second controlled study, 67 patients with moderate to severe COPD (mean age ~ 67 yrs, FEV1 ~43% pred) were randomized to three months of clarithromycin (500 mg of sustained release preparation daily) or placebo (CitationBanerjee et al 2004). In contrast to the previous study, all patients were taking inhaled corticosteroids. Clarithromycin was associated with a significant improvement in the St. George’s Respiratory Questionnaire (SGRQ) symptom score (10.2 units) and the SF-36 Physical Function Score (12.9 units) but no difference in overall SGRQ score, shuttle walk distance, spirometry or CRP levels. During the relatively short time of follow-up there were five total AECOPDs (3 in the clarithromycin and 2 in the placebo group). No difference was seen in quantitative sputum cultures obtained in the stable state (). In contrast to the previous study, sputum IL-8, TNF-α, or LTB4 levels did not differ between clarithromycin versus placebo treatment, although there was a modest macrolide-associated improvement in sputum neutrophil differential and neutrophil chemotaxis (CitationBanerjee et al 2004).
Figure 4 Levels of induced-sputum inflammatory markers in clarithromycin- and placebo-treated COPD patients before and after treatment. AT, after treatment; BT, before treatment; IL-8, interleukin-8; LTB4, leukotriene B4; TNF-α, tumor necrosis factor-α. *p < 0.05 before versus after treatment. Copyright © 2004. Reproduced with permission from CitationBasyigit I, Yildiz F, Ozkara SK, et al. 2004. The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data. Ann Pharmacother, 38:783–92.
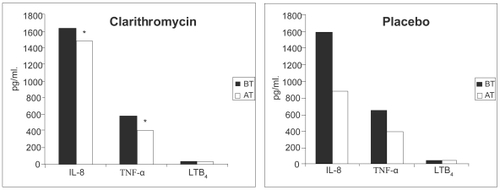
Figure 5 The effect of clarithromycin and placebo on sputum colony forming units (Cfu) numbers/bacterial (PPM) isolate. Cfu numbers are logged. Weighted bars indicate the mean for the whole group. There was no statistically significant difference between pre- and post-therapy Cfu numbers for both clarithromycin and placebo groups. NS indicates not significant. Copyright © 2004. Reproduced with permission from CitationBanerjee D, Honeybourne D, Khair OA. 2004. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trial. Treat Respir Med, 3:59–65.
Other investigators have proposed mechanistic rationale for improvement with chronic macrolide therapy in COPD patients. CitationTagaya and colleagues (2002) suggested that chronic bronchitis or bronchiectasis patients treated with clarithromycin (400 mg qd for seven days) experienced greater decrease in sputum volume than patients treated with a β-lactam. CitationParnham and colleagues (2005) treated 16 COPD patients (mean FEV1 1.8 liters) with azithromycin (500 mg daily) for three days and eight with placebo. Azithromycin resulted in an early transient increase in overall nitrite plus nitrate concentration, but later (days 11–18) decreases in total blood leukocyte count, serum CRP, IL-8- and oxidative burst of isolated blood granulocytes. No difference was noted in sputum inflammatory markers between azithromycin- treated and placebo-treated patients.
There is potential risk to chronic macrolide therapy in this patient population. Macrolide use may be associated with an increased risk of subsequent infection with macrolide nonsusceptible bacteria, particularly S. pneumoniae (CitationKlugman and Lonks 2005; CitationVanderkooi et al 2005). An increase in macrolide-nonsusceptible S. pneumoniae has been reported with chronic erythromycin (CitationAberg et al 2001; CitationKasahara et al 2005), clarithromycin (CitationKasahara et al 2005) and azithromycin therapy (CitationGomez et al 2000; CitationAberg et al 2001). Similarly, these agents may be associated with gastrointestinal intolerance (CitationSaiman et al 2003; CitationBasyigit et al 2004; CitationCymbala et al 2005), cardiac toxicity (CitationIannini 2002; CitationMilberg et al 2002), and ototoxicity (CitationHaydon et al 1984; CitationSwanson et al 1992; CitationWallace et al 1994). The frequency and severity of these toxicities varies with the agent, the dose and duration of therapy. These potential complications and the high prevalence of medical comorbitidies in COPD patients highlight the potential risk of chronic empiric macrolide therapy in this patient population.
Conclusions
Macrolide antibiotics have assumed an important role in the management of AECOPDs because of their broad-spectrum coverage and excellent safety profile. Increasingly, data are becoming available that macrolides have beneficial effects that are independent of their antibacterial activity. These potent anti-inflammatory effects may be particularly important in chronic inflammatory airway disorders. These latter actions may be of greatest benefit for selected patients with COPD, a disorder characterized by augmented pulmonary inflammation at baseline, frequent bacterial colonization/infection, and recurrent exacerbations of disease which further increase lung inflammation.
The role of chronic macrolide therapy in these patients requires additional study to better define a series of concerns, including:
What is the treatment-effect of chronic macrolide therapy in COPD patients treated maximally with conventional therapies?
Do macrolides attenuate baseline inflammation or do they prevent or attenuate AECOPD episodes?
Does the therapeutic effect reflect antimicrobial activity, an immunomodulatory effect, or both?
Does the therapeutic effect vary by host factors, including disease severity, presence of bronchiectasis, presence of baseline colonization with typical (including P. aeruginosa) or atypical pathogens (including C. pneumoniae), or the presence of comorbidity?
What is the toxicity of chronic therapy in COPD patients?
What is the risk-benefit of macrolide therapy in COPD patients?
Resolution of these vital questions will require additional, well-designed, placebo-controlled trials. To our knowledge three large studies are currently ongoing which should yield answers to all or most of these vital questions before widespread use of macrolide prophylaxis can be recommended in COPD patients.
Acknowledgments
Supported by NIH 2K24 HL004212, U10 HL074422 and HL082480 and HL56309 from the USPHS; and by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs.
References
- AaronSDAngelJBLunauM2001Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1633495511179105
- AbergJAWongMKFlammR2001Presence of macrolide resistance in respiratory flora of HIV-infected patients receiving either clarithromycin or azithromycin for Mycobacterium avium complex prophylaxisHIV Clin Trials2453911742432
- AdachiTMotojimaSHirataA1996Eosinophil apoptosis caused by theophylline, glucocorticoids, and macrolides after stimulation with IL-5J Allergy Clin Immunol98S207158977529
- AgenCDanesiRBlandizziC1993Macrolide antiboitics as antiinflammatory agents: roxithromycin in an unexpected roleAgents Actions3885908480541
- AgustiAGN2005COPD, a multicomponent disease: implicationis for managementRespir Med996708215878483
- AkiraMKitataniFLeeYS1988Diffuse panbronchiolitis: evaluation with high-resolution CTRadiology1684383380982
- AllegraLBlasiFde BernardiB2001Antibiotic treatment and baseline severity or disease in acute exacerbations of chronic bronchitis: a re-evaluation of previously published data of a placebo-controlled randomized studyPulm Pharmacol Therapeut1414955
- AllegraLBlasiFDianoPL2005Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary diseaseRespir Med99742715878491
- AmayasuHYoshidaSEbanaS2000Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthmaAnn Allergy Asthma Immunol84594810875487
- AminKEkberg-JanssonALofdahlCG2003Relationship between inflammatory cells and structural changes in the lungs of asymptomatic and never smokers: a biopsy studyThorax581354212554896
- AnderssonFBorgSJanssonSA2002The costs of exacerbations in chronic obstructive pulmonary disease (COPD)Respir Med96700812243316
- AngrillJAgustiCDe CelisR2001Bronchial inflammation and colonization in patients with clinically stable bronchiectasisAm J Respir Crit Care Med16416283211719301
- AnthonisenNManfredaJWarrenC1987Antibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Int Med1061962043492164
- AnzuetoARizzoJAGrossmanRF1999The infection-free interval: its use in evaluating antimicrobial treatment of acute exacerbations of chronic bronchitisClin Inf Dis2813445
- AoshibaKNagaiAKonnoK1995Erythromycin shortens neutrophil survival by accelerating apoptosisAntimicrob Agents Chemother3987277785987
- AshitaniJDoutsuYTaniguchiH1992A case of diffuse panbronchiolitis relieved rapidly by the treatment of roxithromycin (Japanese)J Jpn Assoc Infect Dis666578
- BachPBBrownCGelfandSE2001Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidenceAnn Intern Med1346002011281745
- BakriFBrauerALSethiS2002Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbations of chronic obstructive pulmonary diseaseJ Infect Dis1856324011865420
- BalterMSLa ForgeJLowDE2003Canadian guidelines for the management of acute exacerbations of acute exacerbations of chronic bronchitisCan Respir J10Suppl B3B32B
- BandivJakubowyczMKinyonC2003Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzaeFEMS Immunol Med Microbiol10697512770762
- BanerjeeDHoneybourneDKhairOA2004The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trialTreat Respir Med3596515174894
- BanerjeeDKhairOAHoneybourneD2004Impact of sputum bacteria on airway inflammation and health status in clinical stable COPDEur Respir J236859115176680
- BarkerAF2003BronchiectasisNew Eng J Med34613839311986413
- BarnesPJShapiroSDPauwelsRA2003Chronic obstructive pulmonary disease: molecular and cellular mechanismsEur Respir J226728814582923
- BasyigitIYildizFOzkaraSK2004The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary dataAnn Pharmacother3878392
- BaumannUFischerJJGudowiusP2001Buccal adherence of Pseudomonas aeruginosa in patients with cystic fibrosis under long-term therapy with azithromycinInfection2971111261763
- BeatyCDGraystonJTWangSP1991Chlamydia pneumoniae, strain Twar, infection in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis1441408101741558
- BlackPNBlasiFJenkinsCR2001Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniaeAm J Respir Crit Care Med1645364111520711
- BlasiFDamatoSCosentiniR2002Chlamydia pneumoniae and chronic bronchitis: association with severity and bacterial clearance following treatmentThorax57672612149525
- BlasiFLegnaniDLombardoVM1993Chlamydia pneumoniae infection in acute exacerbations of COPDEur Respir J619228425589
- BlasiFEwigSTorresA2006A review of guidelines for antibacterial use in acute exacerbations of chronic bronchitisPulm Pharmacol Ther19361916289762
- BresserPOutTAvan AlphenL2000Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infectionAm J Respir Crit Care Med1629475210988111
- CaramoriGAdcockI2003Pharmacology of airway inflammation in asthma and COPDPulm Pharmacol Ther162477712877818
- ChinCLManzelLJLehmanEE2005Haemophilus influenzae from COPD patients with exacerbation induce more inflammation than colonizersAm J Respir Crit Care Med172859115805181
- ChodoshS2005Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitisChest1272231615947342
- ChodoshSMcCartyJFarkasS1998Randomized, double-blind study of ciprofloxacin and cefuroxime axetil for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study GroupClin Infect Dis2772299798024
- Christ-CrainMJaccard-StolzDBingisserR2004Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded interventionl trialLancet363600714987884
- CulicOErakovicVCepelakI2002Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjectsEur J Pharmacol
- CymbalaAAEdmondsLCBauerMA2005The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasisTreat Respir Med41172215813663
- DaviesGWilsonR2004Prophylactic antibiotic treatment of bronchiectasis with azithromycinThorax59540115170047
- DesakiMOkazakiHSunazukaT2004Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-κB activationAntimicrob Agent Chemother4815815
- DesakiMTakizawaHOhtoshiT2000Erythromycin suppresses nuclear factor-kB and activator protein-1 activation in human bronchial epithelial cellsBiochim Biophys Res Com2671248
- DevDWallaceESankaranR1998Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary diseaseRespir Med9266479659534
- DevaliaJLRusznakCHerdmanMJ1994Effect of nitrogen dioxide and sulphur dioxide on airway response of mild asthmatic patients to allergen inhalationLancet3441668717996960
- Di StefanoACapelliALusuardiM2001Decreased T lymphocyte infiltration in bronchial biopsies of subjects with severe chronic obstructive pulmonary diseaseClin Exp Allergy3189390211422154
- Di StefanoACaramoriGRicciardoloFLM2004Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overviewClin Exp Allergy3411566715298554
- Di StefanoATuratoGMaestrelliP1996Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosaAm J Respir Crit Care Med153629328564109
- DiMangoERatnerAJBryanR1998Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cellsJ Clin Invest10125986059616231
- DonaldsonGCSeemungalTABhowmikA2002Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax578475212324669
- DouwesJGibsonPPekkanenJ2002Non-eosinophilic asthma: importance and possible mechanismsThorax57643812096210
- DrostEMSkwarskiKMSauledaJ2005Oxidative stress and airway inflammation in severe exacerbations of COPDThorax6029330015790984
- DrussBGMarcusSCOlfsonM2002The most expensive medical conditions in AmericaHealth Aff (Millwood)211051112117121
- EllerJEdeASchabergT1998Infective exacerbations of chronic obstructive pulmonary disease. Relation between bacteriologic etiology and lung functionChest113154289631791
- EquiABalfour-LynnIMBushA2002Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trialLancet3609788412383667
- EvansDJCullinanPGeddesDM2003Troleandomycin as an oral corticosteroid sparing agent in stable asthmaCochrane Database Syst Rev3
- FagonJYChastreJTrouilletJL1990Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis: use of the protected specimen brush technique in 54 mechanically ventilated patientsAm Rev Respir Dis142100482240819
- FinkelsteinRFraserRSGhezzoH1995Alveolar inflammation and its relation to emphysema in smokersAm J Respir Crit Care Med1521666727582312
- FostDALeungDYMartinRJ1999Inhibition of methylprednisolone elimination in the presence of clarithromycin therapyJ Allergy Clin Immunol1031031510359882
- FujiiTKadotaJKawakamiK1995Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infectionThorax501246528553295
- FujimotoKYasuoMUrushibataK2005Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary diseaseEur Respir J25640615802337
- GagaMBentleyAMHumbertM1998Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasisThorax53685919828857
- Garcia-AymerichJTobiasAAntoJM2000Air pollution and mortality in a cohort of patients with chronic obstructive pulmonary diseaseJ Epidemiol Community Health5473410692968
- GareyKWRubinsteinIGotfriedMH2000Long-term clarithromycin decreases prednisone requirements in elderly patients with prednisone-dependent asthmaChest1181826711115481
- GillisRJIglewskiBH2004Azithromycin retards Pseudomonas aeruginosa biofilm formationJ Clin Microbiol425842515583321
- GomezJBañosVSimarroE2000Estudio prospective y comparative (1994–1998) sobre la influencia del tratamiento corto profilactico con azitromicina en pacientes con EPOC evolucionadaRev Esp Quimioterap1337983
- GompertzSO’BrienCBayleyDL2001Changes in bronchial inflammation during acute exacerbations of chronic bronchitisEur Respir J1711121911491152
- GonzalesRMaloneDCMaselliJH2001Excessive antibiotic use for acute respiratory infections in the United StatesClin Inf Dis3375762
- GreenbergSB2002Viral respiratory infections in elderly patients and patients with chronic obstructive pulmonary diseaseAm J Med1126A28S32S11955457
- GrumelliSCorryDBSongLZ2004An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysemaPLOS Med17583
- GulsvikA2001The global burden and impact of chronic obstructive pulmonary disease worldwideMonaldi Arch Chest Dis56261411665507
- GumpDWPhillipsCAForsythBR1976Role of infections in chronic bronchitisAm Rev Respir Dis113465731267252
- HalbertRJIsonakaSGeorgeD2003Interpreting COPD prevalence estimamtes: what is the true burden of disease?Chest12316849212740290
- HalpernMTHigashiMKBakstAW2003The economic impact of acute exacerbations of chronic bronchitis in the United States and Canada: A literature reviewJ Manages Care Pharm93539
- HalpernMTStanfordRHBorkerR2003The burden of COPD in the USA: results from the Confronting COPD surveyRespir Med97Suppl CS81912647946
- HamelinMECoteSLaforgeJ2005Human metapneumo-virus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary diseaseClin Infect Dis4149850216028158
- HashimotoNKawabeTHaraT2001Effect of erythromycin on matrix metalloproteinase-9 and cell migrationJ Lab Clin Med1371768311241027
- HaydonRCThelinJWDavisWE1984Erythromycin ototoxicity: analysis and conclusions based on 22 case reportsOtolaryngol Head Neck Surg92678846440087
- HillATCampbellEJBayleyDL1999Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with a1-antitrypsin deficiency (PiZ)Am J Respir Crit Care Med16019687510588615
- HillATCampbellEJHillSL2000Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitisAm J Med1092889510996579
- HiratsukaTMukaeHLliboshiH2003Increased concentration of human β-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitisThorax584253012728165
- HirschmannJV2000Do Bacteria cause exacerbations of COPD?Chest11819320310893379
- HoPLChanKNIpMSM1998The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasisChest114159489872194
- HoggJCChuFUtokaparchS2004The nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Eng J Med350264553
- HommaHYamanakaATanimotoS1983Diffuse panbronchiolitis: a disease of the transitional zone of the lungChest836396848335
- HurstJRPereraWRWilkinsonTMA2006Systemic, upper and lower airway inflammation at exacerbation of COPDAm J Respir Crit Care Med173717816179639
- IanniniPB2002Cardiotoxicity of macrolides, ketolides and fluoroquinolones that prolong the QTc intervalExpert Opin Drug Saf1121812904146
- IchikawaYNinomiyaHKogaH1993Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patientsAm Rev Respir Dis147106458466109
- IchimiyaTTakeokaKHiramatsuK1996The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitroChemotherapy42186918983885
- ImamuraYHigashiyamaYTomonoK2005Azithromycin exhibits bactericidal effects on Pseudomonas aeruginosa through interaction with the outer membraneAntimicrob Agent Chemother49137780
- ImamuraYYanagiharaKMizutaY2004Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa auto-inducer N-(3-Oxododecanoyl) homoserine lactone in NCI-H292 cellsAntimicrob Agent Chemother48345761
- InamuraKOhtaNFukaseS2000The effects of erythromycin on human peripheral neutrophil apoptosisRhinology38124911072658
- InoueMOkamotoROkuboT1992Comparative in-vitro activity of RP 59500 against clinical bacterial isolatesJ Antimicrob Chemother30Suppl A45511399950
- IshimatsuYKadotaJIwashitaT2004Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitroInt J Antimicrob Agents242475315325428
- IshizawaKSuzukiTYamayaM2005Erythromycin increases bactericidal activity of surface liquid in human airways epithelial cellsAm J Physiol Lung Mol Physiol289L56573
- ItoKItoMElliottWM2005Decreased histone deacetylase activity in chronic obstructive pulmonary diseaseN Eng J Med352196776
- JaffeABushA2001Anti-inflammatory effects of macrolides in lung diseasePediatric Pulmonology314647311389580
- JaffeAFrancisJRosenthalM1998Long-term azithromycin may improve lung function in children with cystic fibrosisLancet3514209482305
- JainRDanzigerLH2004The macrolide anbiotics: a pharmacokinetic and pharmacodynamic overviewCurr Pharm Des1030455315544496
- JohnstonSL2005Overview of virus-induced airway diseaseProc Am Thorac Soc2150616113484
- KadotaJMukaeHIshiiH2003Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitisResp Med9784450
- KadotaJMukaeHTomonoK2001High concentrations of β-chemokines in BAL fluid of patients with diffuse panbronchiolitisChest120602711502665
- KadotaJSakitoOKohnoS1993A mechanism of erythromycin treatment in patients with diffuse panbronchiolitisAm Rev Respir Dis14715398420410
- KadotaJIMizunoeSKishiK2005Antibiotic-induced apoptosis in human activated peripheral lymphocytesInt J Antimicrob Agent2521620
- KamoiHKuriharaNFujiwaraH1995The macrolide antibacterial roxithromycin reduces bronchial hyperresponsiveness and superoxide anion production by polymorphonuclear leukocytes in patients with asthmaJ Asthma3219177759458
- KanaiKAsanoKHisamitsuT2004Suppression of matrix metal-loproteinase production from nasal fibroblasts by macrolide antibiotics in vitroEur Respir J23671815176678
- KannerREAnthonisenNRConnettJE2001Lower respiratory illnesses decline in current smokers but not ex-smokers with promote FEV1 mild chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1643586411500333
- KarnakDBengsunSBederS2001Chlamydia pneumoniae infection and acute exacerbation of chronic obstructive pulmonary disease (COPD)Respir Med958111611601747
- KasaharaKKitaEMaedaK2005Macrolide resistance of Streptococcus pneumoniae isolated during long-term macrolide therapy: difference between erythromycin and clarithromycinJ Infect Chemother11112415856383
- Kawamura-SatoKIinumaYHasegawaT2000Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilisAntimicrob Agent Chemother44286972
- KeichoNKudohSYotsumotoH1994Erythromycin promotes monocyte to macrophage differentiationJ Antibiot (Tokyo)478098119865
- KemenyDMVyasBVukmanovic-StejicM1999CD8(+) T cell subsets and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med160S33710556167
- KhanAASliferTRAraujoFG1999Effect of clarithromycin and azithromycin on production of cytokines by human monocytesInt J Antimicrob Agent1112132
- KhanTZWagenerJSBostT1995Early pulmonary inflammation in infants with cystic fibrosisAm J Respir Crit Care Med1511075827697234
- KikuchiTHagiwaraKHondaY2002Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factorsJ Antimicrob Chemother497455512003967
- KlugmanKPLonksJR2005Hidden epidemic of macrolide-resistant pneumococciEmer Inf Dis118027
- KobayashiHTakedaHSakayoriS1995Study on azithromycin in treatment of diffuse panbronchiolitis (English abstract)J Jpn Assoc Infect Dis6971122
- KobayashiH1995Biofilm disease: its clinical manifestation and therapeutic possibilities of macrolidesAm J Med9926S30S8585531
- KohYYLeeMHSunYH1997Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled studyEur Respir J1099499163637
- KohyamaTTakizawaHKawasakiS1999Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donorsAntimicrob Agent Chemother4390711
- KostadimaETsiodrasSAlexopoulosEK2004Clarithromycin reduces the severity of bronchial hyperresponsiveness in patients with asthmaEur Respir J237141715176685
- KoyamaHGeddesDM1997Erythromycin and diffuse panbronhiolitisThorax52915189404381
- KraftMCassellGHPakJ2002Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma. Effect of clarithromycinChest1211782812065339
- KudohSAzumaAYamamotoM1998Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycinAm J Respir Crit Care Med1571829329620913
- KudohSKimuraKUetakeK1984Abstract of annual meetingJpn J Thorac Dis22254
- KudohSUetakeTHagiwaraK1987Clinical effect of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis (English Abstract)Jpn J Thorac Dis2563242
- LabroMT2004Cellular and molecular effects of macrolides on leukocyte functionCurr Pharm Des1030678015544498
- LaForceCFSzeflerSJMillerMF1983Inhibition of methylprednisolone elimination in the presence of erythromycin therapyJ Allergy Clin Immunol723496602160
- LiebermanDBen-YaakovMLazarovichZ2001Chlamydia pneumoniae infection in acute exacerbations of chronic obstructive pulmonary disease: analysis of 250 hospitalizationsEur J Clin Microbiol Infect Dis2069870411757970
- LodeHEllerJLinnhoffA2004Levofloxacin versus clarithromycin in COPD exacerbation: focus on exacerbation-free intervalEur Respir J249475315572537
- MajoJGhezzoHCosioMG2001Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysemaEur Respir J179465311488331
- ManninoDMHomaDMAkinbamiLJ2002Chronic obstructive pulmonary disease surveillance – United States, 1971–2000MMWR51116
- MartinezFJ2004Acute bronchitis: state of the art diagnosis and therapyCompr Ther30556915162593
- MartinezFJGrossmanRFZadeikisN2005Patient stratification in the management of acute bacterial exacerbation of chronic bronchitis: the role of levofloxacin 750 mgEur Respir J2005
- MartinezFJSimonRH2004Clinical implications of macrolide therapy in chronic sinopulmonary diseasesCurr Pharm Des10
- McCroryDCBrownCGelfandSE2001Management of acute exacerbations of COPD. A summary and appraisal of published evidenceChest119119020911296189
- McGuireAIrwinDEFennP2001The excess of acute exacerbations of chronic bronchitis in patients aged 45 and older in England and WalesValue Health4370511705127
- MilbergPEckardtLBrunsHJ2002Diveregent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointesJ Pharmacol Exp Therapeut30321825
- MiravitllesMEspinosaCFernandez-LasoE1999Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPDChest11640610424501
- MiravitllesMFerrerMPontA2004Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary diseasee: a 2 year follow up studyThorax593879515115864
- MiravitllesMLlorCNaberanK2004Effect of various antimicrobial regimens on the clinical course of exacerbations of chronic bronchitis and chronic obstructive pulmonary disease in primary careClin Drug Invest246372
- MiravitllesMTorresA2004No more equivalence trials for antibiotics in exacerbations of COPD, pleaseChest1258111315006934
- MiravitllesMMurioCGuerreroT2002Pharmacoeconomic Evaluation of Acute Exacerbations of Chronic Bronchitis and COPD*Chest1211449145512006427
- MiyatakeHTakiFTaniguchiH1991Erythromycin reduces the severity of bronchial hyperresponsiveness in asthmaChest9967031995224
- MogulkocNKarakurtSIsalskaB1999Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infectionAm J Respir Crit Care Med1603495310390424
- MolinariGGuzmanCAPesceA1993Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibioticsJ Antimicrob Chemother3168188392997
- MonsoEGarcia-AymerichJSolerN2003Bacterial infection in exacerbated COPD with changes in sputum characteristicsEpidemiol Infect13179980412948381
- MonsoERuizJRosellA1995Bacterial infection in chronic obstructive pulmonary disease: A study of stable and exacerbated outpatients using the protected specimen brushAm J Respir Crit Care Med152131613207551388
- MotojimaSAdachiTManakaK1996Eosinophil peroxidase stimulates the release of granulocyte-macrophage colony-stimulating factor from bronchial epithelial cellsJ Allergy Clin Immunol98S216238977530
- MukaeHKadotaJKohnoS1995Increase in activated CD8+ cells in bronchoalveolar lavage fluid in patients with diffuse panbronchiolitisAm J Respir Crit Care Med15261387633715
- MurphyTFBrauerALGrantBJB2005Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune reponseAm J Respir Crit Care Med
- MurphyTFSethiSNiedermanMS2000The role of bacteria in exacerbations of COPD. A constructive viewChest118204910893380
- MusherDMKubitschekKRCrennanJ1983Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzaeAnn Intern Med99444506605104
- NagaiAShishidoHYonedaR1991Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitisRespiration5814591745845
- NagataTMukaeHKadotaJ2004Effect of erythromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine modelAntimicrob Agents Chemother482251915155229
- NakamuraHFujishimaSInoueT1999Clinical and immunoregulatory effects of roxithromycin therapy for chronic respiratory tract infectionEur Respir J131371910445614
- NguyenTLouieSGBeringerPM2002Potential role of macrolide antibiotics in the management of cystic fibrosis lung diseaseCurr Opin Pulm Med8521812394161
- NiedermanMSMcCombsJSUngerAN1999Treatment cost of acute exacerbations of chronic bronchitisClinical Therapeutics2157659110321424
- NishimuraKKitaichiMIzumiT1992Diffuse panbronchiolitis: correlation of high resolution CT and pathologic findingsRadiology184779851509067
- NivenASArgyrosG2003Alternate treatments in asthmaChest12312546512684319
- NouiraSMarghliSBelghithM2001Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trialLancet3582020511755608
- O’BrienCGuestPJHillSL2000Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary careThorax556354210899238
- OdaHKadotaJKohnoS1994Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitisChest1061116237924482
- OgawaNSugawaraYFujiwaraY2003Roxithromycin promotes lymphocyte apoptosis in Dermatophagoides-sensitive asthma patientsEur J Pharmacol4742738112921874
- OhnoSSugiyamaYKitamuraS1993Clinical effects of low-dose and long-term erythromycin in diffuse panbronchiolitis with chronic respiratory failure (Engish Abstract)Jpn J Thorac Dis3112516
- OhtoshiTTakizawaHOkazakiH1998Diesel exhaust particulates stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitroJ Allergy Clin Immunol101778859648705
- OishiKSonodaFKobayashiA1994Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseasesInfect Immun624145527927669
- OishiKSonodaFKobayashiS1994Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseasesInfect Immun624145527927669
- OostenbrinkJBRuttenvan MolkenM2004Resource use and risk factors in high-cost exacerbations of COPDRespir Med988839115338802
- O’ShaughnessyTCAnsariTWBarnesNC1997Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1Am J Respir Crit Care Med15585279117016
- ParkerCMVoducNAaronSD2005Physiological changes during symptom recovery from moderate exacerbations of COPDEur Respir J26420816135722
- ParnhamMJCulicOErakovicV2005Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatmentEur J Pharmacol5171324315964564
- PatelISSeemungalTARWilksM2002Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbationsThorax577596412200518
- PauwelsRCalverleyPBuistAS2004COPD exacerbations: the importance of a standard definitionRespir Med989910714971871
- PauwelsRABuistASCalverleyPMA2001Committee GOLD Scientific. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop SummaryAm J Respir Crit Care163125676
- PechereJC2001New perspectives on macrolide antibioticsInt J Anti-microb Agents18S937
- PelaRMarchesaniFAgostinelliC1998Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigationMonaldi Arch Chest Dis5326279785808
- PettyTL2000Scope of the COPD Problem in North AmericaChest117326S331S10843971
- PietilaMPThomasCF2003Inflammation and infection in exacerbations of chronic obstructive pulmonary diseaseSem Resp Inf18916
- PletzMWMcGeeLJorgensenJ2004Levofloxacin-resistant invasive Streptococcus pneumoniae in the United States: evidence for clonal spread and the impact of conjugate pneumococcal vaccineAntimicrob Agents Chemother483491715328116
- QiuYZhuJBandiV2003Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1689687512857718
- ReatoGCuffiniAMTullioV2004Immunomodulating effect of antimicrobial agents on cytokine production by human polymorpho-nuclear neutrophilsInt J Antimicrob Agent231504
- ReichenbergerFWyserCGononM2001Pulmonary mucosa-associated lymphoid tissue lymphoma in a patient with common variable immunodeficiency syndromeRespiration681091211223743
- RhodeGWiethegeABorgI2003Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control studyThorax58374212511718
- RicheldiLFerraraGFabbriL2005Macrolides for chronic asthmaCochrane Database Syst Rev4
- Richman-EisenstatJBYJorensPGHebertCA1993Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseasesAm J Physiol264L413188476069
- Rodrigues-RoisinR2000Toward a consensus definition for COPD exacerbationsChest117398S401S10843984
- RolandMBhowmikASapsfordRJ2001Sputum and plasma endothelin-a levels in exacerbations of chronic obstructive pulmonary diseaseThorax5630511120901
- RosellAMonsoESolerN2005Microbiologic determinants of exacerbation in chronic obstructive pulmonary diseaseArch Intern Med165891715851640
- RubinBKDruceHRamirezOE1997Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitisAm J Respir Crit Care Med1552018239196110
- RudellBBlombergAHelledayR1999Bronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particulate trap filtered exhaustOccup Environ Med565273410492649
- RussellRECulpittSVDeMatosC2002Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol26602911970913
- RussellREThorleyACulpittSV2002Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteasesAm J Physiol Lung Cell Mol Physiol283L8677312225964
- RutgersSRPostmaDSHackenNH2000Ongoing airway inflammation in patients with COPD who do not currently smokeThorax55121810607796
- SaettaMBaraldoSCorbinoL1999CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med160711710430750
- SaettaMDi StefanoAMaestrelliP1993Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitisAm Rev Respir Dis14730168430952
- SaettaMDi StefanoATuratoG1998CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med15782269517597
- SaettaMMarianiMPanina-BordignonP2002Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1651404912016104
- SaettaMTuratoGCorbinoL1997Mechanisms of damage in COPDMonaldi Arch Chest Dis5258689550872
- SaimanLMarshallBCMayer-HamblettN2003Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trialJAMA29017495614519709
- SaimanLMayer-HamblettNCampbellP2005Heterogeneity of treatment response to azithromycin in patients with cystic fibrosisAm J Respir Crit Care Med17210081216040785
- SaintSBentSVittinghoffE1995Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysisJAMA273957607884956
- SaintSFlahertyKRAbrahamseP2001Acute exacerbations of chronic bronchitis: disease-specific issues that influence the cost-effectiveness of antimicrobial therapyClin Ther2349951211318083
- SakitoOKadotaJKohnoS1996Interleukin 1β, tumor necrosis factor-α, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapyRespiration634288833992
- SawakiMMikamiRMikasaK1986The long term chemotherapy with erythromycin in chronic lower respiratory tract infections - Second report: including cases with Pseudomonas infections (English Abstract)J Jpn Assoc Infect Dis604550
- ScaglioneFRossoniG1998Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycinJ Antimicrob Chemother41Suppl B47509579712
- SchmierJKHalpernMTHigashiMK2005The quality of life impact of acute exacerbations of chronic bronchitis (AECB): A literature reviewQual Life Res143294715892423
- SeemungalTHarper-OwenRBhowmikA2001Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1641618162311719299
- SeemungalTAWedzichaJAMacCallumPK2002Chlamydia pneumoniae and COPD exacerbationThorax5710878 author reply 1088–912454307
- SeemungalTARDonaldsonGCBhowmikA2000Time course and recovery of exacerbations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16116081310806163
- SethiS1999Infectious exacerbations of chronic bronchitis: diagnosis and managementJ Antimicrob Chemother43Suppl A9710510225579
- SethiS2000Infectious etiology of acute exacerbations of chronic bronchitisChest117380S385S10843981
- SethiS2004Bacteria in exacerbations of chronic obstructive pulmonary disease. Phenomenon or epiphenomenon?Proc Am Thorac Soc11091416113422
- SethiSEvansNGrantBJB2002New strains of bacteria and exacerbations of chronic obstructive pulmonary diseaseNew Eng J Med3474657112181400
- SethiSMurphyTF2001Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art reviewClin Microbiol Rev143366311292642
- ShapiroSDIngenitoEP2005The pathogenesis of chronic obstructive pulmonary disease. Advances in the past 100 yearsAm J Respir Cell Mol Biol323677215837726
- ShimizuTKatoMMochizukiH1994Roxithromycin reduces the degree of bronchial hyperresponsiveness in children with asthmaChest106458617774320
- ShimizuTShimizuSHattoriR2003In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cellsAm J Respir Crit Care Med168581712829454
- ShojiTYoshidaSSakamotoH1999Anti-inflammatory effect of roxithromycin in patients with aspirin-intolerant asthmaClin Exp All299506
- ShryockTRMortensenJEBaumholtzM1998The effects of macrolides on the expression of bacterial virulence mechanismsJ Antimicrob Chemother41505129630404
- SnowVLascherSMottur-PielsonC2001Evidence base for management of acute exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med134595911281744
- SolerNEwigSTorresA1999Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary diseaseEur Respir J1410152210596683
- SolerNTorresAEwigS1998Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilationAm J Respir Crit Care Med157149815059603129
- SouthernKWBarkerPMSolisA2005Macrolide antibiotics for cystic fibrosisCochrane Database Syst Rev4
- SpencerSJonesPWfor the GLOBE Study Group2003Time course of recovery of health status following an infective exacerbation of chronic bronchitisThorax585899312832673
- StevensonNJCostelloPRCalverleyPMA2005Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med17215101616166620
- StockleyRAO’BrienCPyeA2000Relationship of sputum color to nature and outpatient management of acute exacerbations of COPDChest11716384510858396
- StollerJK2002Acute exacerbations of chronic obstructive pulmonary diseaseN Eng J Med34698894
- StottEJGristNREadieMB1968Rhinovirus infections in chronic bronchitis: isolation of eight possible new rhinovirus serotypesJ Med Microbiol1109174318937
- StrasselsSASmithDHSullivanSD2001The costs of treating COPD in the United StatesChest1193445211171708
- SunyerJSaezMMurilloC1993Air pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year studyAm J Epidemiol13770158484361
- SunyerJSchwartzJTobiasA2000Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysisAm J Epidemiol15150610625173
- SutherlandERMartineRJ2003Airway inflammation in chronic obstructive pulmonary disease: comparisons with asthmaJ Allergy Clin Immunol1128192714610463
- SuzakiHAsanoKOhkiS1999Suppressive activity of a macrolide antibiotic, roxithromycin, on pro-inflammatory cytokine production in vitro and in vivoMediators Inflamm819920410704073
- SuzukiTYanaiMYamayaM2001Erythromycin and common cold in COPDChest120730311555501
- SwansonDJSungRJFineMJ1992Erythromycin ototoxicity: prospective assessment with serum concentrations and audiograms in a study of patients with pneumoniaAm J Med926181731511
- TagayaETamaokiJKondoM2002Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretionChest1222131812114361
- TakakiMUshikaiMDeguchiK2003Interleukin-8 expression by human adenoidal fibroblastsLaryngoscope11313788512897563
- TakedaHMiuraHKawahiraM1989Long-term administration study on TE-031 (A-56268) in treatment of diffuse panbronchiolitis (English Abstract)J Jpn Assoc Infect Dis63718
- TakeokaKIchimiyaTYamasakiT1998The in vitro effect of macrolides on the interaction of human polymorphonuclear leukocytes with Pseudomonas aeruginosa in biofilmChemotherapy4419079612609
- TakizawaHDesakiMOhtoshiT1997Erythromycin modulates IL-9 expression in normal and inflammed human bronchial epithelial cellsAm J Respir Crit Care Med156266719230759
- TamaokiJTakeyamaKTagayaE1995Effect of clarithromycin on sputum production and its rheological properties in chronic respiratory tract infectionsAntimicrob Agent Chemother39168890
- TanWCXiangXQiuD2003Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary diseaseAm J Med115272712967691
- TatedaKComteRPechereJC2001Azithromycin inhibits quorum sensing in Pseudomonas aeruginosaAntimicrob Agents Chemother451930311353657
- TravesSLDonnellyLE2005Chemokines and their receptors as targets for the treatment of COPDCurr Respir Med Rev11532
- TrulockEPEdwardsLBTaylorDO2003The Registry of the International Society for Heart and Lung Transplantation: Twentieth official adult lung and heart-lung transplant report – 2003J Heart Lung Trans2262535
- TsaiWCRodriguezMLYoungKS2004Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infectionAm J Respir Crit Care Med1701331915361366
- TsaiWCStandifordTJ2004Immunomodulatory effects of macrolides in the lung: lessons from in-vitro and in-vivo modelsCurr Pharm Des1030819315544499
- TsangKWChanKNHoPL2000Sputum elastase in steady-state bronchiectasisChest117420610669685
- TsangKWNgPHoPL2003Effects of erythromycin on Pseudomonas aeruginosa adherence to collagen and morphology in vitroEur Respir J21401612661992
- TsangKWTHoPIChanKN1999A pilot study of low-dose erythromycin in bronchiectasisEur Resp J133614
- TsoumakidouMTzanakisNChrysofakisG2005Nitrosative stress, hemo oxygenase-1 expression and airway inflammation during severe exacerbations of COPDChest1271911815947302
- TuratoGZuinRMiniatiM2002Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysemaAm J Respir Crit Care Med1661051012091179
- VanderkooiOGLowDEGreenK2005Predicting antimicrobial resistance in invasive pneumococcal infectionsClin Inf Dis40128897
- VillagrasaVBertoLCortijoJ1997Effects of erythromycin on chemoattractant-activated human polymorphonuclear leukocytesGen Pharmac296059
- WagnerTSoongSSokolS2005Effects of azithromycin on clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patientsChest1282162016002938
- WallaceMRMillerLKNguyenMT1994Ototoxicity with azithromycinLancet3432417904701
- WardMMJavitzHSSmithWM2000Direct medical cost of chronic obstructive pulmonary disease in the U.S.AResp Med9411239
- WedzichaJA2000The heterogeneity of chronic obstructive pulmonary diseaseThorax5631210899236
- WedzichaJA2002Exacerbations. Etiology and pathophysiologic mechanismsChest121136S41S12010842
- WedzichaJA2004Role of viruses in exacerbations of chronic obstructive pulmonary diseaseProc Am Thorac Soc11152016113423
- WhiteAJGompertzSBayleyDL2003Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitisThorax58680512885984
- WilkinsonTMADonaldsonGCHurstJR2004Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary diseaseAm J Resp Crit Care Med169129830314990395
- WilsonR2005Treatment of COPD exacerbations: antibioticsEur Respir Rev14328
- WilsonRAllegraLHuchonG2004Short-term and Long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitisChest1259536415006954
- WilsonRKubinRBallinI1999Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitisJ Antimicrob Chemother445011310588312
- WilsonRSchentagJJBallP2002A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomesClin Ther246395212017408
- WolterJSeeneySBellS2002Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trialThorax57212611867823
- WoodheadMBlasiFEwigS2005Guidelines for the management of adult lower respiratory tract infectionsEur Respir J26113980
- WoutersEFM2003Economic analysis of the Confronting COPD survey: an overview of resultsRespir Med97Suppl CS31412647938
- WoutersEFM2005Local and systemic inflammation in chronic obstructive pulmonary diseaseProc Am Thorac Soc2263316113466
- WozniakDJKeyserR2004Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosaChest1252 Suppl
- YamamotoM1991Therapeutic effect of erythromycin in patients with DPB-double blind trial (Japanese)Annual report on the study of diffuse disseminated interstitial lung disease, Grant-in-aid from the Ministry of Health and Welfare in Japan1820
- YamasawaHOshikawaKOhnoS2004Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor releaseAm J Respir Cell Mol Biol305697514551160
- YasunamiJHayashiS2001Fourtenn-membered ring macrolides as anti-angiogenic compoundsAnticancer Res214253811908678
- YiKSethiSMurphyTF1997Human immune response to non-typable Haemophilus influenzae in chronic bronchitisJ Infect Dis1761247529359725
- Zalewska-KaszubskaJGorskaD2001Anti-inflammatory capabilities of macrolidesPharmacol Res44451411735349
- ZhuJQiuYSMajumdarS2001Exacerbations of bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractantsAm J Respir Crit Care Med1641091611435248