Abstract
Background:
Traditional NSAIDs (tNSAIDs) and COX-2 inhibitors (COX-2s) are important agents for the treatment of a variety or arthritic conditions. The purpose of this study was to systematically review the effectiveness of misoprostol, H2-receptor antagonists (H2RAs), and proton pump inhibitors (PPIs) for the prevention of tNSAID related upper gastrointestinal (GI) toxicity, and to review the upper gastrointestinal (GI) safety of COX-2s.
Methods:
An extensive literature search was performed to identify randomized controlled trials (RCTs) of prophylactic agents used for the prevention of upper GI toxicity, and RCTs that assessed the GI safety of the newer COX-2s. Meta-analysis was performed in accordance with accepted techniques.
Results:
39 gastroprotection and 69 COX-2 RCTs met inclusion criteria. Misoprostol, PPIs, and double doses of H2RAs are effective at reducing the risk of both endoscopic gastric and duodenal tNSAID-induced ulcers. Standard doses of H2RAs are not effective at reducing the risk of tNSAID-induced gastric ulcers, but reduce the risk of duodenal ulcers. Misoprostol is associated with greater adverse effects than the other agents, particularly at higher doses. COX-2s are associated with fewer endoscopic ulcers and clinically important ulcer complications, and have fewer treatment withdrawals due to GI symptoms than tNSAIDS. Acetylsalicylic acid appears to diminish the benefit of COX-2s over tNSAIDs. In high risk GI patients, tNSAID with a PPI or a COX-2 alone appear to offer similar GI safety, but a strategy of a COX-2 with a PPI appears to offer the greatest GI safety.
Conclusion:
Several strategies are available to reduce the risk of upper GI toxicity with tNSAIDs. The choice between these strategies needs to consider patients’ underlying GI and cardiovascular risk.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to treat arthritis, menstrual, musculoskeletal and post-operative pain, as well as headache and fever. NSAIDs include acetylsalicylic acid (ASA), traditional NSAIDs (tNSAIDs) (eg, diclofenac, ibuprofen, indomethacin, and naproxen) and inhibitors of the COX-2 isoform of cyclo-oxygenase (referred to here as COX-2s, eg, celecoxib, lumiracoxib, etoricoxib, rofecoxib).
One cohort study found that about 25% of Canadians in 2001 were prescribed short-term NSAIDs (a rise of 28% over 1999 when COX-2s were first introduced), and about 4% were prescribed these agents long-term (defined in this study as ≥6 months);Citation1 this equates to approximately 6.2 million short-term users, and 1.0 million long-term users of NSAID therapy. However, this substantially underestimates the true magnitude of NSAID uses since it does not include use of over the counter NSAIDs. A US cohort study, reported the point prevalence of daily prescription NSAID use as 8.7% between 2002 and 2003 with 46% being COX-2s.Citation2 Low-dose ASA is extensively used for cardiovascular risk reduction.
There are increasing concerns over the risks of gastrointestinal and cardiovascular adverse events with these medications. The increased risks of upper gastrointestinal ulcers and complications with tNSAIDs and ASA are well documented,Citation3–Citation7 and while the risks are reduced by about 50% with COX-2s, they continue to be important since this risk is not reduced to baseline.Citation8–Citation10 Furthermore with the introduction of COX-2s in the late 1990, overall NSAID prescriptions rose with COX-2s overtaking tNSAIDs suggesting that individuals not previously on NSAIDs were being prescribed COX-2s. Over the same time frame, there was a 75.9% increase in the rate of non-fatal digestive perforations and hemorrhages in the presence of NSAIDs. Moreover, the benefits of COX-2s are attenuated when COX-2s are co-prescribed with ASACitation10 although to a lesser extent than when tNSAIDs are co-prescribed with ASA. In addition, extensive data associate COX-2s and non-naproxen tNSAIDs with an increased risk of cardiovascular events,Citation11,Citation12 which has led regulatory authorities to introduce warning statements and advisories Additionally, the COX-2s, rofecoxib, valdecoxib, and lumiracoxib have been withdrawn from the market because of cardiovascular, cutaneous, and hepatic adverse events respectively.Citation1,Citation2,Citation13–Citation15 Health Canada and the Food and Drug Administration (FDA) require the product information for tNSAIDs and COX-2s to include a warning of the increased incidence of cardiovascular (eg, heart attack, stroke) and gastrointestinal (eg, ulcer, bleeding) adverse events, as well as recommendations to limit use of the drug to the lowest effective dose for the shortest possible duration of treatment.Citation2,Citation15
The purpose of this study was to systematically review the literature on interventions to prevent tNSAID related upper gastrointestinal (GI) toxicity, and on the GI safety of COX-2s.
Methods
This review was conducted in accordance with the methods of the Cochrane Collaboration.Citation16
Literature search strategy
The search strategy and methods have been previously described elsewhere. These were updated to May 2009.Citation10,Citation17
Inclusion criteria
Types of studies
RCTs of COX-2s (celecoxib [Celebrex®], rofecoxib [Vioxx®], etoricoxib [Arcoxia®], valdecoxib [Bextra®], lumi-racoxib [Prexige®]) were considered eligible for inclusion if the upper GI toxicity of these agents was compared to that of a non-selective NSAID or to placebo. RCTs of prostaglandin analogues (misoprostol), H2-receptor antagonists (H2RA), and proton pump inhibitors (PPI) in the prevention of NSAID-induced upper GI toxicity were also considered if these agents were used alongside an NSAID compared to an NSAID alone. Further the RCTs had to meet the following additional criteria.
Participants were 18 years or older and had osteoarthritis, rheumatoid arthritis or another arthritic condition; NSAID exposure was 4 weeks or longer (chronic NSAID exposure); the proportion of patients with endoscopic ulcers, significant clinical GI events (eg, perforation, obstruction, bleeding, symptomatic ulcers), and/or symptom based clinical events (adverse GI symptoms, withdrawals due to GI symptoms) could be determined; endoscopic ulcers were defined as being at least 3 mm in diameter or could be distinguished from erosions based on the authors’ descriptions; and it was noted whether endoscopy was performed based on symptoms or as part of a protocol.
Types of interventions
The interventions included the following COX-2s: celecoxib (Celebrex®), rofecoxib (Vioxx®), etoricoxib (Arcoxia®), valdecoxib (Bextra®), lumiracoxib (Prexige®). For this review, low-dose COX-2s were defined as celecoxib 200 mg bid or less, rofecoxib 25 mg daily or less, etoricoxib 60 mg daily or less, valdecoxib 10 mg daily or less, and lumiracoxib 100 to 200 mg. High-dose COX-2s were defined as celecoxib 400 mg bid, rofecoxib 50 mg daily, etoricoxib 90 mg daily or more, valdecoxib 20 mg daily or more, and lumiracoxib 400 mg or more. For prophylaxis against tNSAID induced upper GI toxicity we included: the prostaglandin antagonist misoprostol (Cytotec®) (low dose 400 μg/day, intermediate dose 600 μg/day; high dose 800 μg/day); the PPIs omeprazole, esomeprazole, pantoprazole, and lansoprazole (Losec®, Nexium®, Pantoloc®, Prevacid®, respectively); and the H2RAs cimetadine, ranitidine, nizatidine, and famotidine (Tagamet®, Zantac®, Axid®, and Pepcid®, respectively). Double doses of H2RAs were defined as a dose equivalent to or greater than 300 mg of ranitidine twice daily, and standard dose of PPIs were considered the equivalent of 20 mg of omeprazole once daily.
Types of outcome measures
The primary outcomes were: endoscopically detected ulcer in endoscopy trials; and clinical GI events. Clinically important adverse events were categorized in two ways: 1) strict ulcer complications, which are referred to as “POB” (for perforation, obstruction or bleeding), and 2) ulcer complications and/or ulcer-related symptoms that lead to the identification of an ulcer (so called symptomatic ulcer), which are referred to as “PUB” (for perforation, obstruction, bleeding or the presence of a symptomatic ulcer). Efficacy/tolerability trials were defined as studies that focused on clinical efficacy or effectiveness of COX-2s but also reported on adverse symptoms or other clinical adverse events. Secondary outcomes were: adverse GI symptoms (dyspepsia, nausea, abdominal pain, or diarrhea); and treatment withdrawals due to GI symptoms.
Quality assessment
All RCTs were scored for quality by 2 independent reviewers using the Jadad scale.Citation18 The quality of allocation concealment was also assessed.Citation19 Differences were resolved by consensus.
Statistical analysis
Data were analyzed using Review Manager (RevMan) version 5.0. Endoscopic, clinical and symptom-based outcomes were analyzed separately. The primary analyses were expressed as relative risks using a fixed effects model. A random-effects model was used to combine “heterogeneous trials” only if it was clinically and statistically appropriate. The absolute risk reduction (ARR) was calculated for appropriate clinical endpoints.
Subgroup analyses
Studies were grouped by interventions (eg, COX-2s vs tNSAIDs, and COX-2s vs placebo), dosage (low-dose and high-dose), and duration of therapy. Additionally, within each of the three main outcome analyses (endoscopic ulcer, clinical ulcer, and symptoms), studies were analyzed as all COX-2s vs all tNSAIDs, individual COX-2s vs all comparator tNSAIDs, individual tNSAIDs vs all comparator COX-2s, and individual COX-2s vs individual tNSAIDs.
Heterogeneity
Sources for clinical and statistical heterogeneity were sought prior to statistical analyses. Logical analyses subgroups were created (see above) to allow for more homogeneous analyses groups. Heterogeneity was tested using the I2 statistic and a chi-square test. An I2 > 50% or a chi-square p value of less than 0.10 is considered to be evidence of statistical heterogeneity.Citation20
Sensitivity analyses
In addition to the published reports, unique studies were identified from the FDA web site, and in the form of published “combined analyses” studies. The latter studies combined published and unpublished primary patient data from the endoscopic studies, as well as the safety and tolerability studies to allow sample sizes large enough to comment on clinical ulcer complications. We carefully examined these studies by their ID number, their sample size, patient demographics and list of authors and cross referenced with the FDA web site in order to ensure that their use in the ulcer complication analyses would not create duplication of individual patient data. Sensitivity analyses were conducted removing or adding FDA studies, and the combined analyses studies. Additionally, sensitivity analyses were used to assess the impact of supplemental FDA data on published study results when available (eg, CLASS study). Sensitivity analysis was also performed removing studies with quality scores of 2 or less.
Results
Part I – tNSAID prophylaxis
Of a total of 1205 references with 256 being potentially relevant, 39 RCTs met the inclusion criteria: 23 misoprostol trials (includes 6 head to head studies); 12 H2RA (9 standard dose, 3 double dose, 1 head to head); and 9 PPI trials (6 direct, 5 head to head). Some studies considered more than one active intervention. summarizes the characteristics of the included studies. Effects of interventions are summarized below.
Table 1 Included studies of gastro-protection
Misoprostol
We found 23 studies that assessed the long term effect of misoprostol on the prevention of tNSAID ulcers.Citation14,Citation21–Citation42
Endoscopic ulcers
Eleven studies with 3,641patients compared the incidence of endoscopic ulcers, after at least 3 months, of misoprostol to that of placebo.Citation21,Citation22,Citation25,Citation29–Citation33,Citation36,Citation38,Citation42 The cumulative incidence of endoscopic gastric and duodenal ulcers with placebo were 15% and 6% respectively. Misoprostol (any dose combined) significantly reduced the relative risk of gastric ulcer and duodenal ulcers by 74% relative risk [RR] 0.26; 95% confidence interval [CI] 0.17 to 0.39, random effects), and 58% (RR 0.42; 95% CI 0.22 to 0.81, random effects). These relative risks correspond to a 12.0%, and 3% absolute risk reductions for gastric and duodenal ulcers respectively. The observed heterogeneity in these estimates was due to inclusion of all misoprostol doses in the analyses. Analysis of the misoprostol studies stratified by dose eliminated this heterogeneity.
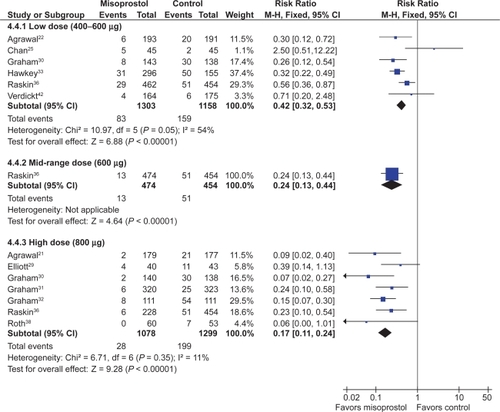
Analysis by dose
All the studied doses of misoprostol significantly reduced the risk of endoscopic ulcers, and a dose response relationship was demonstrated for endoscopic gastric ulcers. Six studies with 2,461 patients used misoprostol 400 μg.Citation22,Citation25,Citation30,Citation33,Citation36,Citation42 1 study with 928 patients used 600 μg daily,Citation36 and 7 with 2,423 patients used 800 μg daily.Citation21,Citation29–Citation32,Citation36,Citation38 Misoprostol 800 μg daily was associated with the lowest risk (RR 0.17; 95% CI 0.11 to 0.24) of endoscopic gastric ulcers when compared to placebo, whereas misoprostol 400 ug daily was associated with a relative risk of 0.42 (95% CI 0.28 to 0.67, random effects model for heterogeneity) (). This difference between high- and low-dose misoprostol reached statistical significance (P0.0055). The intermediate misoprostol dose (600 μg daily) was not statistically different from either the low or high dose. The pooled relative risk reduction of 78% (4.7% absolute risk difference, RR 0.21; 95% CI 0.09 to 0.49) for duodenal ulcers with misoprostol 800 μg daily was not statistically different from those of the lower daily misoprostol dosages.
Studies including data with less than 3 months tNSAID exposure
Eight studies, with 2,206 patients, assessed the rates of endoscopic ulcers with misoprostol compared to placebo at 1 to 1.5 months.Citation14,Citation23,Citation24,Citation26,Citation28,Citation29,Citation34,Citation39 The pooling of these studies revealed an 81% relative risk reduction of gastric ulcers with misoprostol (RR 0.17; 95% CI 0.09 to 0.31) and an 72% relative risk reduction of duodenal ulcers (RR 0.28; 95% CI 0.14 to 0.56).
One study compared misoprostol to a newer cytoprotective agent, dosmafate, for tNSAID prophylaxis and found no statistically significant difference in ulcer rates between the two agents.Citation27
Clinical ulcers
Only 1 RCT, the MUCOSA trial, evaluated the efficacy of misoprostol prophylaxis against clinically important TNSAID induced ulcer complications as the powered primary endpoint. In this study, of 8,843 patients studied over 6 months, the overall GI event incidence was about 1.5% per year.Citation40 Misoprostol 800 μg/day was associated with a statistically significant 40% risk reduction (odds ratio0.598; 95% CI 0.364 to 0.982) in combined GI events (P0.049), representing a risk difference of 0.38% (from 0.95% to 0.57%).
Adverse effects
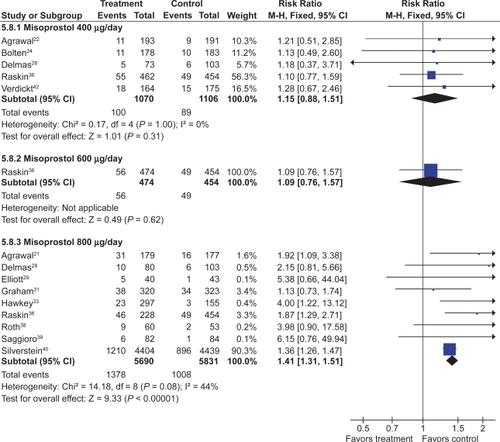
Misoprostol was associated with a small but statistically significant 1.6 fold excess risk of drop out due to drug induced side effects, and an excess risk of drop-outs due to nausea (RR 1.30; 95% CI 1.08 to 1.55), diarrhea (RR 2.36; 95% CI 2.01 to 2.77), and abdominal pain (RR 1.36; 95% CI 1.20 to 1.55). In the MUCOSA trial, 732 out of 4,404 patients on misoprostol experienced diarrhea or abdominal pain, compared to 399 out of 4,439 on placebo for a relative risk of 1.82 associated with misoprostol (P < 0.001). Overall 27% of patients on misoprostol experienced one or more side effects.Citation40
When analyzed by dose, only misoprostol 800 μg daily showed a statistically significant excess risk of drop-outs due to diarrhea (RR 2.45; 95% CI 2.09 to 2.88), and abdominal pain (RR 1.38; 95% CI 1.17 to 1.63). Both misoprostol doses were associated with a statistically significant risk of diarrhea. However, the risk of diarrhea with 800 μg/day (RR 3.25; 95% CI 2.60 to 4.06) was significantly higher than that seen with 400 μg/day (RR 1.81 95% CI 1.52 to 2.16) (P0.0012). The results for overall dropouts due to symptoms analyzed by dose are shown in .
H2RAs
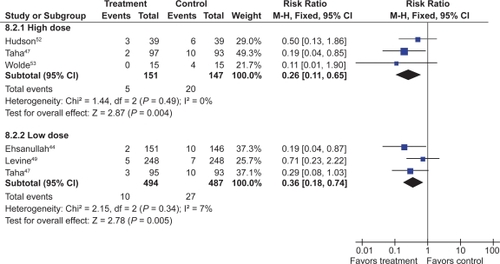
Seven trials with over 900 patients assessed the effect of standard dose H2RAs on the prevention of endoscopic tNSAID ulcers at 1 month,Citation43–Citation48 and 5 trials with 1,005 patients assessed these outcomes at 3 months or longer.Citation44,Citation47,Citation49–Citation51 Standard dose H2RAs are effective at reducing the risk of duodenal ulcers (RR 0.24; 95% CI 0.10 to 0.57, and RR 0.36; 95% CI 0.18 to 0.74 at 1 and 3 or more months respectively), but not of gastric ulcers (NS). One study did not have a placebo comparator and was not included in the pooled estimate.Citation51
Three RCTs with 298 patients assessed the efficacy of double dose H2RA for the prevention of tNSAID induced upper GI toxicity.Citation47,Citation52,Citation53 Double-dose H2RAs when compared to placebo were associated with a statistically significant reduction in the risk of both duodenal (RR 0.26; 95% CI 0.11 to 0.65) and gastric ulcers (RR 0.44; 95% CI 026 to 0.74). This 56% relative risk reduction in gastric ulcer corresponds to a 12% absolute risk difference (from 23.1% to 11.3%) ( and ). Analysis of the secondary prophylaxis studies alone yielded similar results.
Figure 3 H2RAs compared to placebo for the prevention of gastric ulcer. Analysis by dose in studies of 12 weeks or longer duration.
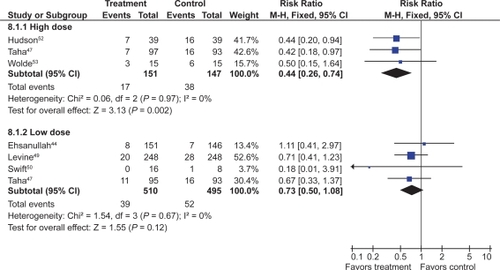
Figure 4 H2RAs compared to placebo for the prevention of duodenal ulcer. Analysis by dose in studies of 12 weeks or longer duration.
Symptoms
H2RA, in standard or double doses, were not associated with an excess risk of total drop-outs, dropouts due to side effects, or symptoms compared to placebo. However, high-dose H2RAs significantly reduced symptoms of abdominal pain when compared to placebo (RR 0.57, 95% CI 0.33 to 0.98).
PPIs
Six RCTs with 1,259 patients assessed the effect of PPIs on the prevention of NSAID-induced upper GI toxicity.Citation32,Citation33,Citation54–Citation57
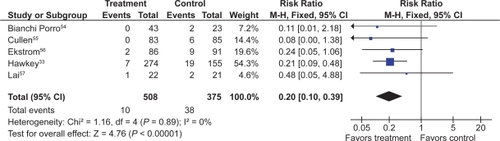
PPIs significantly reduced the risk of both endoscopic duodenal (RR 0.20; 95% CI 0.10 to 0.39) and gastric ulcers (RR 0.39; 95% CI 0.31 to 0.50) compared to placebo ( and ).Citation32,Citation33,Citation54–Citation57 The results were similar for both primary and secondary prophylaxis trials.
Figure 5 Proton pump inhibitors compared to placebo for the prevention of gastric ulcer in studies of 8 weeks or longer duration.
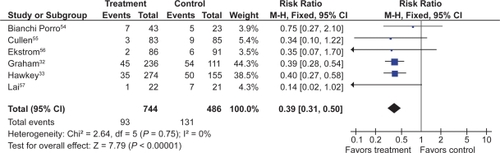
Figure 6 Proton pump inhibitors compared to placebo for the prevention of duodenal ulcer in studies of 8 weeks or longer duration.
Symptoms
Four omeprazole trials used the same composite endpoints to define treatment success.Citation33,Citation55,Citation56,Citation58 In these trials omeprazole significantly reduced “dyspeptic symptoms” as defined by the authors. In the combined analysis, drop-outs overall (RR 0.89; 95% CI 0.62 to 1.29) and drop-outs due to side effects (RR 1.20; 95% CI 0.66 to 2.15) were not different from placebo.
Head to head comparisons of gastroprotective agents
Misoprostol vs H2RAs
Two trials with 600 patients compared misoprostol (400 to 800 μg) to ranitidine 150 mg twice daily.Citation36,Citation41 Misoprostol appears superior to standard dose ranitidine for the prevention of tNSAID induced gastric ulcers (RR 0.12; 95% CI 0.03 to 0.51) but not for duodenal ulcers (RR 1.00; 95% CI 0.14 to 7.14).
PPI vs H2RAS
Yeomans et al in a 12-week study of 425 patients, compared omeprazole 20 mg daily to ranitidine 150 mg twice daily for tNSAID prophylaxis (various tNSAIDs used).Citation58 In this study, omeprazole was superior to standard-dose ranitidine for the prevention of both gastric (RR 0.32; 95% CI 0.17 to 0.62) and duodenal ulcers (RR 0.11; 95% CI 0.01 to 0.89).
PPI vs misoprostol
Four trials with a total of 1,478 patientsCitation13,Citation32,Citation33,Citation35 compared a PPI to misoprostol. Two studies compared low-dose misoprostol (400 μg) daily to a standard-dose PPICitation13,Citation33 while the Graham study compared high-dose misoprostol (800 μg) to lansoprazole 15 or 30 mg daily. PPIs are superior to misoprostol for the prevention of duodenal (RR 0.25; 95% CI 0.11 to 0.056), but not gastric (RR 1.61; 95% CI 0.88 to 3.06, random effects) or total gastroduodenal ulcers (RR 0.90; 95% CI 0.47 to 1.72, random effects).
Symptoms
In the two head to head comparison of omeprazole and misoprostol,Citation32,Citation33 PPIs were associated with significantly less drop-outs overall (RR 0.71; 95% CI 0.52 to 0.97), as well as significantly less drop-outs due to side effects (RR 0.48; 09% CI 0.29 to 0.78). Compared to H2RA used for less than 2 months, misoprostol caused significantly more drop-outs due to abdominal pain (RR 3.00, 95% CI 1.11 to 8.14) and more symptoms of diarrhea (RR 2.03, 95% CI 1.38 to 2.99). There were no significant differences in drop-outs due to side effects (RR 1.90, 95% CI 0.77 to 4.67) or symptoms of abdominal pain or diarrhea between low-dose H2RAs and PPIs.
Part II – COX-2 inhibitors
The search strategy identified 1,169 studies. Of these, 255 references were rated as potentially relevant and the full articles were retrieved. Sixty studies met the inclusion criteria, including 4 unique studies obtained from the new drug submission documents on the FDA web site.Citation59–Citation63 An additional 5 “combined analyses studies” were identified by the search strategy and were included for the clinical ulcer complication endpoint ().Citation64–Citation68
Table 2 COX-2 included studies
Quality scores of the 60 included trials ranged between 4 to 5 in 47 and between 2 to 3 in 22 studies. Removal of quality score 2 studies did not influence overall results. The use of allocation concealment was implied in all of the included trials, but was adequately described in only 6 studies.
Endoscopic ulcers were the measured endpoints of 17 studies.Citation59–Citation61,Citation63,Citation69–Citation81 Eleven COX-2 studies,Citation78,Citation82–Citation91 and 5 combined analysesCitation65–Citation68,Citation92 reported on the outcome of clinical GI events (POBs or PUBs).
The remaining trials were either safety or tolerability studies or examined the clinical efficacy of COX-2s compared to tNSAIDS, but allowed for extraction of GI tolerability data.Citation62,Citation67,Citation88,Citation93–Citation111 FDA study data are only presented as part of sensitivity analyses. Results specifically pertaining to meloxicam are not included herein.
Endoscopic ulcer trials
CoX-2s vs non-selective NSAIDs
Seventeen studies with over 10,000 patients assessed the proportion of patients who developed endoscopic ulcers while taking a COX-2 compared to those taking a tNSAID.Citation59–Citation61,Citation63,Citation69–Citation79,Citation81 Seven studies assessed celecoxib,Citation59,Citation60,Citation69–Citation71,Citation75,Citation81 3 assessed rofecoxib,Citation72–Citation74 2 assessed etoricoxib,Citation78,Citation79 5 that assessed valdecoxib,Citation61,Citation63,Citation76,Citation77,Citation80 and 2 assessed lumiracoxib.Citation75,Citation81 Some studies assessed more than one intervention.Citation75,Citation81
Endoscopically detected gastro-duodenal ulcers
Thirteen studies with a total of 7,839 patients showed a 74% relative risk reduction (RRR) in combined gastro-duodenal ulcers with COX-2s vs tNSAIDs (RR 0.26; 95% CI 0.23 to 0.30).Citation69–Citation80,Citation112 This represented a 16% absolute risk reduction (ARR). Addition of the FDA studies did not significantly alter the results (RR 0.28; 95% CI 0.24 to 0.32). The results analyzed by the dose of COX-2s gave similar results. Results below are for “any dose” combined.
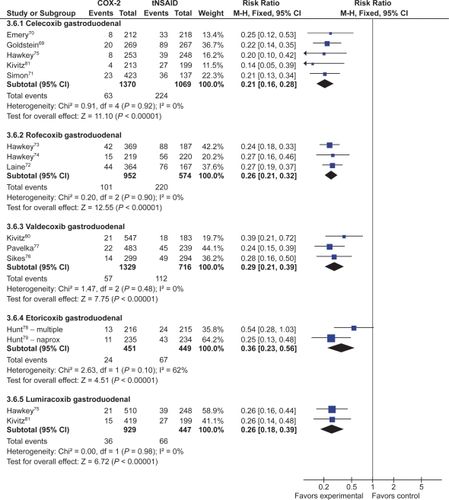
Eleven studies with a total of 6,726 patients compared the safety of a COX-2 to a comparator tNSAID for endoscopic gastric ulcers.Citation69–Citation77,Citation80,Citation112 The use of a COX-2 in this setting was associated with a 79% RRR in gastric ulcers (RR 0.21; 95% CI 0.18 to 0.25) (). This represented a 14% ARR in gastric ulcers with the use of COX-2s compared with tNSAIDs. Addition of the FDA studies did not significantly alter the results (RR 0.26; 95% CI 0.22 to 0.30).
The same 11 studies also compared the proportions of duodenal ulcers that occurred while using a COX-2 vs a tNSAID.Citation69–Citation77,Citation80,Citation112 Compared to using a tNSAID, the use of a COX-2 was associated with a 66% RRR in duodenal ulcers (RR 0.34; 95% CI 0.25 to 0.45) (). This represented a 3% ARR. Addition of the FDA studies did not significantly alter the results (RR 0.29; 95% CI 0.23 to 0.38) Keeping in mind that tNSAID related gastric ulcers were more commonly observed than duodenal ulcer, a trend was observed for greater RRR and ARR in gastric ulcers than for duodenal ulcers with COX-2s, compared to tNSAIDs (RR 0.21 vs 0.34, ARR of 14% vs 3%). This trend was consistent when celecoxib, rofecoxib and valdecoxib were analyzed separately. Analysis by duration The data presented above are for any dose and duration up to 6 months. Subgroup analysis of these studies on the basis of duration (1 to 3 months and 3 to 6 months) did not significantly alter the results.
Analysis by COX-2
Analyses stratified by the individual COX-2s showed that each of the studied agents were safer than comparator tNSAIDs ().
Celecoxib
Five studies with a total of 2,439 patients compared celecoxib to non-selective NSAIDs, showing a 79% RRR in total gastro-duodenal ulcers (RR 0.21; 95% CI 0.16 to 0.28) with celecoxib.Citation69–Citation71,Citation75,Citation112 Similar RRR were observed for gastric ulcers (RR 0.20; 95% CI 0.14 to 0.28) and duodenal ulcers alone (RR 0.29; 95% CI 0.18 to 0.47), as well as when the FDA studies were included (RR 0.26; 95% CI 0.21 to 0.32).
Rofecoxib
Three studies with a total of 1,526 patients compared rofecoxib to non-selective NSAIDs.Citation72–Citation74 In this case, a 74% RRR was seen with rofecoxib (RR 0.26; 95% CI: 0.21 to 0.32). The results were similar when FDA studies were added to the analysis as well as when the analysis was done only for gastric ulcers (RR 0.20; 95% CI 0.15 to 0.26) and duodenal ulcers alone (RR 0.36; 95% CI 0.14 to 0.93, random effects).
Etoricoxib
Two studies, with a total of 900 patients compared etoricoxib to non-selective NSAIDs using the endpoint of endoscopic gastro-duodenal ulcers.Citation78,Citation79 These trials demonstrated a 64% RRR (RR 0.37; 95% CI 0.18 to 0.77, random effects) with etoricoxib.
Valdecoxib
Three studies compared valdecoxib to non-selective NSAIDs in 2,045 patients and demonstrated a 70% RRR in gastro-duodenal ulcers (RR 0.29; 95% CI 0.21 to 0.39) with valdecoxib.Citation76,Citation77,Citation80 Similar RRR were observed when the analysis was done for gastric ulcers (RR 0.24; 95% CI 0.18–0.37) and duodenal ulcers alone (RR 0.39; 95% CI 0.21 to 0.70), and when the FDA studies were included in the gastro-duodenal ulcers analysis (RR 0.30; 95% CI 0.24 to 0.39).
Lumiracoxib
Two studies with a total of 1,376 patients compared lumiracoxib to non-selective NSAIDs.Citation112,Citation113 Lumiracoxib was associated with a 74% RRR in gastro-duodenal ulcers (RR 0.26; 95% CI 0.18 to 0.39). Similar results were observed for gastric ulcers (RR 0.25; 95% CI 0.16 to 0.40) and duodenal ulcers (RR 0.20; 95% CI 0.09 to 0.43) when they were considered alone.
Analysis by comparator NSAIDs
Naproxen
Five studies compared either celecoxib or valdecoxib to naproxen in 2,734 patients. These showed a 75% RRR in endoscopic gastro-duodenal ulcers in favor of the COX-2s (RR 0.25; 95% CI 0.20 to 0.32). Results were similar when the FDA studies were included in the analysis (RR 0.27; 95% CI: 0.22 to 0.32).Citation69,Citation71,Citation74,Citation79,Citation80
Ibuprofen
Six studies which enrolled over 3,800 patients (2 rofecoxib,Citation72,Citation73 1 etoricoxib,Citation78 2 lumiracoxib,Citation112,Citation113 and 1 valdecoxibCitation76) showed a 73% RRR in gastro-duodenal ulcers with COX-2s compared with ibuprofen (RR 0.27; 95% CI 0.23 to 0.32). Results were similar when the FDA studies were included in the analysis (RR 0.28; 95% CI 0.23 to 0.32).
Diclofenac
Three studies which enrolled a total of 1,596 patients demonstrated a 75% RRR in gastro-duodenal ulcers with COX-2s compared to diclofenac (RR 0.25; 95% CI 0.18 to 0.35). This effect was somewhat reduced when the FDA studies were included in the analysis (RR 0.36; 95% CI 0.27 to 0.47).Citation70,Citation76,Citation77
Similar results were obtained when individual COX-2s were compared with the individual non-selective NSAIDs.
COX-2s vs placebo
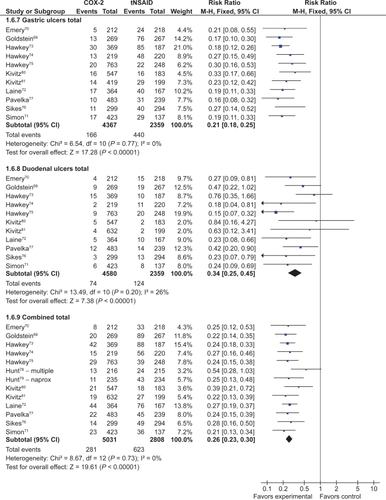
Eight studies with a total of 4,081 patients compared low- and high-dose COX-2s to placebo.Citation71–Citation74,Citation76,Citation78–Citation80 Low dose COX-2s appeared to demonstrate no greater risk of gastric or gastro-duodenal ulcers than placebo. However, high doses of COX-2s appeared to raise the relative risk of gastric (RR 1.22; 95% CI 0.83 to 1.80), duodenal (RR 1.29; 95% CI 0.63 to 2.66), and combined gastro-dudenal ulcers (RR 1.57; 95% CI 0.96 to 2.56, random effects), though these trends missed statistical significance. Clinical GI events COX-2s vs non-selective NSAIDs Nine studies with a total of 94,294 patients assessed the safety of COX-2s by using the clinically important endpoint of ulcer complication, POB.Citation65,Citation66,Citation68,Citation82,Citation83,Citation92,Citation114–Citation116 Three of these trials studied celecoxib,Citation82,Citation92,Citation115 2 studied rofecoxib,Citation66,Citation83 2 trials evaluated etoracoxib,Citation68,Citation116 and 1 each evaluated valdecoxibCitation65 and lumiracoxibCitation114 separately. Overall, the use of these COX-2s was associated with a 57% RRR in POBs (RR, 0.43; 95% CI 0.28 to 0.67, random effects), compared with using tNSAIDs. Removal of the combined analyses studies had no influence on the result (RR 0.39; 0.29 to 0.53) and the inclusion of the FDA 12-month CLASS study dataCitation117 did not alter the results (RR 0.42; 95% CI 0.33 to 0.54). The 60% RRR in these analyses represents an ARR of 0.4% ().
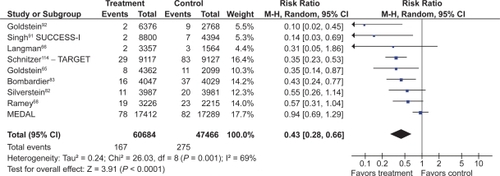
Fourteen studies compared COX-2s with tNSAIDs by using PUB as the study endpoint.Citation65,Citation66,Citation68,Citation78,Citation82,Citation83,Citation87–Citation90,Citation92,Citation114–Citation116 In this analysis, the use of a COX-2 was associated with a 57% RRR in PUBs (RR 0.43; 95% CI 0.34 to 0.55, random effects). Removal of the combined analyses studies eliminated the observed heterogeneity but had little effect on the point estimate (RR 0.49; 95% CI 0.41 to 0.58). Similarly, the use of the FDA CLASS data did not significantly alter the estimate (RR 0.42; 95% CI 0.33 to 0.53, random effects) ().
Analyses stratified by cyclooxygenase-2s
Celecoxib
Four studies with 31,106 assessed the effect celecoxib vs non-selective NSAIDs on clinical GI events (POBs or PUBs).Citation82,Citation89,Citation92 Celecoxib use was associated with a 77% RRR in POBs (RR 0.23; 95% CI 0.07 to 0.76, random effects) and a 61% RRR in PUBs (RR 0.39; 95% CI 0.21 to 0.73, random effects). Removal of the combined analyses studyCitation92 eliminated the heterogeneity observed in both the POB (RR 0.42; 95% CI 0.22 to 0.80) and PUBs (RR = 0.34; 95% CI 0.22 to 0.80) analyses. The use of the FDA 12-month CLASS data did not alter the RR estimates for POBs or PUBs significantly.
Rofecoxib
Four studies with 19,288 patients assessed the effect of rofecoxib vs non-selective NSAIDs on clinical GI events (POBs or PUBs).Citation66,Citation83,Citation88,Citation90 Rofecoxib use reduced the relative risk of POBs by 58% (RR 0.42; 95% CI 0.24 to 0.73) and the relative risk of PUBs by 56% (RR 0.44; 95% CI 0.34 to 0.58). Removal of the combined analysis study did not alter the point estimates.
Valdecoxib
One combined analysis study with 6,461 patients evaluated the effect of valdecoxib on POBs and PUBs.Citation65 Valdecoxib reduced the relative risk of POBs by 65% (RR 0.35; 95% CI 0.14 to 0.87) and the relative risk of PUBs by 77% (RR 0.23; 95% 0.15 to 0.36).
Etoricoxib
Four studies with 10,856 patients evaluated the effect of etoricoxib on POBsCitation68,Citation116 and PUBs.Citation78,Citation87 Etoricoxib demonstrated a nonsignificant trend in reducing the risk of POBs (RR 0.82; 95% CI 0.44 to 1.51, random effects), but it significantly reduced the RR of PUBs by 46% (RR 0.64; 95% CI 0.42 to 0.96).
Lumiracoxib
One study with 18,244 patients demonstrated a significant 64% RRR in POBs (RR 0.36; 95% CI 0.24 to 0.55) and a 44% RRR in PUBs (RR 0.56; CI 0.41 to 0.78) with the use of lumiracoxib, compared with using non-selective NSAIDs.Citation114
Analysis by comparator NSAIDs
In general COX-2s appeared to maintain their safety advantage regardless of the comparator non-selective NSAID. COX-2s were statistically superior to naproxen (RR 0.34; 95% CI 0.24 to 0.48), and ibuprofen (RR 0.46; 95% CI 0.30 to 0.71) for the POB endpoint. The data comparing COX-2s to diclofenac are predominately derived from 2 studies and heavily influenced by the CLASS trial data which showed no significant difference between celecoxib vs diclofenac.Citation82,Citation92 In the current analysis, celecoxib demonstrated a non-significant trend towards fewer POBs than diclofenac (RR 0.31; 95% CI 0.06 to 1.61) while a statistically significant 59% RRR in PUBs was observed (RR 0.41; 95% CI 0.30 to 0.55).
COX-2s vs placebo
There are limited data, mostly derived from the combined analyses studies, comparing COX-2s with placebo for the clinical outcomes of POBsCitation65,Citation92 and PUBs.Citation65,Citation66,Citation87,Citation89,Citation92 In these analyses, the use of COX-2s was associated with non-significant trends toward an increased RR of POBs (RR 2.66; 95% CI 0.34 to 20.95) and PUBs (RR 2.26; 95% CI 0.96 to 5.33) (). These findings are supported by the APPROVe polyp prevention study which demonstrated that over a 3-year period, rofecoxib was associated with a statistically significant 4.9-fold increased risk of clinical ulcer complications compared to placebo.Citation9 This study was not included in the main results since its population did not include arthritis patients.
Influence of acetylsalicylic acid co-administration on clinically important ulcer complications
Five trials allowed assessment of the effects of the co-administration of ASA with a COX-2.Citation65,Citation82,Citation91,Citation114,Citation116 In a pooled subgroup analysis of over 18,000 patients taking ASA, there was no statistically significant difference in the relative risk of ulcer complications (POBs) between those in the COX-2 arms and those in the non-selective arms of these trials (RR 0.93; 95% CI 0.68 to 1.27 for POBs). A small advantage of COX-2s over tNSAIDs cannot be ruled out by these results because this subgroup analysis might be under-powered The PUB analysis showed a statistically significant benefit for COX-2 + ASA vs tNSAID +ASA (RR 0.72; 95% CI 0.62 to 0.95), but data from one study could not be used in this analysis. In more than 40,000 patients in the COX-2 arms, patients taking ASA had a 3.46 (95% CI 2.44 to 4.91) greater relative risk of POBs than COX-2 users not taking ASA. Among 34,000 patients in the tNSAID arms of these studies, those taking ASA had a 1.65 greater relative risk of POBs than those not taking ASA, although this result did not reach statistical significance (95% CI 0.76 to 3.57). One must keep in mind that these are post-hoc subgroup analyses that might be subject to bias. Furthermore, the subgroup analysis within an tNSAID treatment group (eg, COX-2 vs COX-2 + ASA) represents a nonrandomized comparison in which differences could be influenced by factors other than ASA use ( to ).
Figure 11 Clinical ulcers (PUBs [perforation, obstruction, bleeding or the presence of a symptomatic ulcer]) with COX-2 + ASA vs tNSAID + ASA.
![Figure 11 Clinical ulcers (PUBs [perforation, obstruction, bleeding or the presence of a symptomatic ulcer]) with COX-2 + ASA vs tNSAID + ASA.](/cms/asset/bf78bf2b-b273-49c9-922d-171e1891703a/dhps_a_4334_f0011_c.jpg)
Addition of a PPI to COX-2s
The comparative safety of a COX-2s compared to a tNSAID with a PPI has been addressed in high-risk patients with recent ulcer bleeding who were enrolled after ulcer healing and H. pylori eradication. Chan et alCitation118 found recurrent ulcer bleeding at 6 months to be 4.9% with celecoxib 200 mg twice daily and 6.4% with diclofenac 75 mg twice daily plus omeprazole 20 mg daily. Lai et alCitation119 found recurrent ulcer complications (bleeding and 1 case of severe pain) in 3.7% with celecoxib 200 mg daily and 6.3% with naproxen 750 mg daily plus lansoprazole 30 mg daily at a median follow-up of 24 weeks. These results suggest high-risk patients have high rates of recurrent bleeding even with the protective strategy of a coxib or a tNSAID + PPI.
The combination of a coxib and PPI was assessed in the same high-risk population in a subsequent 1-year study by Chan et alCitation120 Recurrent ulcer bleeding occurred in 9% with celecoxib alone vs zero with celecoxib plus twice daily esomeprazole. The MEDAL Program also demonstrated that a coxib plus PPI had significantly fewer upper GI clinical events (again, driven by a decrease in uncomplicated events) than a tNSAID plus PPI (RR 0.62, 0.45 to 0.83).Citation116
Symptoms and treatment withdrawals
Treatment withdrawals as a result of GI side effects: COX-2s vs nonselective NSAIDs.
Twenty-one studies with close to 47,000 patients assessed the effect of COX-2s on patient withdrawals due to GI symptoms.Citation61,Citation69–Citation71,Citation79,Citation82,Citation83,Citation87–Citation90,Citation95,Citation98,Citation101,Citation106,Citation109,Citation110,Citation111,Citation115,Citation121–Citation123 Overall, compared to tNSAIDs, COX-2s were associated with a significantly lower relative risk of withdrawals due to GI side effects (RR 0.65; 95% CI 0.57 to 0.73, random effects), withdrawals due to dyspepsia (RR 0.37; 95% CI 0.18 to 0.74), and due to abdominal pain (RR 0.25; 95% CI 0.13 to 0.49). Compared to placebo, low-dose COX-2s showed no statistically significant difference for these same endpoints, while high-dose COX-2s were associated with a small but significantly increased relative risk of drop-outs due to GI side effects (RR 1.74; 95% CI 1.13 to 2.68).
Adverse GI symptoms with COX-2s compared with non-selective NSAIDs
Twenty-eight studies with close to 60,000 patients assessed the effect of low- or high-dose COX-2s compared to tNSAIDs for treatment related overall GI side effects, dyspepsia, nausea, and abdominal pain.Citation69,Citation70,Citation75–Citation77,Citation82,Citation86,Citation87,Citation89,Citation90,Citation96–Citation98,Citation101,Citation104,Citation106,Citation107,Citation111,Citation112,Citation114,Citation122,Citation124 Low-dose COX-2s were associated with a lower relative risk of GI symptoms (RR 0.78; 95% CI 0.74 to 0.82); dyspepsia (RR 0.83; 95% CI 0.75 to 0.90); nausea (RR 0.72; 95% CI 0.64 to 0.82); and abdominal pain (RR 0.64; 95% CI 0.58 to 0.70). The results for high-dose COX-2s were similar.
Adverse GI symptoms with COX-2s compared with placebo
Twenty studies with over 10,000 patients compared the occurrence of adverse GI symptoms between COX-2s and placebo. Low-dose COX-2s were associated with a slight but statistically significant increased relative risk of overall GI symptoms (RR 1.26; 95% CI 1.13 to 1.42); dyspepsia (RR 1.28; 95% CI 1.08 to 1.51); nausea (RR 1.24; 95% CI 1.01 to 1.53); and abdominal pain (RR 1.24; 95% CI 1.02 to 1.52).Citation76,Citation80,Citation86,Citation87,Citation89,Citation90,Citation94,Citation96,Citation97,Citation99,Citation100,Citation104,Citation106–Citation108,Citation122,Citation123–Citation126 The results for high-dose COX-2s were similar.
Discussion
The results of this systematic review demonstrate that there are several therapeutic strategies available to reduce the incidence of tNSAID related upper GI harms. Large, well powered, studies have shown that strategies using a tNSAID with misoprostol, or the use of a COX-2 instead of a tNSAID, each reduce the incidence of endoscopically detected upper GI ulcerations, and clinically important upper GI events such as bleeding. Misoprostol in doses that prevent upper GI ulcer complications is associated with important adverse effects which may limit its long-term use. Standard doses of H2RAs reduce the incidence of duodenal ulcers but are not effective at reducing the incidence of gastric ulcers. Double doses of H2RAs and standard-dose PPIs reduce the incidence of duodenal as well as gastric ulcers, but because tachyphylaxis can occur with chronic H2RA use, a standard-dose PPI strategy is preferred. H2RAs and PPIs have not been directly assessed in large primary prevention clinical outcome studies powered to detect ulcer complications. However, in secondary prevention studies of high-risk GI patients, tNSAIDs with a PPI appear as effective as a COX-2 strategy at preventing clinical ulcer complications. In these high-risk patients, these strategies were still associated with important ulcer relapse rates, suggesting that both strategies may provide incomplete protection for the secondary prevention of tNSAID-related ulcers. However, a recent study has shown that a strategy of combining a PPI with a COX-2 was superior to a COX-2 alone for the secondary prevention of ulcer complications, suggesting that a COX-2 + PPI strategy is the preferred strategy in high-risk GI patients. Further, the current meta-analysis, supported by the APPROVe polyp prevention study,Citation9 has shown that while COX-2 offer greater GI safety than tNSAIDs as a group, COX-2 are associated with a statistically greater risk of clinical upper GI complications than those taking placebo.
The discovery that COX-2s are associated with important cardiovascular harm has complicated the clinical use of NSAIDs significantly. Further, in Canada, all COX-2 save celecoxib have been withdrawn from the market due to cardiovascular and other harms and it is unlikely that a new COX-2 would be released to market unless it is truly cardiovascularly neutral or it is combined with a GI-safe antithrombotic agent. During this time of uncertainty, when physicians were actively switching patients back to tNSAIDs + a gastropropective agent such as a PPI, it became increasingly clear that non-naproxen tNSAIDs were also associated with important CVS harms.Citation11 A meta-analysis by Kearney et al using an extensive set of RCT data derived from published and unpublished studies has suggested that, as a group, COX-2s are associated with an increased risk of CV outcomes when compared with placebo or naproxen, but not when compared with non-naproxen, non-ASA tNSAIDsCitation11 suggesting that non-naproxen-tNSAIDs share the cardiovascular harms of COX-2s.
In light of the cardiovascular harm data relating to COX-2s, it is tempting to suggest combining these agents with ASA. However, the available data from this meta-analysis suggest that this strategy would likely undermine the GI safety advantage of COX-2s. In patients taking ASA, we found no statistically significant difference in POBs or PUBs in patients randomized to a COX-2 or a tNSAID; however, the analyses did not stratify the randomization for ASA use. Thus, it is possible that other patient-related factors played a role in this result. Furthermore, although the analysis included about 7000 patients, it is still possible that a protective effect of COX-2s over tNSAIDs in this setting is present but not detected because of insufficient statistical power. We also found that the addition of ASA to a COX-2 significantly increased the risk of a POB 4.12-times over a COX-2 alone, and that the addition of ASA to a tNSAID demonstrated a nonsignificant 1.27 increased risk of POBs over the use of a tNSAID alone. One needs to note that these analyses represent nonrandomized comparisons, and that the group sizes were somewhat uneven (more patients in the COX-2 or tNSAID alone groups than in the groups with ASA). Nonetheless, the results are not entirely unexpected, because it has been known for some time that concomitant use of multiple NSAIDs increases the risk of GI complications over a single NSAID alone. These results are also in keeping with an RCT by Laine et alCitation127 revealing that the incidence of endoscopically detected ulcers with rofecoxib and low-dose ASA was not lower than that seen with ibuprofen alone. However, it is clear that further study in this area is required to verify the above findings, such as through a dedicated RCT or from individual patient data systematic reviews. Further, adding ASA to a COX-2 implies that the COX-2s will not interfere with the effect of ASA. However, this hypothesis also requires further study because there are suggestions that the use of a tNSAID might interfere with the action of ASA in this setting, although there appears to be less interference with selective COX-2s.Citation128–Citation132
When COX-2s were released, they promised an era of improved GI safety, as well as an era of greater clinical simplicity, with the option of prescribing a single low risk agent when chronic NSAID use was required. However, with the greater understanding of the GI, cardiovascular, and other end organ safety profile of tNSAIDs and COX-2s, clinicians must now stratify their patients on the basis of GI, cardiovascular, and other organ system risk factors and choose an NSAID strategy, that minimizes a patient’s overall risk. This has become especially difficult, for patients who are know to be at high risk of GI and cardiovascular harms.
When considering the treatment of an arthritic patient with a tNSAID or a COX-2, a clinician must consider the patient’s underlying GI, cardiovascular, and other organ risks factors. Further, low-dose ASA is recommended for patients at increased cardiovascular risk;Citation133,Citation134 therefore an algorithm considering-high cardiovascular risk patients needs to assume the use of low-dose ASA in such patients. The recent Canadian Consensus Conference on NSAIDs proposed the following recommendations;Citation135 For patients with both low GI and cardiovascular risk, a tNSAID alone may be acceptable. For patients with low GI risk and high cardiovascular risk, naproxen may be preferred because of the potential lower cardiovascular risk than with other tNSAIDs or COX-2s. However, since these patients are assumed to be on low-dose ASA therapy, the combination of naproxen plus ASA would increase the GI risk, and therefore, the addition of a gastro-protective agent such as a PPI should be considered.
Long-term NSAID therapy can be more complex in patients with high GI risk. Testing for and eradicating Helicobacter pylori in patients at high risk of NSAID-related GI bleeding should be considered but will be insufficient without ongoing gastroprotection.Citation57,Citation136–Citation139 In these patients, if cardiovascular risk is low, a COX-2 alone or a tNSAID with a PPI appear to offer similar protection from recurrent GI bleeding, but this protection is incomplete. Therefore, for patients at very high risk of upper GI events, a combination of a COX-2 plus a PPI may offer the best GI safety profile. When both GI and cardiovascular risks are high, the optimal strategy is to avoid NSAID therapy if at all possible. If the NSAID therapy is deemed necessary, then the clinician must prioritize the cardiovascular and GI risks, recognizing that these patients are likely taking ASA for their cardiovascular risk. If GI risk is the primary concern (ie, a very high-risk GI patient), a COX-2 plus a PPI is recommended. If the primary concern is cardiovascular risk, naproxen plus a PPI in patients on ASA would be preferred; however, GI risk should be closely monitored, as this strategy carries a higher GI risk than a COX-2 plus a PPI in patients on ASA.Citation135
Disclosures
Dr. Rostom participated in an AstraZeneca advisory board in 2008. Dr. Lanas is or has been involved in advisory boards of studies sponsored by Pfizer, AstraZeneca and Bayer, and has also received funds for institutional research from Pfizer and AstraZeneca. In the past 5 years Dr. Tugwell has acted as a paid consultant for: AstraZeneca, Bristol-Myers Squibb, Chelsea, Eli Lilly, GlaxoSmithKline, Merck & Co, Pennside, Pfizer, Scios, Solvay, UCB, and Wyeth Ayerst. The other authors report no conflicts of interest.
References
- BarnardLLavoieDLajeunesseNIncrease in nonfatal digestive perforations and haemorrhages following introduction of selective NSAIDs: a public health concernDrug Saf200629761362016808553
- RoumieCLArbogastPGMitchelEFJrGriffinMRPrescriptions for chronic high-dose cyclooxygenase-2 inhibitors are often inappropriate and potentially dangerousJ Gen Intern Med2005201087988316191131
- GarciaRLJickHRisk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugsLancet199434389007697727907735
- DerrySLokeYKRisk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysisBMJ200032172701183118711073508
- RostomAWellsGTugwellPWelchVDubeCMcGowanJThe prevention of chronic NSAID induced upper gastrointestinal toxicity: a Cochrane collaboration metaanalysis of randomized controlled trialsJ Rheumatol20002792203221410990235
- RostomADubéCJolicoeurEBoucherMJoyceJ Gastroduodenal ulcers associated with the use of non-steroidal anti-inflammatory drugs: a systematic review of preventive pharmacological interventions. Technology report no 37. 2003. Ottawa, Canadian Coordinating Office for Health Technology Assessment.
- RostomAMoayyediPHuntRSystematic Review: Canadian Consensus guidelines on long-term NSAID therapy and the need for gastroprotectionAliment Pharmacol Ther200929548149619053986
- LanasABaronJASandlerRSPeptic ulcer and bleeding events associated with rofecoxib in a 3-year colorectal adenoma chemoprevention trialGastroenterology2007132249049717258718
- BaronJASandlerRSBresalierRSA randomized trial of rofecoxib for the chemoprevention of colorectal adenomasGastroenterology200613161674168217087947
- RostomAMuirKDubeCGastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic reviewClin Gastroenterol Hepatol20075781882817556027
- KearneyPMBaigentCGodwinJHallsHEmbersonJPatronoCDo selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of athero-thrombosis? Meta-analysis of randomised trialsBMJ20063321302130816740558
- McGettiganPHenryDCardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2JAMA2006296131633164416968831
- StupnickiTDietrichKGonzalez-CarroPEfficacy and tolerability of pantoprazole compared with misoprostol for the prevention of NSAID-related gastrointestinal lesions and symptoms in rheumatic patientsDigestion200368419820814707396
- MeloGARothSHZeehJBruynGAWoodsEMGeisGSDouble-blind comparison of efficacy and gastroduodenal safety of diclofenac/misoprostol, piroxicam, and naproxen in the treatment of osteoarthritisAnn Rheum Dis199352128818858311540
- WatsonDJRahmeESantanelloNCIncrease in nonfatal digestive perforations and haemorrhages following introduction of selective NSAIDs: a public health concernDrug Saf2007301899017194174
- HigginsJGreenS Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Higgins J, Green S, editors. The Cochrane Collaboration. 2008. The Cochrane Collaboration.
- RostomAWellsGTugwellPWelchVDubeCMcGowanJPrevention of NSAID-induced gastroduodenal ulcers [update of Cochrane Database Syst Rev. 2000;(3):CD002296; 10908548.].Cochrane Database Syst Rev20024CD00229612519573
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials19961711128721797
- SchulzKChalmersIHayesRAltmanDEmperical evidence of bias: dimension of the methodological quality associated with estimates of treatment effects in controlled trialsJAMA19952734084127823387
- PetittiDApproaches to heterogeneity in meta-analysisStat Med20012023224233
- AgrawalNMRothSGrahamDYMisoprostol compared with sucralfate in the prevention of nonsteroidal anti-inflammatory drug-induced gastric ulcer. A randomized, controlled trialAnn Intern Med199111531952001905501
- AgrawalNMVanKHErhardtLJGeisGSMisoprostol coadministered with diclofenac for prevention of gastroduodenal ulcers. A one-year studyDig Dis Sci1995405112511317729275
- BocanegraTSWeaverALTindallEADiclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or hip: a randomized, placebo controlled trial. Arthrotec Osteoarthritis Study GroupJ Rheumatol1998258160216119712107
- BoltenWGomesJASteadHGeisGSThe gastroduodenal safety and efficacy of the fixed combination of diclofenac and misoprostol in the treatment of osteoarthritisBr J Rheumatol199231117537581450797
- ChanFKSungJJChingJYRandomized trial of low-dose misoprostol and naproxen vs. nabumetone to prevent recurrent upper gastrointestinal haemorrhage in users of non-steroidal anti-inflammatory drugsAliment Pharmacol Ther2001151192411136274
- ChandrasekaranANSambandamPRLalHMDouble blind, placebo controlled trial on the cytoprotective effect of misoprostol in subjects with rheumatoid arthritis, osteoarthritis and seronegative spondarthropathy on NSAIDsJ Assoc Physicians India199139129199211816217
- Cohen de LaraAGompelHBaranesCTwo comparative studies of dosmalfate vs misoprostol in the prevention of NSAID-induced gastric ulcers in rheumatic patientsDrugs Today200036Suppl A7378
- DelmasPDLambertRCapronMHMisoprostol in the prevention of gastric erosions caused by nonsteroidal anti-inflammatory agentsRevue du Rhumatisme1994 Edition(2):1261317920500
- ElliottSLYeomansNDBuchananRRSmallwoodRAEfficacy of 12 months’ misoprostol as prophylaxis against NSAID-induced gastric ulcers. A placebo-controlled trialScand J Rheumatol19942341711768091141
- GrahamDYAgrawalNMRothSHPrevention of NSAID-induced gastric ulcer with misoprostol: multicentre, double-blind, placebo-controlled trialLancet198828623127712802904006
- GrahamDYWhiteRHMorelandLWDuodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study GroupAnn Intern Med199311942572628328732
- GrahamDYAgrawalNMCampbellDRUlcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazoleArch Intern Med2002162216917511802750
- HawkeyCJKarraschJASzczepanskiLOmeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study GroupN Engl J Med1998338117277349494149
- HenrikssonKUribeASandstedtBNordCEHelicobacter pylori infection, ABO blood group, and effect of misoprostol on gastroduodenal mucosa in NSAID-treated patients with rheumatoid arthritisDig Dis Sci1993389168816968359082
- JensenDMHoSHamamahSA randomized study of omeprazole compared to misoprostol for prevention of recurrent ulcers and ulcer hemorrhage in high risk patients ingesting aspirin or NSAIDs [abstract]Gastroenterology20001184 Suppl 2 Pt 1 AGA A892
- RaskinJBWhiteRHJacksonJEMisoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimensAnn Intern Med199512353443507625622
- RaskinJBWhiteRHJaszewskiRKorstenMASchubertTTFortJGMisoprostol and ranitidine in the prevention of NSAID-induced ulcers: a prospective, double-blind, multicenter studyAm J Gastroenterol19969122232278607484
- RothSHTindallEAJainAKA controlled study comparing the effects of nabumetone, ibuprofen, and ibuprofen plus misoprostol on the upper gastrointestinal tract mucosaArch Intern Med199315322256525718239849
- SaggioroAAlvisiVBlasiADobrillaGFioravantiAMarcolongoRMisoprostol prevents NSAID-induced gastroduodenal lesions in patients with osteoarthritis and rheumatoid arthritisItal J Gastroenterol1991233119123 Erratum in Ital J Gastroenterol. 1991;23(5):273.1742504
- SilversteinFEGrahamDYSeniorJRMisoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trialAnn Intern Med199512342412497611589
- ValentiniMCannizzaroRPolettiMNonsteroidal antiinflammatory drugs for cancer pain: comparison between misoprostol and ranitidine in prevention of upper gastrointestinal damageJ Clin Oncol19951310263726427595718
- VerdicktWMoranCHantzschelHFragaAMSteadHGeisGSA double-blind comparison of the gastroduodenal safety and efficacy of diclofenac and a fixed dose combination of diclofenac and misoprostol in the treatment of rheumatoid arthritisScand J Rheumatol199221285911570496
- BerkowitzJMRogenesPRSharpJTWarnerCWRanitidine protects against gastroduodenal mucosal damage associated with chronic aspirin therapyArch Intern Med198714712213721393689065
- EhsanullahRSPageMCTildesleyGWoodJRPrevention of gastro-duodenal damage induced by non-steroidal anti-inflammatory drugs: controlled trial of ranitidineBMJ19882976655101710213142593
- RobinsonMMillsRJEulerARRanitidine prevents duodenal ulcers associated with non-steroidal anti-inflammatory drug therapyAliment Pharmacol Ther1991521431501832313
- RobinsonMGGriffinJJBowersJEffect of ranitidine on gastroduodenal mucosal damage induced by nonsteroidal antiinflam-matory drugsDig Dis Sci19893434244282646087
- TahaASHudsonNHawkeyCJFamotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugsN Engl J Med199633422143514398618582
- Van GroenendaelJHMarkusseHMDijkmansBABreedveldFCThe effect of ranitidine on NSAID related dyspeptic symptoms with and without peptic ulcer disease of patients with rheumatoid arthritis and osteoarthritisClin Rheumatol19961554504568894357
- LevineLRCloudMLEnasNHNizatidine prevents peptic ulceration in high-risk patients taking nonsteroidal anti-inflammatory drugsArch Intern Med199315321244924548215749
- SwiftGLHeneghanMWilliamsGTWilliamsBDO’SullivanMMRhodesJEffect of ranitidine on gastroduodenal mucosal damage in patients on long-term non-steroidal anti-inflammatory drugsDigestion198944286942693162
- SimonBMullerPNizatidine in therapy and prevention of non-steroidal anti-inflammatory drug-induced gastroduodenal ulcer in rheumatic patientsScand J Gastroenterol Suppl199420625287863248
- HudsonNTahaASRussellRIFamotidine for healing and maintenance in nonsteroidal anti-inflammatory drug-associated gastroduodenal ulcerationGastroenterology19971126181718229178671
- WoldeSDijkmansBAJanssenMHermansJLamersCBHigh-dose ranitidine for the prevention of recurrent peptic ulcer disease in rheumatoid arthritis patients taking NSAIDsAliment Pharmacol Ther19961033473518791962
- Bianchi PorroGLazzaroniMImbesiVMontroneFSantagadaTEfficacy of pantoprazole in the prevention of peptic ulcers, induced by non-steroidal anti-inflammatory drugs: a prospective, placebo-controlled, double-blind, parallel-group studyDig Liver Dis200032320120810975769
- CullenDBardhanKDEisnerMPrimary gastroduodenal prophylaxis with omeprazole for non-steroidal anti-inflammatory drug usersAliment Pharmacol Ther19981221351409692687
- EkstromPCarlingLWetterhusSPrevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic multicentre studyScand J Gastroenterol19963187537588858742
- LaiKCLamSKChuKMLansoprazole reduces ulcer relapse after eradication of Helicobacter pylori in nonsteroidal anti-inflammatory drug users – a randomized trialAliment Pharmacol Ther200318882983614535877
- YeomansNDTulassayZJuhaszLRaczIHowardJA comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugsN Eng J Med199833811719726
- FDA – Study 21-Celebrex. Available from http://www.fda.gov/cder/foi/adcomm/98/celebrex.htm). Accessed Aug 31, 2009.
- FDA – Study 71-Celebrex. Available from http://www.fda.gov/cder/foi/adcomm/98/celebrex.htm. Accessed Aug 31, 2009.
- FDA – Study 47-FDA Review (ND21-341) – Bextra. Available from http://www.fda.gov/cder/foi/nda/2001/21-341_Bextra.htm. Accessed Aug 31, 2009.
- FDA – Study 61-FDA Drug Review (ND21-341) – Bextra. Available from http://www.fda.gov/cder/foi/nda/2001/21-341_Bextra.htm. Accessed Aug 31, 2009.
- FDA – Study 63-FDA Drug Review (ND21-341) – Bextra. Available from http://www.fda.gov/cder/foi/nda/2001/21-341_Bextra.htm). Accessed Aug 31, 2009.
- GoldsteinJLSilversteinFEAgrawalNMReduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitorAm J Gastroenterol20009571681169010925968
- GoldsteinJLEisenGMAgrawalNStensonWFKentJDVerburgKMReduced incidence of upper gastrointestinal ulcer complications with the COX-2 selective inhibitor, valdecoxibAliment Pharmacol Ther200420552753815339324
- LangmanMJJensenDMWatsonDJAdverse upper gastrointestinal effects of rofecoxib compared with NSAIDsJAMA1999282201929193310580458
- DistelMMuellerCBluhmkiEGlobal analysis of gastrointestinal safety of a new NSAID, meloxicamInflammopharmacology1996417181
- RameyDRWatsonDJYuCBologneseJACurtisSPReicinASThe incidence of upper gastrointestinal adverse events in clinical trials of etoricoxib vs non-selective NSAIDs: An updated combined analysisCurr Med Res Opin200521571572215974563
- GoldsteinJLCorreaPZhaoWWReduced incidence of gastro-duodenal ulcers with celecoxib, a novel cyclooxygenase-2 inhibitor, compared to naproxen in patients with arthritisAm J Gastroenterol20019641019102711316141
- EmeryPZeidlerHKvienTKCelecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparisonLancet199935491962106211110609815
- SimonLSWeaverALGrahamDYAnti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial.[see comment]JAMA1999282201921192810580457
- LaineLHarperSSimonTA randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis. Rofecoxib Osteoarthritis Endoscopy Study GroupGastroenterology1999117477678310500058
- HawkeyCLaineLSimonTComparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. The Rofecoxib Osteoarthritis Endoscopy Multinational Study GroupArthritis Rheum200043237037710693877
- HawkeyCJLaineLSimonTIncidence of gastroduodenal ulcers in patients with rheumatoid arthritis after 12 weeks of rofecoxib, naproxen, or placebo: a multicentre, randomised, double blind studyGut2003526820826 erratum in Gut. 2003;52(12):1800.12740337
- HawkeyCCSvobodaPFiedorowicz-FabrycyIFGastroduodenal safety and tolerability of lumiracoxib compared with Ibuprofen and celecoxib in patients with osteoarthritisJ Rheumatol20043191804181015338504
- SikesDHAgrawalNMZhaoWWKentJDReckerDPVerburgKMIncidence of gastroduodenal ulcers associated with valdecoxib compared with that of ibuprofen and diclofenac in patients with osteoarthritisEur J Gastroenterol Hepatol200214101101111112362101
- PavelkaKReckerDPVerburgKMValdecoxib is as effective as diclofenac in the management of rheumatoid arthritis with a lower incidence of gastroduodenal ulcers: results of a 26-week trialRheumatology200342101207121512810937
- HuntRHHarperSWatsonDJThe gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal eventsAm J Gastroenterol20039881725173312907325
- HuntRHHarperSCallegariPComplementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxibAliment Pharmacol Ther200317220121012534404
- KivitzAEisenGZhaoWWBevirtTReckerDPRandomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritisJ Fam Pract200251653053712100776
- KivitzAJNayiagerSSchimanskyTGimonaAThurstonHJHawkeyCReduced incidence of gastroduodenal ulcers associated with lumiracoxib compared with ibuprofen in patients with rheumatoid arthritisAliment Pharmacol Ther200419111189119815153172
- SilversteinFEFaichGGoldsteinJLGastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety StudyJAMA2000284101247125510979111
- BombardierCLaineLReicinAComparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study GroupN Engl J Med2000343211520152811087881
- DequekerJHawkeyCKahanAImprovement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritisBr J Rheumatol19983799469519783758
- HawkeyCKahanASteinbruckKGastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large-scale International Study Safety AssessmentBr J Rheumatol1998379937945 erratum in Br J Rheumatol. 1998; 37(10):1142.9783757
- SchnitzerTJFrickeJJrGittonXJayawardeneSSloanVSLumiracoxib in the treatment of osteoarthritis, rheumatoid arthritis and acute postoperative dental pain: Results of three dose-response studiesCurr Med Res Opin200521115116115881487
- LeungATMalmstromKGallacherAEEfficacy and tolerability profile of etoricoxib in patients with osteoarthritis: A randomized, double-blind, placebo and active-comparator controlled 12-week efficacy trialCurr Med Res Opin2002182495812017209
- LisseJRPerlmanMJohanssonGGastrointestinal tolerability and effectiveness of rofecoxib versus naproxen in the treatment of osteoarthritis: a randomized, controlled trialAnn Intern Med2003139753954614530224
- ZhaoSZMcMillenJIMarkensonJAEvaluation of the functional status aspects of health-related quality of life of patients with osteoarthritis treated with celecoxibPharmacotherapy199919111269127810555933
- GeusensPPTruittKSfikakisPA placebo and active comparator-controlled trial of rofecoxib for the treatment of rheumatoid arthritisScand J Rheumatol200231423023812369656
- SinghGFortJGGoldsteinJLCelecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I StudyAm J Med2006119325526616490472
- GoldsteinJLSilversteinFEAgrawalNMReduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitorAm J Gastroenterol20009571681169010925968
- YocumDFleischmannRDalginPCaldwellJHallDRoszkoPSafety and efficacy of meloxicam in the treatment of osteoarthritis: a 12-week, double-blind, multiple-dose, placebo-controlled trial. The Meloxicam Osteoarthritis InvestigatorsArch Intern Med2000160192947295411041902
- WilliamsGW ERREHRLMYSZTreatment of osteoarthritis with a once-daily dosing regimen of celecoxib: A randomized, controlled trialJ Clin Rheumatol200062657419078452
- BensenWGFiechtnerJJMcMillenJITreatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trialMayo Clin Proc199974111095110510560596
- TruittKESperlingRSEttingerWHJrA multicenter, randomized, controlled trial to evaluate the safety profile, tolerability, and efficacy of rofecoxib in advanced elderly patients with osteoarthritisAging Clin Exp Res2001132112121
- DayRMorrisonBLuzaAA randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Rofecoxib/Ibuprofen Comparator Study GroupArch Intern Med2000160121781178710871971
- CannonGWCaldwellJRHoltPRofecoxib, a specific inhibitor of cyclooxygenase 2, with clinical efficacy comparable with that of diclofenac sodium: results of a one-year, randomized, clinical trial in patients with osteoarthritis of the knee and hip. Rofecoxib Phase III Protocol 035 Study GroupArthritis Rheum200043597898710817549
- EhrichEWSchnitzerTMMcIlwainHEffect of specific COX-2 inhibition in osteoarthritis of the knee: A 6 week double-blind, placebo controlled pilot study of rofecoxibJ Rheumatol199926112438244710555907
- SchnitzerTJTruittKFleischmannRThe safety profile, tolerability, and effective dose range of rofecoxib in the treatment of rheumatoid arthritisClin Ther199921101688170210566565
- SaagKvan derHDFisherCRofecoxib, a new cyclooxygenase 2 inhibitor, shows sustained efficacy, comparable with other nonsteroidal anti-inflammatory drugs: a 6-week and a 1-year trial in patients with osteoarthritis. Osteoarthritis Studies GroupArch Fam Med20009101124113411115219
- GebaGPWeaverALPolisABDixonMESchnitzerTJVioxxACTVGEfficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trialJAMA200228716471 erratum in JAMA. 200227;287(8):989.11754710
- WheltonACyclooxygenase-2 – specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patientsAm J Ther200182859511304662
- McKennaFBorensteinDWendtHWallemarkCLefkowithJBGeisGSCelecoxib versus diclofenac in the management of osteoarthritis of the kneeScand J Rheumatol2001301111811252686
- McKennaFCOX-2 specific inhibitors in the management of osteoarthritis of the knee: A placebo-controlled, randomized, double-blind studyJ Clin Rheumatol20017315115917039120
- MakarowskiWZhaoWWBevirtTReckerDPEfficacy and safety of the COX-2 specific inhibitor valdecoxib in the management of osteoarthritis of the hip: a randomized, double-blind, placebo-controlled comparison with naproxenOsteoarthritis Cartilage200210429029611950252
- BensenWWeaverAEspinozaLEfficacy and safety of valdecoxib in treating the signs and symptoms of rheumatoid arthritis: a randomized, controlled comparison with placebo and naproxenRheumatology20024191008101612209034
- GottesdienerKSchnitzerTFisherCResults of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritisRheumatology (Oxford)200241910521061 Erratum in Rheumatology (Oxford). 2003;42(6):814.12209041
- ZacherJFeldmanDGerliRA comparison of the therapeutic efficacy and tolerability of etoricoxib and diclofenac in patients with osteoarthritisCurr Med Res Opin2003198725736 Erratum in Curr Med Res Opin. 2004;20(10):1689.14687444
- CollantesECurtisSPLeeKWA multinational randomized, controlled, clinical trial of etoricoxib in the treatment of rheumatoid arthritis [ISRCTN25142273]BMC Fam Pract2002311012033987
- MatsumotoAKMelianAMandelDRA randomized, controlled, clinical trial of etoricoxib in the treatment of rheumatoid arthritisJ Rheumatol20022981623163012180720
- KivitzAJNayiagerSSchimanskyTGimonaAThurstonHJHawkeyCReduced incidence of gastroduodenal ulcers associated with lumiracoxib compared with ibuprofen in patients with rheumatoid arthritisAliment Pharmacol Ther200419111189119815153172
- HawkeyCCSvobodaPFiedorowicz-FabrycyIFGastroduodenal safety and tolerability of lumiracoxib compared with Ibuprofen and celecoxib in patients with osteoarthritisJ Rheumatol20043191804181015338504
- SchnitzerTJBurmesterGRMyslerEComparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trialLancet2004364943566567415325831
- GoldsteinJLAgrawalNEisenG Significantly improved upper gastrointestinal (UGI) tolerability with celecoxib, a COX-2 specific inhibitor, compared with conventional NSAIDS. The Success-1 trial [abstract]. Abstracts in Perspective 5[1]2001
- LaineLCurtisSPCryerBKaurACannonCPAssessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparisonLancet2007369956046547317292766
- U.S. Food and Drug Administration Arthritis Drugs Advisory Committee Meeting 12/1/1998. CLASS – 12 Month Data. 1998. Available from.
- ChanFKHungLCSuenBYCelecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: results of a randomized double-blind trialGastroenterology200412741038104315480981
- LaiKCChuKMHuiWMCelecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complicationsAm J Med2005118111271127816271912
- ChanFKWongVWSuenBYCombination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trialLancet200736995731621617499604
- Myllykangas-LuosujarviRLuHSChenSLComparison of low-dose rofecoxib versus 1000 mg naproxen in patients with osteoarthritis. Results of two randomized treatment trals of six weeks durationScand J Rheumatol200231633734412492248
- KivitzAJMoskowitzRWWoodsEComparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hipJ Int Med Res200129646747911803730
- WiesenhutterCWBoiceJAKoAEvaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: A randomized, double-blind, placebo-controlled trialMayo Clin Proc200580447047915819283
- GeusensPAltenRRovenskyJEfficacy, safety and tolerability of lumiracoxib in patients with rheumatoid arthritisInt J Clin Pract200458111033104115605667
- GrifkaJKZacherJBrownJPEfficacy and tolerability of lumiracoxib versus placebo in patients with osteoarthritis of the handClin Exp Rheumatol200422558959615485012
- LehmannRBrzoskoMKopsaPEfficacy and tolerability of lumiracoxib 100mg once daily in knee osteoarthritis: A 13-week, randomized, double-blind study vs. placebo and celecoxibCurr Med Res Opin200521451752615899100
- LaineLMallerESYuCQuanHSimonTUlcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double-blind trialGastroenterology2004127239540215300570
- Catella-LawsonFReillyMPKapoorSCCyclooxygenase inhibitors and the antiplatelet effects of aspirinN Engl J Med2001345251809181711752357
- OuelletMRiendeauDPercivalMDA high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirinProc Natl Acad Sci U S A20019825145831458811717412
- BaigentCPatronoCSelective cyclooxygenase 2 inhibitors, aspirin, and cardiovascular disease: a reappraisalArthritis Rheum2003481122012528099
- PatronoCAspirin resistance: definition, mechanisms and clinical read-outsJ Thromb Haemost2003181710171312911581
- CipolloneFRoccaBPatronoCCyclooxygenase-2 expression and inhibition in atherothrombosisArterioscler Thromb Vasc Biol200424224625514592854
- KhanNAMcAlisterFARabkinSWThe 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – TherapyCan J Cardiol200622758359316755313
- PearsonTABlairSNDanielsSRAHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating CommitteeCirculation2002106338839112119259
- RostomAMoayyediPHuntRfor the Canadian Association of Gastroenterology Consensus groupCanadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risksAliment Pharmacol Ther200929548149619053986
- ChanFKChungSCSuenBYPreventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxenN Engl J Med20013441396797311274623
- VergaraMCatalanMGisbertJPCalvetXMeta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID usersAliment Pharmacol Ther200521121411141815948807
- LabenzJBlumALBoltenWWPrimary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trialGut200251332933512171952
- HuntRFalloneCVeldhuyzan vanZSCanadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori–an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infectionCan J Gastroenterol200418954755415457293
- RothSHBennettREMitchellCSHartmanRJCimetidine therapy in nonsteroidal anti-inflammatory drug gastropathy. Double-blind long-term evaluationArch Intern Med198714710179818013310942
- TannenbaumHBerenbaumFReginsterJYLumiracoxib is effective in the treatment of osteoarthritis of the knee: a 13 week, randomised, double blind study versus placebo and celecoxibAnn Rheum Dis200463111419142615020310
- WilliamsGHubbardRYuSS ZComparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the kneeClin Ther200123221322711293555
![Figure 10 PUBs (POBs [perforation, obstruction or bleeding] or symptomatic ulcer) with COX-2s vs tNSAIDs.](/cms/asset/139f5ab0-8c88-4b6c-8d20-e9d9ea5ef904/dhps_a_4334_f0010_c.jpg)
![Figure 12 Clinical ulcers (PUBs [perforation, obstruction, bleeding or the presence of a symptomatic ulcer]) with COX-2 + ASA vs COX-2 alone.](/cms/asset/9011c375-e895-4d7c-bafe-12d0b40cd05a/dhps_a_4334_f0012_c.jpg)
![Figure 13 Clinical ulcers (PUBs [perforation, obstruction, bleeding or the presence of a symptomatic ulcer]) with tNSAID + ASA vs tNSAID alone.](/cms/asset/4f38c23c-6bbd-43d1-a0e4-037b41a7f7b2/dhps_a_4334_f0013_c.jpg)