Abstract
Type 2 diabetes is a progressive disease associated with high levels of morbidity and mortality and for which there is both a large and growing prevalence worldwide. Lifestyle advice plus metformin is commonly recommended initially to manage hyperglycemia and to minimize the risk of vascular complications. However, additional agents are required when glycemic targets cannot be achieved or maintained due to the progressive nature of the disease. Repaglinide/metformin fixed-dose combination (FDC) therapy (PrandiMet®; Novo Nordisk, Bagsværd, Denmark) has been approved for use in the USA. This FDC is a rational second-line therapy given the complementary mechanisms of action of the components. Repaglinide is a rapidly absorbed, short-acting insulin secretagogue targeting postprandial glucose excursions; metformin is an insulin sensitizer with a longer duration of action that principally regulates basal glucose levels. A pivotal, 26-week, randomized study with repaglinide/metformin FDC therapy has been conducted in patients experiencing suboptimal control with previous oral antidiabetes therapy. Repaglinide/metformin FDC improved glycemic control and weight neutrality without adverse effects on lipid profiles. There were no major hypoglycemic episodes and patients expressed greater satisfaction with repaglinide/metformin FDC than previous treatments. Repaglinide/metformin FDC is expected to be more convenient than individual tablets for patients taking repaglinide and metformin in loose combination, and it is expected to improve glycemic control in patients for whom meglitinide or metformin monotherapies provide inadequate control.
Introduction
Prevalence and burden
Diabetes mellitus is a challenging global health problem, with a large and growing prevalence worldwide and high levels of associated morbidity and mortality. The International Diabetes Federation (IDF) currently forecasts that 380 million people will have diabetes within 20 years, a rise of 55% from current prevalence estimates.Citation1 As 85% to 95% of cases of diabetes in the developed world (and higher proportions in the developing world) are type 2, the prevalence forecast for type 2 diabetes in 2025 is effectively in excess of 323 million people. Similar predictions have been made by the World Health Organization.Citation2 The rise in prevalence will in part be due to increased life expectancy, as type 2 diabetes is more common in older than younger adults.Citation1 However, the rise also reflects ongoing trends towards low levels of physical activity, unhealthy diets and excess body weight. The burden of type 2 diabetes is significant for patients, their carers and healthcare systems. The IDF has commented, “diabetes is expected to cause 3.8 million deaths worldwide in 2007, about 6% of total global mortality, and almost the same as HIV/AIDS.”Citation1 It also estimates that “the equivalent of … 23 million years of life are lost to disability and reduced quality of life caused by the complications of diabetes.” In financial terms, it is believed that at least US$232 billion was spent treating and preventing diabetes and its complications globally in 2007, and this is expected to rise to over US$302 billion by 2025.
Pathology and symptoms
Type 2 diabetes is characterized by two underlying defects: insulin resistance in peripheral tissues and reduced insulin secretion due to pancreatic beta cell failure. Insulin resistance remains relatively stable during the course of the disease, but beta cell function continues to decline and is responsible for the progressive nature of type 2 diabetes.Citation3,Citation4 In the early stages of beta cell failure there is a reduced insulin response to meals, and thus raised postprandial blood glucose levels.Citation5 Chronic elevation of blood glucose levels thereafter has a toxic effect on beta cells, resulting in further reductions in insulin secretion and thus a cycle of increased glucose levels and beta cell damage. Fasting plasma glucose levels become raised as beta cell failure progresses.
The deleterious effects of chronic hyperglycemia are not restricted to pancreatic beta cells. There is also a strong association with micro- and macrovascular complicationsCitation6 and with resultant high levels of mortality and morbidity. The macrovascular complications of coronary heart disease and stroke, for example, are twice as likely to occur in individuals with type 2 diabetes as in those without.Citation7 Of the microvascular complications, retinopathy is particularly common, with 74% of patients with diabetes developing the complication after 10 years or more; neuropathy occurs in approximately half of patients with diabetes and nephropathy in one third.Citation1
Treatment
Stated simply, a key goal of therapy for type 2 diabetes is to achieve and maintain good glycemic control, thus minimizing the risk of diabetes complications. Good glycemic control is generally considered to be a glycated hemoglobin (HbA1c) level of < 7.0%Citation8 or < 6.5%,Citation1,Citation9 but there is increasing emphasis on separate fasting and postprandial plasma glucose targets.Citation9–Citation11 A combination of lifestyle advice (diet and exercise) and metformin is commonly recommended as a first-line therapy for the management of hyperglycemia for patients with type 2 diabetes.Citation8,Citation9 Weight loss and increased activity, the goals of lifestyle advice, are associated with significant short- and long-term benefits, and thus form an important part of the therapeutic strategy throughout the course of the disease. The use of metformin at this early stage acknowledges the fact that lifestyle modifications alone are rarely sufficient to maintain glycemic control. Metformin monotherapy is a highly appropriate first-line pharmaceutical intervention as it reduces hyperglycemia without concomitant hypoglycemia or weight gain – side effects that are problematic with hypoglycemic agents.
As type 2 diabetes is progressive, the repeated appraisal of glycemic control is imperative at all stages in the treatment pathway.Citation8,Citation9 Where hyperglycemia persists after first-line therapy, it is common practice to add a second agent.
The need for combination therapy
Dual therapy has an important place in the management of hyperglycemia in type 2 diabetes. The American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) advise that monotherapy may initially be sufficient to achieve glycemic goals in patients with HbA1c levels < 7.5%, but that dual therapy should be initiated if monotherapy fails.Citation9 For patients with HbA1c levels of 7.6% to 9.0%, the AACE/ACE advise starting treatment with dual therapy and then progressing to triple and insulin therapies thereafter.Citation9
Antihyperglycemic and hypoglycemic drugs with different mechanisms of action are likely to have the greatest efficacy in dual-therapy combinations.Citation8 AACE/ACE recommend that metformin should be the “cornerstone” of dual therapy for most patients. For patients with HbA1c levels < 7.5%, they recommend (in order) incretin-based therapies, glinides or sulfonylureas as second components.Citation9 Glinides are recommended over sulfonylureas because of the need to control postprandial glucose excursions in patients with HbA1c levels < 7.5% and the better safety profile. For patients with HbA1c levels of 7.6% to 9.0%, AACE/ACE recommend (in order) incretin-based therapies, thiazolidinediones, sulfonylureas or glinides as second components.
Combination therapy with metformin (an antihyperglycemic drug) and a short-acting insulin secretagogue (an oral hypoglycemic drug), such as repaglinide, is a rational dual- therapy option for type 2 diabetes. Metformin regulates basal glucose levels and repaglinide targets postprandial glucose excursions. The two agents have complementary mechanisms of action and both are agents recommended by AACE/ACE for the management of hyperglycemia in patients with HbA1c levels < 9.0%. Moreover, clinical data have shown that improvements in glycemic control with a loose combination of repaglinide and metformin over a 4- to 5-month period are both statistically significant and clinically relevant, even after adjusting for improvements in the monotherapy groups.Citation12
The concomitant use of repaglinide and metformin has been approved previously, but experience with other dual therapies suggests that fixed-dose combinations (FDCs) can provide greater patient compliance and thus better glycemic control than loose combinations.Citation13,Citation14 Repaglinide/metformin FDC (PrandiMet®; Novo Nordisk, Bagsværd, Denmark) was approved by the Food and Drug Administration (FDA) in June 2008. Repaglinide/metformin FDC is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are already treated with a meglitinide and metformin or who have inadequate glycemic control on either meglitinide or metformin alone.Citation15 An initial review of the pharmacokinetics, efficacy and safety of this combination product has been published previously.Citation16 This article provides further detail and an update following FDA approval and the full publication of key data. This article also considers the potential disadvantages of FDCs as they pertain to the repaglinide/metformin FDC.
Pharmacology and pharmacokinetics
The mechanisms of action of repaglinide and metformin, and aspects of their pharmacology and pharmacokinetics, have been reviewed previously.Citation16 Additional data are summarized here, along with key points from the previous summary for appropriate context.
Repaglinide
Repaglinide is a rapidly absorbed, short-acting insulin secretagogue that stimulates insulin secretion in the presence of glucose. Peak plasma levels (Cmax) occur in healthy volunteers and patients within 1 hour of administration.Citation15 The mean time to reach maximum plasma concentrations is unchanged when repaglinide is administered with food, but the mean Cmax and area under the time/plasma concentration curve (AUC) are reduced by 20% and 12.4%, respectively. The mean absolute bioavailability of repaglinide is 56% and, after intravenous dosing in healthy volunteers, the volume of distribution at steady state is 31 L and the total body clearance is 38 L/hour. Protein binding and binding to human serum albumin are greater than 98%, and the plasma half-life is short (approximately 1 hour). Repaglinide is completely metabolized by oxidative biotransformation and direct conjugation with glucuronic acid, and the resulting metabolites have no antihyperglycemic activity. As CYP2C8 and CYP3A4 are involved in metabolism, drugs that inhibit or induce 2C8 and/or 3A4 may alter the pharmacokinetics and pharmacodynamics of repaglinide. These drugs include gemfibrozil, trimethoprim, itraconazole, ketoconazole and rifampin. Within 96 hours of dosing with 14C-repaglinide, approximately 90% of the radiolabel is recovered in the feces and approximately 8% in the urine. Only 0.1% of the dose is cleared in the urine as parent compound and less than 2% in feces.
Metformin
Metformin is an insulin sensitizer that inhibits hepatic glucose output and increases peripheral glucose uptake and utilization. Metformin does not affect insulin secretion. In healthy volunteers, metformin reaches peak plasma levels in 2.8 hours (500 mg or 850 mg tablet).Citation17 Metformin Cmax and AUC values are reduced by food (by 40% and 25%, respectively, for a single 850 mg tablet), and the time to maximum plasma concentrations is delayed (by 35 minutes).Citation15 Under fasting conditions, the absolute bioavailability after a 500 mg dose of metformin is approximately 50% to 60%. Dose proportionality is not apparent due to decreased absorption at higher doses. The high apparent volume of distribution following a single 850 mg dose (averaging 654 ± 358 L) reflects negligible binding to plasma proteins. The plasma elimination half-life is 6.2 hours; the longer blood elimination half-life of 17.6 hours reflects partitioning into erythrocytes. Metformin does not undergo hepatic metabolism or biliary excretion but is excreted unchanged in the urine. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours.
Repaglinide/metformin FDC
The bioequivalence and the effects of food on the pharmacokinetics of the repaglinide/metformin FDC have been investigated in randomized, single-blind, crossover trials in healthy volunteers.Citation18,Citation19 Repaglinide and metformin in the higher-dose FDC tablet (2 mg/500 mg) were bioequivalent to individual 2 mg repaglinide and 500 mg metformin tablets in terms of total exposure and Cmax.Citation15,Citation18 Furthermore, repaglinide total exposure and Cmax values for the higher-dose FDC tablet were twice those for the lower-dose tablet (1 mg/500 mg), indicating dose proportionality. In a separate study of volunteers receiving 2 mg/850 mg FDC tablets, food was shown to affect metformin AUC and Cmax and repaglinide Cmax, but not repaglinide AUC.Citation19
Efficacy
This section focuses on the recently published full data from the large, randomized, open-label trial of the repaglinide/metformin FDC. Efficacy data from earlier studies of repaglinide/metformin combination therapy (sometimes referred to as ‘loose combination’) are also relevant, but have been reviewed previouslyCitation16 and are therefore summarized only briefly here.
Co-administration of repaglinide and metformin
Studies involving loose combinations of repaglinide and metformin showed that good glycemic control could be attained in patients failing to reach glycemic targets with diet and exercise only or lifestyle advice with up to two oral antidiabetes treatments. These studies provided a strong rationale for the more widespread use of repaglinide plus metformin combination therapy.Citation16 Key data from three loose-combination studies are summarized in .Citation12,Citation20–Citation22 In brief, loose-combination therapies provided significantly greater reductions in HbA1c levels than repaglinide and metformin monotherapiesCitation12,Citation20,Citation22 and the reduction with one combination therapy was significantly greater than the sum of the reductions for the monotherapies ().Citation12 Reductions in HbA1c levels were also greater with repaglinide + metformin than with nateglinide + metformin combination therapy.Citation21 Similar effects were apparent for comparisons of fasting plasma glucose levels.Citation12,Citation20–Citation22
Figure 1 Mean changes in glycated hemoglobin (HbA1c) levels from baseline in patients receiving repaglinide or metformin monotherapies or repaglinide + metformin combination therapy. Drawn from data of Moses et al, 1999.Citation12
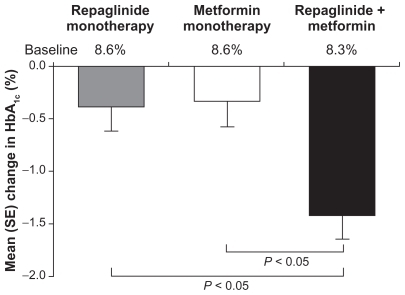
Table 1 Glycemic control in terms of HbA1c levels from key studies involving the co-administration of repaglinide and metformin (loose combination) in patients with type 2 diabetes
Repaglinide/metformin FDC therapy
The efficacy of repaglinide/metformin FDC tablets has been investigated in a pivotal, 26-week study.Citation23,Citation24 The active comparator in this trial was another metformin-based FDC, but with an insulin sensitizer, rosiglitazone, rather than an insulin secretagogue.
Study design
Participants enrolled into the study had been previously receiving up to two oral antidiabetes drugs (for at least 2 months as monotherapy) and experiencing suboptimal glycemic control (HbA1c levels of 7.5% to 11.0% for monotherapy or 7.0% to 10.0% for dual therapy). Any drug other than metformin must have been administered at half-maximal doses or less. A total of 561 patients were randomly allocated 1:1:1 to repaglinide/metformin FDC twice or three times daily, or rosiglitazone/metformin twice daily (n = 187 in each group). The repaglinide/metformin FDC groups received maximum doses of either 4 mg repaglinide/1,000 mg metformin at breakfast and supper (twice daily regimen) or 4 mg repaglinide/1,000 mg metformin at breakfast and supper and 2 mg repaglinide/500 mg metformin at lunch (three times daily regimen). The rosiglitazone/metformin group received maximum doses of 4 mg rosiglitazone/1,000 mg metformin at breakfast and supper. Doses were titrated weekly over a 4-week period up to the maximum doses. Patients who were unable to tolerate dosing were withdrawn from the study. The efficacy of repaglinide/metformin twice daily was compared with those of the other two treatment regimens. Parameters assessed included glycemic control (HbA1c and fasting plasma glucose levels), body weight, waist:hip ratios and lipid profiles. The primary endpoint was the change in HbA1c level from baseline to week 26.
Patient disposition and baseline characteristics
A total of 383 (68.3%) patients completed the study. Discontinuation rates were similar across the treatment groups, but withdrawal because of ineffective therapy was slightly more common with rosiglitazone/metformin and withdrawal for adverse events slightly more common with repaglinide/metformin. Citation24 The treatment groups were comparable in terms of baseline demographic and disease characteristics. The study population had mean ages across the treatment groups of 54.5 to 54.8 years, and mean body mass indices of 32 to 33 kg/m2. The mean durations of diabetes were 7.1 to 7.4 years and the mean baseline HbA1c values were 8.3% to 8.5%.
Repaglinide/metformin FDC twice versus three times daily
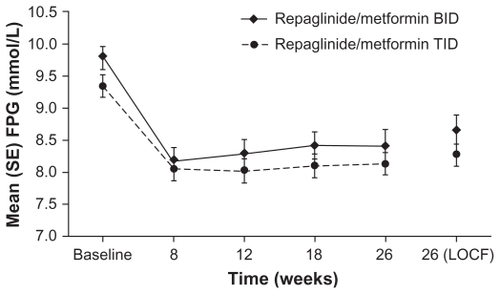
Both regimens improved HbA1c levels during the study (), with overall mean reductions from baseline to week 26 of −0.99% and −1.02% for the twice and three times daily regimens, respectively.Citation23 Importantly, the twice daily regimen was non-inferior to the three times daily regimen (least square mean, 0.122; 95% confidence interval [CI]: −0.104 to 0.349). Improvements in glycemic control were reflected in the proportions of patients reaching target HbA1c levels. At week 26, 42.9% and 48.9% of the twice and three times daily groups, respectively, had HbA1c levels of ≤ 7.0%. There was no significant difference between the treatment regimens in terms of the proportions of patients reaching this or the ≤7.5% or ≤6.5% HbA1c targets. The pattern of reductions in fasting plasma glucose levels was similar to that for HbA1c levels (). Moreover, the changes from baseline to week 26 were not significantly different for the two regimens (P = 0.3472). Mean reductions in fasting plasma glucose levels were −1.13 mmol/L and −1.10 mmol/L for the twice and three times daily regimens, respectively.
Figure 2 Glycated hemoglobin (HbA1c) values by study visit for repaglinide/metformin twice daily (BID) and three times daily (TID) fixed-dose combination regimens. Raskin P, Lewin A, Reinhardt R, Lyness W, for the repaglinide/metformin fixed-dose combination study group. Twice-daily and three-times-daily dosing of a repaglinide/metformin fixed-dose combination tablet provide similar glycemic control. Diab Obes Metab. 2009;11:947–952.Citation23 Reprinted with permission from John Wiley & Sons Inc.
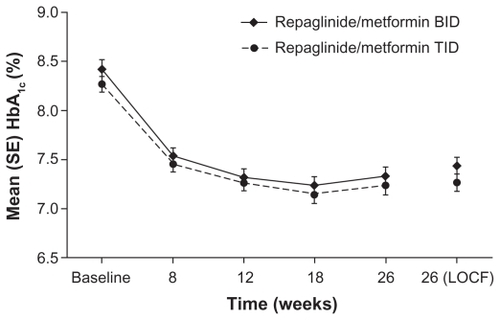
Figure 3 Fasting plasma glucose (FPG) values by study visit for repaglinide/metformin twice daily (BID) and three times daily (TID) fixed-dose combination regimens. Raskin P, Lewin A, Reinhardt R, Lyness W, for the repaglinide/metformin fixed-dose combination study group. Twice-daily and three-times-daily dosing of a repaglinide/metformin fixed-dose combination tablet provide similar glycemic control. Diab Obes Metab. 2009;11:947–952.Citation23 Reprinted with permission from John Wiley and Sons Inc.
In essence, the regimens were weight-neutral, with a small mean weight gain of 0.47 kg in the twice daily group compared with a small mean weight loss of −0.34 kg in the three times daily group. The difference between the groups was statistically significant (P = 0.0333), but not clinically relevant. Moreover, there was no significant difference between the two regimens for waist:hip ratios (P = 0.5438). Long-term weight-neutrality is expected with repaglinide/metformin combination therapy based on extensive clinical experience with metformin monotherapy, and 1-year double- blind clinical trial data with repaglinide monotherapy.Citation25–Citation27
Overall, the efficacy data from the study suggest that repaglinide/metformin FDC can be administered in a twice or three times daily regimen, depending on individual patient needs, without compromising efficacy. This FDC may also be an appropriate therapeutic option for patients with type 2 diabetes who have fears about weight gain when intensifying their oral antidiabetes therapy.
Repaglinide/metformin twice daily versus rosiglitazone/metformin twice daily
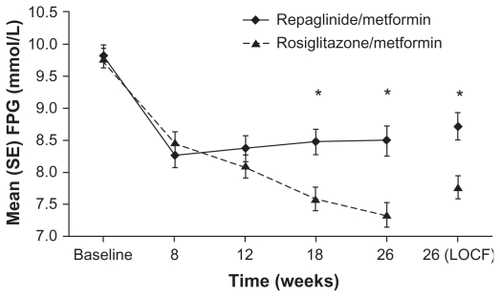
The improvement in HbA1c levels at study end for repaglinide/metformin was non-inferior to that for rosiglitazone/metformin (−0.99% and −1.01%, respectively; P = 0.8186).Citation24 During the course of the study, however, reductions occurred earlier with repaglinide/metformin than rosiglitazone/metformin (). At week 26, 42.9% and 48.9% of the repaglinide/metformin and rosiglitazone/metformin groups, respectively, had HbA1c levels of ≤ 7.0%. There was no significant difference between the treatment regimens in terms of the proportions of patients reaching this or the ≤7.5% or ≤6.5% HbA1c targets. Fasting plasma glucose levels improved in both treatment groups but rosiglitazone/metformin was associated with significantly greater reductions than repaglinide/metformin at weeks 18 and 26 (). At week 26, the changes for the rosiglitazone/metformin and repaglinide/metformin groups were −2.04 mmol/L and −1.13 mmol/L, respectively (P = 0.004). These differences between treatment groups may be due to the mechanisms of action of repaglinide and rosiglitazone. Repaglinide is a short-acting insulin secretagogue commonly used to reduce mealtime glucose excursions, whereas rosiglitazone is an insulin sensitizer, which may be expected to have a greater effect on fasting plasma glucose levels.
Figure 4 Glycated hemoglobin (HbA1c) values by study visit for repaglinide/metformin and rosiglitazone/metformin twice daily fixed-dose combination regimens. Raskin P, Lewin A, Reinhardt R, Lyness W; for the repaglinide/metformin fixeddose combination study group. Twice-daily dosing of a repaglinide/metformin fixeddose combination tablet provides glycemic control comparable to rosiglitazone/metformin tablet. Diab Obes Metab. 2009;11:865–873.Citation24 Reprinted with permission from John Wiley & Sons Inc.
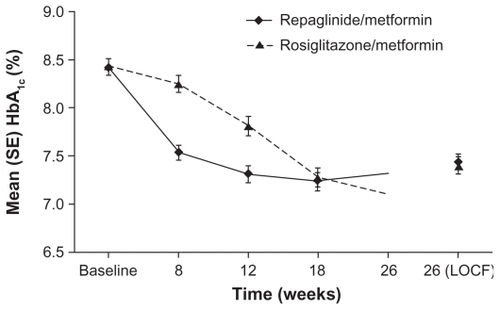
Figure 5 Fasting plasma glucose (FPG) values by study visit for repaglinide/metformin and rosiglitazone/metformin twice daily fixed-dose combination regimens. *P < 0.05 for between-group differences in change in FPG. Raskin P, Lewin A, Reinhardt R, Lyness W; for the repaglinide/metformin fixed-dose combination study group. Twice-daily dosing of a repaglinide/metformin fixed-dose combination tablet provides glycemic control comparable to rosiglitazone/metformin tablet. Diab Obes Metab. 2009;11:865–873.Citation24 Reprinted with permission from John Wiley & Sons Inc.
Changes in body weight and waist:hip ratios were not clinically relevant (<0.5 kg) and not statistically different between groups. There were small (although not significant) reductions from baseline with repaglinide/metformin for all lipids measured but significant increases (P ≤ 0.001) with rosiglitazone/metformin for all lipids measured except triglycerides.
Over a 26-week period, the repaglinide/metformin and rosiglitazone/metformin FDCs used in this study provided similar levels of glycemic control in terms of HbA1c levels and both were weight-neutral. While rosiglitazone/metformin provided better control of fasting plasma glucose levels, there were also adverse effects on lipid profiles.
Appraisal of current research
There are currently few studies with repaglinide/metformin FDC. The key study reported above was a large, randomized, controlled trial and as such provides valuable data about the efficacy and safety of repaglinide/metformin FDC. The open-label nature of the study may have influenced study outcomes, although the key findings are in line with expectations based on clinical experience and clinical trial data with loose combination therapy. It is hoped that future research will investigate whether the efficacy benefits of repaglinide/metformin FDC are maintained in the longer term.
Dosing strategy
The potential benefits afforded by FDCs in terms of patient convenience and compliance must be weighed against the potential disadvantage of reduced dosing flexibility (such that patients are at an increased risk of adverse effects without added benefit). Repaglinide/metformin FDC is available in two dose strengths, 1 mg repaglinide/500 mg metformin and 2 mg repaglinide/500 mg metformin.Citation15 For patients already taking repaglinide and metformin in loose combination, doses should be matched to FDC doses. Patients experiencing suboptimal glycemic control with metformin or meglitinide monotherapy are candidates for dual therapy.Citation9 Where the repaglinide/metformin FDC appears the most appropriate choice for that dual therapy, treatment should usually be started with the low-dose tablet twice daily.Citation15
Physicians will also be familiar, based on their experience with other treatment options, with the need to monitor blood glucose levels to determine the therapeutic response and advise patients on the appropriate use of treatment in relation to meals. In the case of the repaglinide/metformin FDC, treatment should be administered within the 15 minutes preceding a meal, but the timing can vary from immediately preceding the meal to up to 30 minutes before the meal. Patients who skip a meal should be advised to skip the dose for that meal. Maximum doses are 10 mg repaglinide/2500 mg metformin per day or 4 mg repaglinide/1000 mg metformin per meal, and treatment can be administered two or three times daily. Indeed, data from the clinical study conducted by Raskin and colleagues have shown that reductions in HbA1c and fasting plasma glucose over 26 weeks with a twice daily regimen were not significantly different from those for the three times daily regimen.Citation23
Safety and tolerability
Individual components
The individual components of the repaglinide/metformin FDC are generally well tolerated.Citation28,Citation29 As with all insulin secretagogues, repaglinide has the potential to cause hypoglycemia. However, the risk of major hypoglycemia with repaglinide may be lower than that with sulfonylureas,Citation30 and the risk can be minimized by matching the dose with the correct carbohydrate meal portions and by initiating treatment with the lowest available dose in patients naïve to meglitinide therapy. Metformin is not associated with hypoglycemia; moreover, it lowers blood glucose levels without causing weight gain and has neutral-to-positive effects on lipids and blood pressure.Citation31
Metformin is associated with more gastrointestinal symptoms than other oral antidiabetes drugs (eg, nausea, vomiting, diarrhea),Citation32 but these symptoms can be limited by reducing the dose and using slow-release formulations. Additionally, although metformin accumulation can lead to lactic acidosis, this adverse event is rare, with an incidence of 0.03 cases/1,000 patient-years of exposure.Citation33 Moreover, in more than 20,000 patient-years of exposure to metformin in clinical trials, there were no reports of lactic acidosis. As metformin is largely excreted by the kidneys, the risk of metformin accumulation is greater in patients with renal impairment. Metformin is thus contraindicated in patients with renal impairment.
The incidence of total serious cardiovascular adverse events is 4% for repaglinide and 3% for sulfonylurea drugs in 1-year controlled clinical trials.Citation15 Initial data from a nationwide study in Denmark among 100,206 patients with diabetes without prior myocardial infarction suggest repaglinide monotherapy is associated with a risk of cardiovascular death similar to metformin monotherapy, the drug considered to be the safest glucose-lowering agent in terms of cardiovascular disease.Citation34 The risks of cardiovascular death for acarbose and the sulfonylurea gliclazide were also similar to those for metformin, but the risks were higher for the other sulfonylureas tested (glimepiride, glibenclamide, glipizide and tolbutamide).
Repaglinide/metformin combination therapy
Combination therapy is well tolerated. The safety of repaglinide/metformin FDC was investigated in a large, 26-week study of patients experiencing suboptimal glycemic control with one or two oral antidiabetes drugs.Citation23,Citation24 The study had three treatment arms: repaglinide/metformin FDC twice or three times daily, and rosiglitazone/metformin FDC twice daily. A slightly higher proportion of patients withdrew because of adverse events from repaglinide/metformin FDC regimens (9.6% and 9.1% for twice and three times daily, respectively) than the rosiglitazone/metformin FDC regimen (6.4%), but the proportions of patients experiencing adverse events overall were similar across treatment arms (54.3%, 63.4% and 58.9%, respectively). The most common adverse events with the twice daily repaglinide/metformin regimen were diarrhea (10.6%), headache (6.4%), upper respiratory tract infection (4.8%) and nausea (4.8%). Similar rates were observed with the three times daily regimen, although the rate for upper respiratory tract infection was lower (2.2%). Diarrhea and headache were less common with rosiglitazone/metformin FDC, but the adverse-event profile was otherwise similar. Eleven patients receiving repaglinide/metformin FDC experienced serious adverse events, although none were considered likely to be related to study medication. One patient (twice daily regimen) with a history of hypertension, hyperlipidemia and coronary artery disease died during the study, which the investigator considered to be probable sudden cardiac death.
No major hypoglycemic episodes were reported in any treatment arm in the 26-week study. The rates of minor hypoglycemic events (defined as plasma glucose levels < 3.1 mmol/L) were similar for the two repaglinide/metformin regimens (3.79 and 4.46 events/patient/year for twice and three times daily, respectively), with over half of the events experienced by only 18 patients. The rate of minor hypoglycemia was significantly lower in the rosiglitazone/metformin FDC treatment arm (0.27 events/patient/year) compared with the twice daily repaglinide/metformin treatment arm (Poisson regression P = 0.0003), but most patients had only approximately 2 events/year.Citation24 The higher rate of hypoglycemia with the repaglinide/metformin FDC was not unexpected, as insulin secretagogues have greater hypoglycemic potential than thiazolidinediones. As mentioned earlier, the risk of hypoglycemia with repaglinide/metformin FDC therapy can be minimized by matching the dose with the correct carbohydrate meal portions and by initiating treatment with lowest available dose in patients naïve to meglitinide therapy.Citation15 It is also important to skip medication when meals are skipped. Data from studies in which repaglinide and metformin were used in loose combination are broadly consistent with those from the key FDC trial.Citation12,Citation21,Citation22 A higher rate of hypoglycemia was observed with combination therapy compared with repaglinide monotherapy in the study by Moses and colleagues, but none of the events were severe.Citation12
As metformin is a component of the FDC, there is an associated risk of lactic acidosis.Citation15 However, lactic acidosis was not reported in studies involving combination therapyCitation12,Citation21–Citation24 and the incidence of lactic acidosis with metformin overall is rare.Citation33 Repaglinide/metformin FDC, as with metformin alone, is contraindicated in patients with renal impairment because metformin is largely excreted by the kidneys.Citation15
Additional safety information relating to the use of repaglinide/metformin FDC is provided in country-specific prescribing information. The panel summarizes key points of the prescribing information for the USA.
Panel Key features of the prescribing information for PrandiMet® in the USACitation15
Patient perspective
The successful management of type 2 diabetes depends heavily on the behavior of the patients themselves. Oral agents generally compare favorably with injectable therapies used later in the disease process in terms of the perceived burden, but compliance is nonetheless influenced by treatment- related side effects, and the flexibility and convenience of oral antidiabetes regimens. As discussed earlier, the repaglinide/metformin FDC is generally well tolerated.Citation23 Data suggest that both twice and three times daily regimens are weightneutral, Citation23 and experience with repaglinide and metformin as monotherapies suggests this will be the case in the longer term.Citation25–Citation27 Moreover, starting treatment at the lowest doses in patients naïve to meglitinide therapy and skipping medications when meals are skipped will do much to minimize the risk of hypoglycemia.Citation15
The diabetes treatment satisfaction questionnaire was used to gather data on patients’ satisfaction with repaglinide/metformin FDC therapy as part of the 26-week study comparing a twice daily repaglinide/metformin FDC regimen with a three times daily regimen and a twice daily rosiglitazone/metformin FDC regimen.Citation23,Citation24 Patients’ perceptions of therapy were similar for twice daily repaglinide/metformin and twice daily rosiglitazone/metformin,Citation24 and patients were generally more satisfied with the FDC therapies than their previous oral antidiabetes therapies. Perceptions of therapy were similar for the twice and three times daily repaglinide/metformin regimens, except that the three times daily group perceived hypoglycemia and low glucose readings to be more frequent at the end of the study, whereas the twice daily group perceived them to be less frequent.Citation23 The frequency of dosing, twice or three times daily, is likely to be considered convenient by most patients. In a study of patient perceptions of repaglinide monotherapy, 18% of the 1233 patients were taking treatment twice daily with meals and 68% three times daily with meals.Citation35 Overall, 81% of patients considered treatment to be convenient.
How patients’ perceptions of repaglinide/metformin FDC therapy translate into treatment adherence has not been assessed. However, other FDC products have been shown to provide greater patient compliance and thus glycemic control.Citation13,Citation14
Conclusions
The first-line therapy commonly recommended for type 2 diabetes is lifestyle advice in combination with metformin monotherapy. The subsequent failure of therapy is largely due to the progressive nature of type 2 diabetes, with continued deterioration of beta cell function leading to persistent hyperglycemia. The addition of repaglinide to metformin therapy is a rational choice for second-line therapy as the two components have complementary mechanisms of action. Repaglinide is a rapidly absorbed, short-acting insulin secretagogue, which is intended to address mealtime excursions in blood glucose levels. In contrast, metformin is an insulin sensitizer with a longer duration of action, inhibiting hepatic glucose output and increasing peripheral glucose uptake and utilization. Metformin is thus predominantly a basal glucose regulator. The complementary modes of action of repaglinide and metformin suggest that patients with inadequate glycemic control with meglitinide or metformin monotherapy may achieve improved control with repaglinide/metformin FDC therapy. FDC therapy may additionally be more convenient for patients taking repaglinide and metformin in loose combination.
Acknowledgment
The author accepts direct responsibility for this paper but is grateful for the contribution made by Watermeadow Medical (supported by Novo Nordisk A/S) in the preparation and revision of the article.
Disclosure
The author has no conflicts of interest to declare.
References
- International Diabetes Federation 2009 http://www.idf.org/ Accessed December 10, 2009
- World Health Organization 2009 http://www.who.int/diabetes/facts/world_figures/en/ Accessed December 10, 2009
- Levy J Atkinson AB Bell PM McCance DR Hadden DR Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study Diabet Med 1998 15 290 296 9585393
- Rudenski AS Hadden DR Atkinson AB Natural history of pancreatic islet B-cell function in type 2 diabetes mellitus studied over six years by homeostasis model assessment Diabet Med 1988 5 36 41 2964326
- Mitrakou A Kelley D Venerman T Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycaemia in NIDDM Diabetes 1990 39 1381 1390 2121568
- Stratton IM Adler AI Neil HAW on behalf of the UK Prospective Diabetes Study Group Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study BMJ 2000 321 405 412 10938048
- Haffner SM Lehto S Rönnemaa T Pyörälä K Laakso M Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction N Engl J Med 1998 339 229 234 9673301
- Nathan DM Buse JB Davidson MB Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy Diabetes Care 2009 32 193 203 18945920
- Rodbard HW Davidson JA Garber AJ Handelsman Y Lebovitz H Moghissi ES Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control Endocr Pract 2009 15 540 559 19858063
- American Diabetes Association Standards of medical care in diabetes – 2009 Diabetes Care 2009 32 Suppl 1 S13 S61 19118286
- International Diabetes Federation Guideline for management of postmeal glucose Brussels International Diabetes Federation 2007 http://www.idf.org/guideline_postmeal Accessed December 10, 2009
- Moses R Slobodniuk R Boyages S Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes Diabetes Care 1999 22 119 124 10333912
- Melikian C White TJ Vanderplas A Dezii CM Chang E Adherence to oral antidiabetic therapy in a managed care organization: a comparison of monotherapy, combination therapy, and fixed-dose combination therapy Clin Ther 2002 24 460 467 11952029
- Blonde L Wogen J Kreilick C Seymour AA Greater reductions in A1C in type 2 diabetic patients new to therapy with glyburide/metformin tablets as compared to glyburide co-administered with metformin Diabetes Obes Metab 2003 5 424 431 14617228
- Novo Nordisk Inc Prescribing information for PrandiMet (repaglinide and metformin HCl) tablets 2008 www.prandimet.com Accessed December 10, 2009
- Moses R Fixed combination of repaglinide and metformin in the management of type 2 diabetes Diabetes Metab Syndr Obes 2009 2 101 109
- Sambol NC Brookes LG Chiang J Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man Br J Clin Pharmacol 1996 42 510 512 8904626
- Hoelscher D Chu PL Lyness W Fixed-dose combination tablet of repaglinide and metformin is bioequivalent to concomitantly administered individual tablets of repaglinide and metformin Clin Drug Invest 2008 28 573 582
- Reilley S Chang CT Lyness W Single-dose pharmacokinetics of a repaglinide/metformin fixed-dose combination tablet in fasted and fed conditions Diabetes 2008 57 Suppl 1 A589
- Moses R Achieving glycosylated haemoglobin targets using the combination of repaglinide and metformin in type 2 diabetes: a reanalysis of earlier data in terms of current targets Clin Ther 2008 30 552 554 18405794
- Raskin P Klaff L McGill J for the repaglinide vs nateglinide metformin combination study group Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin Diabetes Care 2003 26 2063 2068 12832314
- Reboussin DM Goff DC Lipkin EW The combination and nutritional treatment of late-onset diabetes mellitus (CONTROL DM) trial results Diabet Med 2004 21 1082 1089 15384954
- Raskin P Lewin A Reinhardt R Lyness W for the repaglinide/metformin fixed-dose combination study group Twice-daily and three-times-daily dosing of a repaglinide/metformin fixed-dose combination tablet provide similar glycemic control Diab Obes Metab 2009 11 947 952
- Raskin P Lewin A Reinhardt R Lyness W for the repaglinide/metformin fixed-dose combination study group Twice-daily dosing of a repaglinide/metformin fixed-dose combination tablet provides glycemic control comparable to rosiglitazone/metformin tablet Diab Obes Metab 2009 11 865 873
- Wolffenbuttel BH Landgraf R A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes Diabetes Care 1999 22 463 467 10097930
- Marbury T Huang WC Strange P Lebovitz H Repaglinide versus glyburide: a one-year comparison trial Diabetes Res Clin Pract 1999 43 155 166 10369424
- Derosa G Mugellini A Ciccarelli L Crescenzi G Fogari R Comparison between repaglinide and glimepiride in patients with type 2 diabetes mellitus: a one-year, randomized, double-blind assessment of metabolic parameters and cardiovascular risk factors Clin Ther 2003 25 472 484 12749508
- Black C Donnelly P McIntyre L Royle P Shepherd JJ Thomas S Meglitinide analogues for type 2 diabetes mellitus Cochrane Database Syst Rev 2007 Issue 2 Art.No.:CD004654 10.1002/14651858.CD004654.pub2
- Salpeter SR Greyber E Pasternak GA Salpeter EE Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus Cochrane Database Syst Rev 2006 Issue 1 Art. No.: CD002967 10.1002/14651858.CD002967.pub2
- Owens DR Repaglinide – prandial glucose regulator: a new class of oral antidiabetic drug Diabet Med 1998 15 Suppl 4 S28 S36 9868989
- Saenz A Fernandez-Esteban I Mataix A Ausejo M Roque M Moher D Metformin monotherapy for type 2 diabetes mellitus Cochrane Database Syst Rev 2005 3 CD002966 16034881
- Bolen S Feldman L Vassy J Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus Ann Intern Med 2007 147 386 399 17638715
- Bristol-Myers Squibb Co Package insert for Glucophage® (metformin hydrochloride) tablets and Glucophage® XR (metformin hydrochloride extended-release) tablets 2009 http://packageinserts.bms.com/pi/pi_glucophage.pdf last accessed December 10, 2009
- Schramm TK Gislason G Vaag A Differences in risk of cardiovascular death according to type of oral glucose-lowering therapy in patients with diabetes: a nationwide study Diabetes 2009 58 Suppl 1 A24
- Bonneville M Colgin J Nalesnick JA Perez J Wentz L Patient perceptions of prandial oral therapy for type 2 diabetes Diabetes Educ 2001 27 659 677