Abstract
Mucocutaneous candidiasis is frequently one of the first signs of human immunodeficiency virus (HIV) infection. Over 90% of patients with AIDS will develop oropharyngeal candidiasis (OPC) at some time during their illness. Although numerous antifungal agents are available, azoles, both topical (clotrimazole) and systemic (fluconazole, itraconazole, voriconazole, posaconazole) have replaced older topical antifungals (gentian violet and nystatin) in the management of oropharyngeal candidiasis in these patients. The systemic azoles, are generally safe and effective agents in HIV-infected patients with oropharyngeal candidiasis. A constant concern in these patients is relapse, which is dependent on the degree of immunosuppression commonly seen after topical therapy, rather than with systemic azole therapy. Candida esophagitis (CE) is also an important concern since it occurs in more than 10% of patients with AIDS and can lead to a decrease in oral intake and associated weight loss. Fluconazole has become the most widely used antifungal in the management of mucosal candidiasis. However, itraconazole and posaconazole have similar clinical response rates as fluconazole and are also effective alternative agents. In patients with fluconazole-refractory mucosal candidiasis, treatment options now include itraconazole solution, voriconazole, posaconazole, and the newer echinocandins (caspofungin, micafungin, and anidulafungin).
Introduction
Fungi are found ubiquitously in nature in association with plants and mammals. Accordingly, humans are continually exposed to multiple genera of fungi via various routes, but particularly by the ingestion of food, allowing for the colonization of the gastrointestinal tract. Depending on the interaction between the host’s mucosal defense mechanisms, fungal virulence factors, and antifungal utilization, colonization may be transient or persistent and local disease may ensue.
Of the numerous pathogenic fungi, Candida is the dominant genus responsible for fungal diseases in humans.Citation1 Candida albicans is the species with the highest prevalence among human yeast isolates and is the main opportunistic yeast pathogen in most warm-blooded animals.Citation1
Symptomatic mucosal candidiasis (MC) arises in subjects colonized with Candida who are predisposed by illness, debility, or a local reduction in host resistance to an overgrowth of their own indigenous flora. Candida species are frequently isolated from the oral cavity and are detected in 31%–60% of healthy individuals.Citation1,Citation2 Colonization rates generally increase with the severity of illness and duration of hospitalization.Citation3 In a recent study, the frequency of oral yeast carriage in the competent host varied as a function of age.Citation2 The colonization rates increase from 24% in persons aged 5–7 years, to 59% in persons above the age of 60. In the hospitalized non-HIV infected individual, C. albicans accounted for 70%–80% of oral isolates and C. glabrata and C. tropicalis each represented approximately 5%–8%, while the other non-albicans Candida species were recovered only rarely.Citation4,Citation5
In the past decade, there has been a significant increase in the frequency of non-albicans Candida species isolated from HIV-infected individuals with MC.Citation6,Citation7 In the 1980’s, non-albicans Candida species accounted for 3.4% of oral isolates from HIV-infected patients, while in the 1990’s, 16.8% of isolates recovered from HIV patients were non-albicans Candida species. Citation6,Citation7 The more commonly recovered non-albicans Candida species, include C. glabrata, C. parapsilosis, C. tropicalis, and C. dubliniensis. In 5%–10% of circumstances multiple Candida species may be recovered from a single specimen. The more common combinations include C. albicans with C. glabrata, C. krusei, C. dubliniensis, or C. tropicalis.
Several local and systemic host and exogenous factors increase the prevalence of gastrointestinal (GI) tract Candida carriage and population levels.Citation8 The acuteness and extent of candidal infections increase with the number and severity of predisposing factors. The role of CD4+ T cells is to be the normal gastrointestinal mucosal defense mechanism against Candida species and this relation is highlighted by the frequent occurrence of oropharyngeal candidiasis (OPC) and esophageal candidiasis (EC) in patients with low CD4+ T cells and acquired immunodeficiency syndrome (AIDS).Citation8,Citation9 In HIV infection, oral carriage of yeast and risk of mucosal invasion increase with a progressive reduction in CD4+ T cells.Citation8,Citation10 The anti-Candida protective mechanism of CD4+ T cells at a mucosal level is still incompletely understood. Recently, investigators have shown that cytokines, especially gamma interferon, can inhibit the transformation of Candida blastoconidia to the more invasive hyphal phase.Citation8,Citation11 In addition, several investigators have shown that a decrease in E-cadherin levels and a loss of CD4+ T cells in the mucosa are associated with episodes of acute OPC.Citation12 The most commonly reported cause of higher GI yeast carriage rates and symptomatic oral candidiasis is the use of antibiotics.Citation1,Citation8 Elimination of bacterial competition is almost certainly the important mechanism by which antibiotics affect Candida numbers in vivo.
It is important to note that the introduction of highly active antiretroviral therapy (HAART), including protease inhibitors, has significantly reduced the prevalence of oropharyngeal and esophageal candidiasis in HIV-infected patients. In the first 12–24 months after the introduction of HAART, the prevalence of OPC decreased from 50%–80% down to ~10%.Citation13 In addition, a decrease of 25%–50% in the occurrence of EC was also documented. Unfortunately, there have been no further studies describing the epidemiology, incidence, or significance of either OPC or EC since 2004.
Based upon epidemiological studies, it is apparent that humans are exposed repeatedly to Candida in food and other sources. The natural history of this commensal “ normal” colonization over weeks, months, and years is poorly understood. Nevertheless, one may reasonably conclude that Candida colonization is almost universal.
Oropharyngeal candidiasis
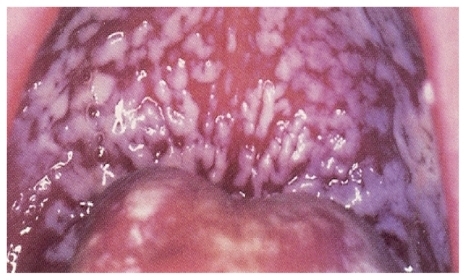
Oral candidiasis has been known since the era of Hippocrates, although Hippocrates used the term “aphthae” to describe this infection.Citation1 Several clinical forms of oral candidiasis exist; thrush is the most commonly and widely recognized and is also called acute pseudomembranous candidiasis (). Oropharyngeal candidiasis remains the most frequent opportunistic fungal infection among HIV-positive patients and is frequently the initial manifestation of HIV infection. Currently, it is estimated that approximately 80%–90% of HIV-infected patients develop OPC at some time during the progression of their disease from HIV infection to AIDS.Citation1,Citation13,Citation14,Citation15
C. albicans is the species responsible for the majority of cases of OPC.Citation1,Citation15 The ability of C. albicans to adhere to buccal epithelial cells is critical in establishing oral colonization. After colonization, the organisms may persist for months or years in low numbers in the absence of inflammation. These low numbers are the result of effective host defense mechanisms in the oral cavity. Genotyping of Candida strains obtained from HIV-infected patients with either OPC or EC indicate a genotype distribution frequency similar to that seen in non-HIV-infected subjects, suggesting that HIV-associated MC is not caused by a unique or particularly virulent strain of Candida, but likely results from defects in host defense mechanisms.Citation16
Symptoms of OPC can be extremely variable and range from asymptomatic oral lesions, to a sore, painful mouth, a burning tongue, and associated dysphagia. Clinical signs include diffuse erythema and white patches that appear as discrete lesions on the surfaces of the buccal mucosa, throat, tongue, and gums.Citation1,Citation14 Severe OPC may ultimately impair quality of life and result in a reduction of fluid or food intake. The most serious complication of untreated OPC is extension of the infection into the esophagus resulting in decreased nutritional intake.
Candida esophagitis
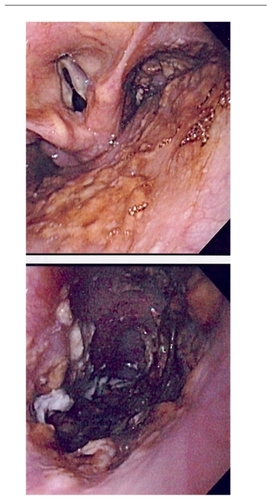
Candida is the most common cause of infectious esophagitis and, after the oropharynx, the esophagus is the second most common site of gastrointestinal candidiasis. The prevalence of Candida esophagitis (CE) has increased mainly because of its association with HIV-infected individuals. Approximately 10%–15% of patients with AIDS will eventually suffer from this entity during their lifetime.Citation1,Citation15,Citation17–Citation19
The same organisms that are recovered from the esophageal surface are generally the same organisms identified in oral secretions. C. albicans remains the most common organism identified in CE. In contrast to oral candidiasis, little is known about host and yeast factors operative in the pathogenesis of esophageal candidiasis and experimental models have not been established. However, it is likely that the usual yeast virulence factors and defects in host defense mechanisms are responsible. Esophageal candidiasis in HIV-positive patients may be the first manifestation of AIDS. The high prevalence of esophagitis in connection to AIDS indicates the critical role of cell-mediated immunity in normally protecting the esophagus from Candida invasion. Candida esophagitis tends to occur later in the natural history of HIV infection and almost invariably at lower CD4+ T cell counts (range 10–105, mean 79, median 30 cells).Citation1,Citation17–Citation19 It is not uncommon for patients with advanced AIDS, and near the end of life, suffering from severe esophageal candidiasis manifested by the inability to have any form of oral intake.
Candida esophagitis commonly causes dysphagia, odynophagia, and retrosternal pain. Although CE may arise as an extension of OPC, in approximately 10% of cases the esophagus maybe the only site involved affecting the distal two-thirds, rather than the proximal one-third, which is the area more commonly affected (). An occasional feature of CE is the complete lack of clinical symptoms despite extensive objective esophageal involvement.Citation1,Citation17–Citation19
A reliable diagnosis can only be made by direct visualization of the esophagus along with histological evidence of tissue invasion in biopsy material.Citation22,Citation21 However, clinical criteria may be accepted as a basis for initiating antifungal therapy in high risk patients. The differential diagnosis of EC must include gastroesophageal reflux disease (GERD), idiopathic HIV-ulcers, and viral esophagitis due to either cytomegalovirus or herpes simplex virus.Citation1,Citation20,Citation21
Therapy
Numerous antifungal agents are available for the treatment of OPC and EC in the HIV-positive patient ( and ). In addition, guidelines for the management of mucosal candidiasis in HIV-infected patients have been published by the Centers for Disease Control, National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America.Citation22 However, several factors should be considered when selecting antifungal agents for patients with HIV infection. In HIV-positive patients, antifungals are frequently less efficacious than in patients with other immunodeficiencies such as cancer. Similarly, the time to clinical response also tends to be delayed in this population.Citation17,Citation22,Citation23 Moreover, the relapse rate is higher in patients with HIV than in any other patient population. Citation1,Citation17,Citation22,Citation23 For unknown reasons, a subgroup of HIV-positive patients experience recurrent episodes of OPC and thus receive numerous courses of antifungals during their lifetime. As their HIV infection progresses they tend to experience shorter disease-free intervals between episodes of mucosal candidiasis and thus have greater antifungal exposure, which may ultimately lead to the development of clinical and in vitro antifungal resistance and its associated morbidity and mortality.
Table 1 Antifungals for oropharyngeal candidiasisTable Footnote*
Table 2 Antifungals for esophageal candidiasis
It is important to note that, as with some opportunistic pathogens in this patient population, antifungal treatment merely reduces the signs and symptoms of infection and thus produces a transient clinical response by lowering the quantity of organisms in the affected area. It is extremely difficult to fully eradicate Candida from the mucosal surfaces of patients who are immunocompromised, especially those who are HIV-positive. Citation8,Citation18,Citation23 The combination of frequent clinical relapses and increased antifungal utilization are frequently associated with antifungal resistance.Citation24 This is reflected, clinically, by the ineffectiveness of antifungals to which a patient has not previously been exposed. Thus, we are becoming increasingly aware that choosing the appropriate agent, even in the early stages of disease, is extremely important because of the future repercussions of these selections in advanced stages of infection.
Classes of agents used in the treatment of MC in HIV includes the polyenes (eg, amphotericin B, nystatin), pyrimidine synthesis inhibitors (flucytosine), azoles (miconazole, clotrimazole, ketoconazole, itraconazole, fluconazole, voriconazole, posaconazole), and, more recently, the echinocandins (caspofungin, micafungin, anidulafungin).Citation17,Citation22,Citation24 Common dosage regimens of these agents are listed in and .
The key trials evaluating antifungals in the treatment of OPC and EC are summarized in . Most controlled studies to date have evaluated the azole antifungal agents and in general, clinical response rates appear to be similar. Clinical response varies widely, as does the rate of relapse. Studies of antifungal treatment in MC can be problematic to evaluate, particularly in the HIV-positive population. Limitations often include a small number of patients, open label design, and a short follow-up time. Additional studies are particularly needed among patients with low CD4+ T lymphocyte counts, as this population tends to have lower clinical and mycological response rates. A recent meta-analysis suggested that larger studies using more consistent outcome measures and reporting would be helpful in applying future research to current clinical practice.Citation25 Additionally, these authors also suggested that future investigations should include less expensive antifungal interventions and, at the same time, evaluate symptom-free periods, quality of life, survival, and the development of clinical and in vitro resistance.
Table 3 Clinical trials of antifungal agents for the treatment of OPC and EC in HIV-positive patients
Therapy of oropharyngeal candidiasis
Prior to the development of the polyenes and the azoles, topical therapy consisted of gentian violet applications that were reasonably effective in localized MC, but were extremely messy because of the purple color.Citation24,Citation26
While topical therapies have historically been effective in less severe disease and are relatively inexpensive compared to some of the systemic therapies, their use has diminished due to poor tolerability and poor adherence. Topical therapy of OPC can be accomplished with a multitude of antifungal agents including nystatin, amphotericin B, clotrimazole, and, more recently, miconazole ().Citation17,Citation22,Citation24,Citation26–Citation31 Nystatin is available in several formulations, including pastilles and suspension. Limitations of topical agents such as nystatin include a bitter taste, GI-adverse effects, and frequent dosing, all of which may contribute to reduced adherence.Citation24,Citation27 Importantly, nystatin has not demonstrated significant efficacy in severely immunocompromised patients, such as those with advanced HIV infection.Citation27,Citation28 For instance, in HIV-positive patients, nystatin exhibited lower rates of both clinical cure (52% vs 87%) and mycological cure (6% vs 60%) when compared to oral fluconazole. Additionally, the 28- day relapse rate was found to be greater with nystatin (44% vs 18%) when compared to fluconazole.Citation27
Amphotericin B is also an option for topical therapy and is available in suspension, lozenge, and tablet form.Citation1,Citation22 Neither nystatin, nor amphotericin B is absorbed from the gastrointestinal tract so administration must be frequent (four times daily) to provide adequate drug exposure to the infected mucosal tissues.Citation1,Citation22 Prospective, comparative studies evaluating amphotericin B oral solution against other antifungals are limited. Topical azole antifungal agents, such as clotrimazole 10 mg troches, administered five times daily, provide another option for OPC patients. Topical clotrimazole has been used successfully in treating mild-to-moderate OPC during the early stages of HIV disease.Citation28–Citation30
The newest topical antifungal is the mucoadhesive buccal tablet (MMBT) containing 50 mg of miconazole (Loramyc®).Citation31 This novel formulation of miconazole has been approved in Europe since 2008 for the treatment of OPC in immucocompromised hosts. The formulation is unique, because 50 mg of miconazole is contained in each mucoadhesive tablet and is applied to the mucosa of the upper gum over the canine fossa, once daily, for 7–14 days. The MMBT adheres to the gum surface because of the milk protein concentrate composition of the tablet. This interaction leads to a rapid and prolonged adhesion to the mucosa due to an adsorption mechanism, followed by a protein-protein interaction. In pharmacokinetic studies, 50 mg of MMBT provides a maximum salivary concentration of 15 μg/mL, up to seven hours after the application of the tablet.Citation31 Three separate clinical studies have evaluated the use of MMBTs for the treatment of OPC in the HIV-positive population and in patients with head and neck cancers.Citation32–Citation34 In a phase III, double-blind, double-dummy, multicenter trial evaluating 578 patients with HIV and OPC, MMBT treatment was compared to clotrimazole troches (10 mg, five times daily, for a period of 14 days).Citation32 The results at the primary endpoint of test of cure (TOC) in both the intent-to-treat (ITT) population and in the per-protocol population (PP) demonstrated that the once daily administration of a MMBT was as effective as the five times daily clotrimazole treatment. Clinical cure rates at TOC in both the ITT (61% vs 65%) and PP (68% vs 74%) populations demonstrated no inferiority to clotrimazole. In addition, secondary endpoints such as safety and tolerability were similar between both treatment groups.
The use of topical antimycotic agents has been replaced with systemic azole antifungals such as ketoconazole, fluconazole, itraconazole, and more recently posaconazole ().Citation17,Citation24 Part of the reason for this is that, although clinical cure rates may be similar, microbiologic cure and long term efficacy are not. In one example, a study comparing systemic oral fluconazole with clotrimazole troches in HIV-positive adults found clinical efficacy to be similar (98% vs 94%). However, microbiological cure rates were greater in patients treated with fluconazole (65% vs 48%) and clinical response sustained through a 2-week follow up was also greater (82% vs 50%).Citation30
The currently available systemic azoles include ketoconazole, fluconazole, itraconazole, and posaconazole. Ketoconazole was the first available oral systemic imidazole antifungal agent with high rates of efficacy in OPC.Citation35,Citation36 However, in comparative trials, ketoconazole was found to be less efficacious then fluconazole in both clinical and mycological cure rates.Citation37 In addition, the use of ketoconazole is further limited by potentially severe adverse reactions including hepatotoxicity, poor oral bioavailability, and a host of drug–drug interactions.Citation36 Ketoconazole is a very potent inhibitor of cytochrome P450 3A4 and is relatively contraindicated with some HIV-protease inhibitors.Citation38 Because the drug’s absorption is dependent upon an acidic pH, there are also concerns that systemic absorption may be inadequate in patients receiving acid-suppressive therapy or with AIDS-related hypochlorhydria.Citation39 In view of the lower clinical efficacy rates and the associated adverse event profile (ie, hepatotoxicity), ketoconazole is not widely used anymore. In contrast to ketoconazole, other azoles such as fluconazole, itraconazole, voriconazole, and posaconazole have demonstrated improved efficacy, as well as excellent safety profiles, and have thus become the drugs of choice for OPC, especially in HIV-positive patients ().Citation1,Citation22,Citation24,Citation30
Fluconazole is the most commonly used antifungal in the treatment of MC in HIV-infected patients. It is available in oral suspension, tablet, and parenteral formulations. Fluconazole is more readily absorbed than other oral azoles without being affected by either food or gastric acidity. The clinical efficacy of fluconazole has been established in many well controlled clinical trials, such that it has become the standard comparator in clinical trials of novel agents.Citation24,Citation30,Citation37,Citation40–Citation42 Most studies of fluconazole have used an initial loading dose of 200 mg followed by 100 mg daily, but clinical success has been achieved with doses as low as 50 mg/day.Citation40 Clinical response is usually apparent within 10 days with 50 mg/day, and 5 to 7 days for doses of 100–200 mg/day.Citation17,Citation40 Of the available azoles, fluconazole is associated with the fewest drug–drug interactions because it has less affinity for the CYP3A4 enzyme.Citation38,Citation43 The pharmacological interactions with fluconazole include concomitantly administered phenytoin, rifampin, rifabutin, cyclosporin A, and possibly some of the protease inhibitors.Citation38,Citation39,Citation43–Citation45 Fluconazole is generally well tolerated, however, as with any other azole being used long term, periodic surveillance of liver enzymes to monitor for hepatotoxicity is useful.Citation24,Citation43
Itraconazole is supplied in a cyclodextrin oral solution or capsule; the parenteral formulation is no longer manufactured in the United States.Citation1,Citation24,Citation46 Like ketoconazole, itraconazole exhibits a strong potential for drug–drug interactions through the CPY3A4 enzyme system.Citation38,Citation46,Citation47 The itraconazole oral solution has greater bioavailability than the capsule and absorption is further enhanced by postprandial administration. Citation40,Citation46–Citation48 One prospective, randomized trial in HIV-positive and AIDS patients with OPC, found itraconazole oral solution to have similar efficacy and safety as fluconazole (clinical response 97% vs 87%).Citation49 However, approximately 50% of the patients in both groups experienced relapses at the 1-month follow-up evaluation.
Posaconazole is the newest triazole on the market and is approved for the treatment of acute OPC and antifungal-refractory MC.Citation50–Citation52 It is an oral extended-spectrum triazole with potent in vitro activity against pathogenic yeast and moulds, including fluconazole- and itraconazole-resistant Candida strains.Citation50 As with other azoles, posaconazole inhibits lanosterol 14-α-demehylase. It appears that mutations near the heme cofactor of CYP51 reduce the binding affinity of compact azoles, such as fluconazole and itraconazole, and may lead to azole crossresistance.Citation50 However, 3-dimensional binding models suggest that the long side chain of posaconazole may result in tighter binding affinity.Citation53,Citation54 Thus, posaconazole may be less susceptible than some azoles to the development of secondary azole resistance. Posaconazole is absorbed in an oral suspension.Citation50 It is important to note that posaconazole absorption is enhanced by coadministration with food, especially high fatty meals, or with a nutritional supplement such as Boost® Plus (Nestlé, Fremont, MI).Citation55,Citation56 When food intake is limited, dividing the daily dose from BID (twice daily) to QID (four times daily) also increases the plasma concentration. Unlike itraconazole and voriconazole, posaconazole is not primarily metabolized by fungal cytochrome P450 enzymes.Citation57 During several drug interaction trials evaluating the effects of posaconazole on the CYP450 enzymes, posaconazole did demonstrate an inhibitory effect on CYP3A4, but did not influence the other isoenzymes. In a large multicenter, randomized clinical trial, a 100 mg daily dose of posaconazole was compared to a 100 mg daily dose of fluconazole in HIV-positive patients with OPC.Citation58 Clinical success was reported in 92% of the posaconazole recipients compared with a success rate of 92% for those patients that received fluconazole. The only difference was in the long-term follow-up, where clinical relapses occurred more frequently in the fluconazole group compared to the posaconazole group (38.2% vs 31.5%). In general, the adverse events reported in both groups were comparable.
Several concerns have been raised about the widespread use of the more potent oral azoles, which may offer only minor advantages for patients. These concerns include drug interactions, side effects, increased expense, and risk of developing antifungal resistance. An increased frequency of C. glabrata isolation has been described by several investigators of HIV-positive patients receiving prolonged courses of fluconazole.Citation59–Citation62 In addition, although azole resistance in C. albicans is rare, several reports describe both clinical failure and in vitro resistance in both non-HIV patients and in HIV-infected patients on long-term azoles.Citation24,Citation63
Therapy of esophageal candidiasis
Systemic antifungal therapy using oral or parenteral fluconazole has been the mainstay in the management of EC for over a two decades ( and ).Citation1,Citation17 Topical antifungals such as nystatin, clotrimazole, and miconazole are of minimal value in EC.Citation22,Citation64 The initial step in the management of EC should always be to attempt to minimize all possible predisposing factors, such as corticosteroids, chemotherapeutic agents, and antimicrobials.Citation1,Citation17,Citation22
Oral fluconazole has an excellent safety profile when compared to ketoconazole, demonstrates excellent gastric absorption, and can also be given intravenously when necessary. Similar to the observations in OPC research, studies comparing fluconazole with either clotrimazole or ketoconazole for CE demonstrate cure rates that are superior to those with other imidazoles. Moreover, fluconazole demonstrated a more rapid onset of action and quicker resolution of symptoms. Citation65–Citation67 Itraconazole has also been shown to be effective in the treatment of EC.Citation68–Citation70 Patients treated with itraconazole oral solution (100–200 mg/day) had clinical response rates comparable to those of patients treated with fluconazole tablets (100–200 mg/day) of 94% and 91%, respectively, without significant adverse effects in either group.Citation68 The mycological cure rates were also similar at 92% and 78%, respectively.
Voriconazole, another broad spectrum systemic triazole is also approved for the treatment of EC at a dose of 200 mg BID for 14–21 days. In vitro, it has been shown to be 10- to 500-fold more potent than fluconazole against a wide array of yeast and moulds, including many isolates that have demonstrated in vitro itraconazole- and/or fluconazole-resistance. Citation71 In a double-blind, randomized, multicenter study, voriconazole 200 mg BID was compared to fluconazole 200 mg daily in EC.Citation72 The overall success rates were comparable at 98.2% for voriconazole vs 95% for fluconazole. Furthermore, the overall safety and adverse event profile of both agents was comparable. As with most azoles, voriconazole has an effect on the CYP450 enzymes, so caution regarding drug-drug interactions is warranted.Citation36,Citation71 A common side effect with voriconazole includes photopsia (visual abnormalities) that may occur in 20%–30% of patients. These abnormalities eventually resolve after 3–4 days of continued usage. Other side effects may include elevations in transaminases (~10%) and skin rashes (~10%), with rare cases of photodermatitis in patients who are exposed to direct sunlight.Citation71
Prior to the availability of azole antifungal agents, amphotericin B deoxycholate was used extensively. However, it is now rarely used in any patient and is generally reserved for antifungal-refractory cases of Candida esophagitis that do not respond to azoles or echinocandins.Citation73 If necessary, low dose amphotericin B (0.15–0.3 mg/kg/day or 10–20 mg/day for 10–14 days) is sufficient for moderate to severe disease.Citation1,Citation17,Citation73 However, because of the improved adverse event profiles of the lipid formulations of amphotericin B, they have also become popular. Oral flucytosine (100–150 mg/kg/day in divided doses), although effective, is rarely used because of the tendency for resistance to develop during therapy and the well described frequency of bone marrow suppression and transaminase elevation.Citation1,Citation74 Furthermore, in one comparative clinical trial, clinical efficacy and mycological cure rates were lower for flucytosine when compared to fluconazole.Citation74
The echinocandin class of antifungals, which includes caspofungin, micafungin, and anidulafungin are a novel class of antifungal agents with a completely different mechanism of action.Citation75 All three agents have demonstrated excellent in vitro activity against a broad array of Candida species, including those that are resistant to fluconazole, itraconazole, or voriconazole.Citation75 There have been a total of five clinical trials evaluating the efficacy of the three echinocandins in treating EC in HIV-positive patients. In all studies, the echinocandins are compared to either fluconazole or amphotericin B. In a clinical trial comparing caspofungin to amphotericin B in both OPC and EC in a population that was predominantly HIV-positive, clinical success rates for caspofungin ranged from 74% to 91%, which was numerically greater than the success rate for amphotericin B (63%).Citation76 In a related study, 177 patients with EC were stratified to receive either fluconazole 200 mg/day or caspofungin 50 mg/ day. Caspofungin was found to have similar response and relapse rates as fluconazole.Citation77 In another clinical trial, the treatment of EC among HIV-positive patients was also found to be similar for micafungin 150 mg/day when compared to fluconazole 200 mg/day.Citation78 In a separate multicenter clinical study in HIV-positive patients (n = 601, 75% with AIDS) evaluating anidulafungin versus fluconazole in patients with EC found this echinocandin to have similar clinical response rates when compared to fluconazole, in terms of both efficacy and safety.Citation79 So, while the echinocandins are not considered first line therapy for EC, they are effective antifungals for the treatment of MC in HIV-positive patients. Their use however, is limited because of the lack of an oral formulation.
Clinical relapse is not uncommon, especially in patients with persistent underlying immunodeficiency (eg, untreated and advanced HIV infection). Relapse appears to depend on the duration of antifungal therapy and degree of immunosuppression and may occur sooner following clotrimazole and ketoconazole therapy than after itraconazole, fluconazole, or posaconazole therapy.Citation30,Citation71,Citation80,Citation81 After several recurrences of symptomatic OPC in patients with AIDS, clinicians may consider maintenance (secondary) prophylaxis.Citation80
Several dosages of fluconazole as the primary prophylaxis have been evaluated. Although most studies documented a reduction in the frequency of MC in treated patients with AIDS, the regimens did not provide complete protection and occasional breakthrough infections occurred.Citation82,Citation84 Powderly et al compared fluconazole 200 mg/day and clotrimazole troches 10 mg five-times/day prophylactically in HIV-positive patients.Citation83 Overall, fluconazole reduced the frequency of MC, superficial fungal infections, and cryptococcal disease when compared to clotrimazole.Citation83 The benefit was greatest in patients with less than 50 CD4+ T cells/mm3. Unfortunately, in this study, the incidence of in vitro resistance and the effect fluconazole had on fungal flora was not evaluated. In addition, although the incidence of fungal infections was reduced, the survival rate was similar in both groups. In this setting, one must weigh the benefits against the cost of daily drug administration, not only in the financial sense, but also the influence of the agent on the patient’s mycoflora.
Schuman et al evaluated fluconazole 200 mg/week vs placebo in HIV-infected women with CD4+ T cell counts < 300 cells/mm.Citation85 The study concluded that weekly fluconazole was effective in preventing symptomatic OPC and vaginal candidiasis while the rates of clinical and in vitro resistance were low. Additionally, women receiving fluconazole had a reduction in the colonization rates of C. albicans, but had an increased isolation of non-albicans Candida species. In a recent multicenter, randomized clinical trial evaluating the role of prophylaxis in HIV-positive patients, Goldman et al showed that the number of episodes of OPC and other invasive fungal infections was statistically lower in HIV-positive patients with CD4+ T counts < 150 cells/mm3, when receiving continuous (three times a week fluconazole) when compared to those patients only receiving episodic treatment with fluconazole for OPC recurrences.Citation84 The study also demonstrated that the incidence of clinically significant resistance was no higher in the group receiving continuous therapy than in the group using episodic administration of fluconazole, provided the patients were on highly active antiretroviral therapy.
In general, when making the clinical decision to initiate secondary prophylaxis the physician should consider several key points: the impact of excessive recurrences on the patients well being and quality of life, the need for prophylaxis of other fungal infections, financial costs, adverse event profiles, and drug-drug interactions.
Management of antifungal-refractory mucosal candidiasis
The clinical impact of antifungal resistance in patients with AIDS has been demonstrated in patients who failed conventional antifungal therapy for MC.Citation24,Citation86 After the development of fluconazole-resistant OPC, patients were noted to have a median survival of approximately 184 days. Moreover, after the onset of clinical resistance to parenteral amphotericin B, patients were found to have an astonishing 83-day median survival rate.Citation24,Citation86 Although MC per se is not fatal, clinical failure is probably a comorbid factor associated with the rapid demise of these patients. Clinical failure may also be a marker of severe immunosuppression and a dysfunctional immune system.
Antifungal resistance can be divided into two categories, clinical and in vitro. Clinical resistance signifies failure of the antifungal to eradicate the infection in the absence of in vitro resistance. Such resistance may occur for a variety of reasons. In vitro resistance can also be subdivided into either primary (innate or intrinsic) or secondary (acquired) resistance.Citation24,Citation86
The usefulness of bacterial in vitro susceptibility testing is well established in the management of patients with infectious diseases. However, despite the fact that a subcommittee for in vitro antifungal susceptibility testing published approved guidelines, the utility of these results in managing fungal infections is still somewhat controversial.Citation87,Citation88 Several studies have revealed a correlation between the in vitro susceptibility minimum inhibitory concentration [MIC] results of C. albicans and the clinical response to antifungal treatment in HIV-infected patients.Citation60,Citation87,Citation88 Investigators have published therapeutic antifungal successes and failures in patients with OPC and Candida isolates for which MICs were both low and high. In fact, one group has reported several HIV-infected patients with documented in vitro fluconazole-resistant MC who were still able to respond to fluconazole therapy.
Initially, it was believed that the trigger for the emergence of resistance was primarily due to the use of low dose fluconazole for acute treatment as well as for prophylaxis.Citation86,Citation89,Citation90 More recently, investigators have demonstrated that MC due to resistant isolates is seen in patients with low CD4+ cell counts (<50 cells/mm3).Citation91 Conversely, another study indicated that a low CD4+ cell count did not predict azoleresistant MC in patients with AIDS.Citation61
Investigators have be able to identify several key risk factors associated with the development of fluconazole-resistant MC in patients with AIDS, compared to those patients without evidence of fluconazole-resistant MC. These risk factors include: greater number of episodes of OPC (6.1 vs 1.8), lower median CD4+ cell count (11 vs 71 cells/mm3), longer median duration of antifungal therapy (419 vs 118 days), and longer duration of systemic azoles (272 vs 14 days).Citation91 When the authors used two controls matched by CD4+ cell count, resistant cases continued to have a greater median exposure time to azoles (272 vs 88 days; P = 0.005) as the significant risk factor.Citation61,Citation91
It is still not clear whether the total dose of antifungals, the duration of therapy, the type of antifungal, and/or the pattern of drug administration (continuous vs episodic) are the most important determinants in the development of antifungal-refractory fungal disease.Citation24,Citation84,Citation90,Citation91 More than likely, the etiology is multifactorial involving a combination of advanced immunosuppression, high fungal burdens, and prolonged exposure time to antifungals.
The management of fluconazole-resistant MC is frequently unsatisfactory and the clinical response is short-lived with periodic and rapid recurrences. In some patients, the refractory candidiasis may respond to increases in the dose of fluconazole (). For example, if patients fail fluconazole 200 mg/day, a dosage increase to 400–800 mg/day will frequently produce a clinical response for a period of time. However, the response is generally transient and the disease recurs rapidly once patients have reached this stage. As noted, clinical and in vitro azole resistance is not uncommon in patients who fail to respond to fluconazole. Occasionally, in some patients with fluconazole-refractory MC, fluconazole suspension may be beneficial.Citation91 Several reports describe improvement in these patients, possibly associated with increased salivary levels of fluconazole, which results when the suspension is taken with a swish-and-swallow technique.Citation91
Table 4 Alternative therapies for the management of antifungal-refractory mucosal candidiasis in patients with AIDS
Several clinical trials evaluating itraconazole oral solution have demonstrated promising results in patients with AIDS who failed fluconazole 200 mg/day.Citation92–Citation95 A clinical cure or improvement occurred in 55%–70% of these patients. As expected, mycological cure rates were low (<30%) and relapses following treatment cessation were rapid, usually within 14 days.
The two newly expanded spectrum triazoles, voriconazole and posaconazole, have been used successfully in patients with refractory MC.Citation96,Citation97 Posaconazole is licensed in the United States for the treatment of refractory OPC/EC. In the largest clinical trial evaluating antifungal agents for refractory OPC/EC, posaconazole 400 mg suspension was given either QD (once daily) or BID and was evaluated in subjects with documented clinical resistance to either fluconazole or itraconazole.Citation96 Of the 176 subjects enrolled, 132 (75%) achieved a clinical response after 28 days of therapy with very few adverse events. Only eight patients discontinued the study drug because of a side effect. As expected, during the 4-week follow up period the overall clinical relapse rate was 74%.
It is important to note that with any fungal infection in patients with advanced HIV infection it is essential to continue suppressive antifungal therapy in an attempt to increase disease-free intervals and avoid morbidity and occasional mortality. These patients generally suffer from advanced-HIV disease and ultimately very low CD4+ T cells (<50) and high viral burdens along with uncontrolled HIV infection.Citation81
It is important not to underestimate the significance of the underlying dysfunctional immune system in patients with AIDS and refractory fungal infections. Although randomized clinical trials have not been done, the initiation of HAART will frequently assist in the resolution of these recalcitrant infections. In fact, treatment with HAART alone without antifungals have eradicated antifungal-refractory OPC in patients with advanced HIV infection.Citation98,Citation99
The classic management of infections in the compromised host has depended upon antimicrobial agents, without taking into account host defects. Several cytokines developed and produced by recombinant technology show promise in assisting the host response to fungal infection.Citation100,Citation101 There have been several reports describing the use of human recombinant granulocyte-macrophage colony stimulating factor (rhuGM-CSF) in patients with OPC or EC refractory to fluconazole and amphotericin B.Citation100,Citation101 Although no large studies have been published, case reports describe good response rates with rhuGM-CSF in patients with advanced HIV infection and refractory mucosal candidiasis.Citation101,Citation102 Further studies evaluating these cytokines are certainly warranted.
Alternative therapeutic modalities using organic substances are also being administered empirically to combat refractory MC. One of these formulations is Melaleuca alternafolia, or Australian tea tree oil that has been formulated into an oral solution. A small, single center pilot study evaluating the melaleuca oral solution in 14 patients with AIDS and fluconazole-refractory OPC has been completed.Citation103 The results appear to indicate relatively good efficacy in these difficult-to-treat patients, demonstrating a good clinical response in 10 of 12 patients after four weeks of antifungal therapy. However, larger comparative studies are necessary to evaluate the role of this agent in refractory candidiasis.
In conclusion, significant advances in antifungal therapy have been made in the last decade. The impressive clinical trial results and the low grade side effect profile of the azole compounds continue to make them attractive choices in the management of fungal infections in any immunocompromised patient, especially HIV-positive patients. However, difficulties in managing these infections should remind us that we can not rely solely on antifungals. We must continue to find ways to improve the body’s dysfunctional immune system. In addition, we must continue to develop more effective antifungal agents with different mechanisms of action and different modes of administration.
Disclosure
The author reports no conflict of interest in this work.
References
- VazquezJASobelJDCandidiasisDismukesWEPappasPGSobelJDClinical MycologyOxfordOxford University Press2003143187
- KleineggerCLLockhartSRVargasKSollDRFrequency, intensity, species, and strains of oral Candida vary as a function of host ageJ Clin Microbiol199634224622548862593
- JohnstonRDChickEWJohnstonNSJarvisMAAsymptomatic quantitative increase of Candida albicans in the oral cavity: predisposing conditionsSouth Med J196760124412474862923
- StenderupAPedusenGTYeast of human originActa Pathol Microbiol Scand19625446247213916820
- MackenzieDWRYeast from human sourcesSabouraudia1962181513764908
- van’t WoutJWFluconazole treatment of candidal infections caused by non-albicans Candida speciesEur J Clin Microbiol Infect Dis1996152382428740860
- BarchiesiFMorbiducciVAncaraniFScaliseGEmergence of oropharyngeal candidiasis caused by non-albicans species of Candida in HIV-infected patientsEur J Epidemiol199394554568243605
- Cunha VillarCDongari-BagtzoglouAImmune defence mechanisms and immunoenhancement strategies in oropharyngeal candidiasisExpert Rev Mol Med200810e29118
- ChandlerFWPathology of the mycoses in patients with the acquired immune deficiency syndrome (AIDS)Curr Topics Med Mycol19851123
- MelbyeMSchonheyderHKestersLCarriage of oral Candida albicans associated with a high number of circulating suppressor T lymphocytes. [Letter]J Infect Dis1985152135613572933473
- Kalo-KleinAWitkinSSProstaglandin E2 enhances and gamma interferon inhibits germ tube formation in Candida albicansInfect Immunity1990582602622152888
- FidelPLLillyERufenerJBLongitudinal analysis of local immune function in HIV(+) subjects with oropharyngeal candidiasisProgram and Abstracts of the 49th Annual Interscience Conference on Antimicrobial Agents and ChemotherapySan Francisco, CA2009 September 12–15 Abstract# M-365
- de RepentignyLLewandowskiDJolicoeurPImmunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infectionClin Microbiol Reviews200417729759
- SilvermanSJrGalloJWMcKnightMLMayerPdeSanzSTanMMClinical characteristics and management responses in 85 HIV- infected patients with oral candidiasisOral Surg Oral Med Oral Pathol Oral Radiol Endod1996824024078899777
- FeigalDWKatzMHGreenspanDThe prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohortsAIDS199155195251863403
- PowderlyWGRobinsonKKeathEJMolecular typing of Candida albicans isolated from oral lesions of HIV-infected individualsAIDS1992681841543569
- DarouicheROOropharyngeal and esophageal candidiasis in immunocompromised patients: treatment issuesClin Infect Dis1998262592749502438
- SelikRMStarcherETCurranJWOpportunistic diseases reported in AIDS patients: frequencies, associations, and trendsAIDS198711751822831912
- ClotetBGrifolMParroBAsymptomatic esophageal candidiasis in acquired-immunodeficiency-syndrome-related complex. [Letter]Ann Intern Med19861051453717801
- KodsiBEWickremesinghePCKozinnPJIswaraKGoldbergPKCandida esophagitis: a prospective study of 27 casesGastroenterology197671715719964563
- ScottBBJenkinsDGastro-oesophageal candidiasisGut1982231371397068036
- Centers for Disease Control, National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of AmericaGuidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescentsMMWR200932858
- GlattAEChirgwinKLandesmanSHTreatment of infections associated with human immunodeficiency virusN Engl J Med1988318143914483285211
- VazquezJADiagnosing and managing oropharyngeal candidiasisInfect in Med200724427436
- PienaarEDYoungTHolmesHInterventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and childrenCochrane Database of Syst Rev20061
- KozinnPJTaschdjianCLDragutskyDTherapy of oral thrush: a comparative evaluation of gentian violet, mycostatin and amphotericin BMonogr Ther195721624
- PonsVGreenspanDLozada-NurFOropharyngeal candidiasis in patients with AIDS: randomized comparison of fluconazole versus nystatin oral suspensionsClin Infect Dis199724120412079195083
- QuintilianiROwensNJQuerciaRAKlimekJJNightingaleCHTreatment and prevention of oropharyngeal candidiasisAm J Med199477Suppl 4D44486093531
- ShectmanLBFunaroLRobinTBottoneEJCuttnerJClotrimazole treatment of oral candidiasis in patients with neoplastic diseaseAm J Med1984769194
- PonsVGreenspanDDebruinMTherapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, prospective multicenter study of oral fluconazole versus clotrimazole trochesJ Acquir Immune Defic Syndr19936131113168254467
- CardotJMChaumontCDubrayCCostantiniDAiacheJMComparison of the pharmacokinetics of miconazole after administration via a bioadhesive slow release tablet and an oral gel to healthy male and female subjectsBr J Clin Pharmacol20045834535115373926
- VazquezJAEpsteinJAttaliPA multicenter, randomized trial evaluating the efficacy and safety of miconazole mucoadhesive buccal tablets (MMBT) versus clotrimazole troches (CT) for the treatment of oropharyngeal candidiasis (OPC) in subjects with HIV/AIDS:SMiLES TrialProgram and Abstracts of the 49th Annual Interscience Conference on Antimicrobial Agents and ChemotherapySan Francisco, CA2009 September 12–15 Abstract# M-1245
- AttaliPVazquezJBensadounRJDupontBEvaluation of miconazole mucoadhesive buccal tablet: a novel, once daily, antifungal regimen for the treatment of oropharyngeal candidiasis44th American Society of Health System Pharmacist Mid-Year Clinical Meeting and exhibitionThe Venetian Hotel, Las Vegas, NV2009 Dec 6–10 Abstract 6–068
- BensadounRJDaoudJEl GueddariRComparison of the efficacy and safety of miconazole 50-mg mucoadhesive buccal tablets with miconazole 500-mg gel in the treatment of oropharyngeal candidiasisCancer200811220421118044772
- HughesWTBartleyDLPattersonGGTufenkejiHKetoconazole and candidiasis: a controlled studyJ Infect Dis1983147106010636304203
- ComoJADismukesWEOral azole drugs as systemic antifungal therapyN Eng J Med1994330263272
- DeWitSWeertsDGoossensHClumeckNComparison of fluconazole and ketoconazole for oropharyngeal candidiasis in AIDSLancet198917467482564563
- MeyerJMRodvoldKADrug biotransformation by the cytochrome P-450 enzyme systemInfect Med19966452464
- PiscitelliSCFlexnerCMinorJRPolisMAMasurHDrug interactions in patients infected with human immunodeficiency virusClin Infect Dis1996236856938909827
- HayRJOverview of studies of fluconazole in oropharyngeal candidiasisRev Infect Dis199012 Suppl 3S334S3372184511
- MeunierFAounMGerardMTherapy of oropharyngeal candidiasis in the immunocompromised host: a randomized double-blind study of fluconazole vs ketoconazoleRev Infect Dis199012 Suppl 3S364S3682184513
- KoletarSLRussellJAFassRJPlouffeJFComparison of oral fluconazole and clotrimazole troches as treatment for oral candidiasis in patients infected with human immunodeficiency virusAntimicrob Agents Chemother199034226722682073120
- KowalskySFDixonDMFluconazole: a new antifungal agentClin Pharm1991101791942040125
- ZimmermannTYeatesRALaufenHPfaffGWildfeuerAInfluence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazoleEur J Clin Pharmacol1994461471508039534
- TuckerRMDenningDWHansonLHInteractions of azoles with rifampin, phenytoin, and carbamazepine: in vitro and clinical observationsClin Infect Dis1992141651741315160
- Van de VeldeVJSVan PeerAPHeykantsJJPEffect of food on the pharmacokinetics of a new hydroxypropyl-β-cyclodextrin formulation of itraconazolePharmacotherapy1996164244288726601
- Data on FileJanssen PharmaceuticaBeerse, Belgium
- BaroneJAMoskovitzBLGuarnieriJHassellAEEnhanced bio-availability of itraconazole in hydroxypropyl-β-cyclodextrin solution compared with capsules in healthy volunteersAntimicrob Agents Chemother199842186218659661037
- GraybillJRVazquezJDarouicheROItraconazole oral solution: a novel and effective treatment for oropharyngeal candidiasis in HIV/ AIDS patientsAm J Med199810433399528717
- HerbrechtRPosaconazole: a potent extended spectrum triazole anti-fungal for the treatment of serious fungal infectionsInt J Clin Practice200458612624
- VazquezJAPosaconazole for the management of mucosal candidiasisFuture Microbiol2007224525617661697
- VazquezJARole of posaconazole in the management of oropharyngeal and esophageal candidiasisTherap Clin Risk Management20073533542
- LiXBrownNChauASChanges in susceptibility to posaconazole in clinical isolates of Candida albicansJ Antimicrob Chemother200453748014657086
- XiaoLMadisonVChauASLoebenbergDPalermoREMcNicholasPMThree-dimensional models of wild-type and mutated forms of cytochrome P450 14α-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole bindingAntimicrob Agents Chemother2004482124213115155210
- CourtneyRSansoneACalzettaAThe effect of a nutritional supplement (Boost® Plus) on the oral bioavailability of posaconazoleProgram and Abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy2003 Sept 14–17Chicago, IL
- CourtneyRWexlerDRadwanskiEEffect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adultsBr J Clin Pharmacol20045721822214748822
- WexlerDLaughlinMCourtneyREffect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way cross-over studyEur J Pharm Sci20042164565015066665
- VazquezJASkiestDJNietoLA multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDSClin Infect Dis2006421179118616575739
- ChavanetPLopezJGrappinMCross-sectional study of the susceptibility of Candida isolates to antifungal drugs and in vitroin vivo correlation in HIV-infected patientsAIDS199489459507946104
- QueredaCPolancoAMGinerCCorrelation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patientsEur J Clin Microbiol Infect Dis19961530378641300
- MaenzaJRMerzWGRomagnoliMJKerulyJCMooreRDGallantJEInfection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiologyClin Infect Dis19972428348994752
- DrondaFAlonso-SanzMLagunaFMixed oropharyngeal candidiasis due to Candida albicans and non-albicans Candida strains in HIV-infected patientsEur J Clin Microbiol Infect Dis1996154464528839637
- ChryssanthouETorssandeerJPetriniBOral Candida albicans isolates with reduced susceptibility to fluconazole in Swedish HIV-infected patientsScand J Infect Dis1995273913958658076
- GinsburgCHBradenGLTauberAITrierJSOral clotrimazole in the treatment of esophageal candidiasisAm J Med1981718918957304661
- De WitSUrbainDRahirFWeertsDClumeckNEfficacy of oral fluconazole in the treatment of AIDS associated oesophageal candidiasisEur J Clin Microbiol Infect Dis1991105035051915385
- LaineLDretlerRHConteasCNFluconazole compared with ketoconazole for the treatment of Candida esophagitis in AIDS. A randomized trialAnn Intern Med19921176556601308663
- LaineLRabeneckLProspective study of fluconazole suspension for the treatment of oesophageal candidiasis in patients with AIDSAliment Pharmacol Ther199595535568580277
- WilcoxCMDarouicheROLaineLMoskovitzBLMallegolIWuJA randomized, double-blind comparison of itraconazole oral solution and fluconazole tablets in the treatment of esophageal candidiasisJ Infect Dis19971762272329207371
- BarbaroGBarbariniGDi LorenzoGFluconazole compared with itraconazole in the treatment of esophageal candidiasis in AIDS patients: a double-blind, randomized, controlled clinical studyScand J Infect Dis1995276136178685642
- BarbaroGBarbariniGCalderonWGrisorioBAlciniPDiLorenzoGFluconazole versus itraconazole for Candida esophagitis in acquired immunodeficiency syndromeGastroenterol199611111691177
- SheehanDJHitchcockCASibleyCMCurrent and emerging azole antifungal agentsClin Microbiol Rev19991240799880474
- AllyRSchurmannDKreiselWA randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patientsClin Infect Dis2001331447145411577374
- LakeDEKunzweilerJBeerMBuellDNIslamMZFluconazole versus amphotericin B in the treatment of esophageal candidiasis in cancer patientsChemotherapy1996423083148804799
- BarbaroGBarbariniGDi LorenzoGFluconazole vs flucytosine in the treatment of esophageal candidiasis in AIDS patients: a double-blind, placebo controlled studyEndoscopy1995273773837588352
- CappellettyDEiselstein-McKitrickKThe echinocandinsPharmacother200727369388
- ArathoonEGGotuzzoENoriegaLMBermanRSDiNubileMJSableCARandomized, double-blind, multicenter study of caspofungin versus Amphotericin B for treatment of oropharyngeal and esophageal candidiasisAntimicrob Agents Chemother20013315291535
- VillanuevaAGotuzzoEArathoonEGA randomized, double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasisAm J Med200211329429912361815
- de WetNTEBesterAJViljoenJJA randomized, double blind, comparative trial of micafungin (FK463) vs fluconazole for the treatment of oesophageal candidiasisAliment Pharmacol Ther20052189990715801925
- KrauseDSSimjeeAEvan RensburgCA randomized, double- blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasisClin Infect Dis20043977077515472806
- ReefSEMayerKHOpportunistic candidal infections in patients infected with human immunodeficiency virus: prevention issues and prioritiesClin Infect Dis199521 Suppl 1S99S1028547520
- VazquezJASkiestDJTissot-DupontHLennoxJLBoparaiNIsaacsRSafety and efficacy of posaconazole in the long-term treatment of azole-refractory oropharyngeal and esophageal candidiasis in patients with HIV infectionHIV Clin Trials20078869717507324
- StevensDAGreeneILangOSThrush can be prevented in patients with acquired immunodeficiency syndrome and the acquired immunodeficiency syndrome-related complex. Randomized, double-blind, placebo-controlled study of 100-mg oral fluconazole dailyArch Intern Med1991151245824641747004
- PowderlyWGFinkelsteinDMFeinbergJA randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infectionN Engl J Med19953327007057854376
- GoldmanMCloudGAWadeKDA randomized study of the use of fluconazole in continuous versus episodic therapy in patients with advanced HIV infection and a history of oropharyngeal candidiasisClin Infect Dis2005411473148016231260
- SchumanPCappsLPengGWeekly fluconazole for the prevention of mucosal candidiasis in women with HIV infection. A randomized, double-blind, placebo-controlled trialAnn Intern Med19971266896969139554
- VazquezJASobelJDEpidemiologic overview of resistance to oral antifungal agents in the immunocompromised hostExcerpta Medica Abstract1997111
- Clinical and Laboratory Standards InstituteReference method for broth dilution antifungal susceptibility testing of yeasts3rd EditionApproved Standard M27-A3, Clinical and Laboratory Standards InstituteWayne, PA2008
- CameronMLSchellWABruchSBartlettJAWaskinHAPerfectJRCorrelation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1Antimicrob Agents Chemother199337244924538285632
- RevankarSGDibOPKirkpatrickWRClinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patientsClin Infect Dis1998269609639564483
- HornCAWashburnRGGivnerLBPeacockJEJrPegramPSAzole-resistant oropharyngeal and esophageal candidiasis in patients with AIDSAIDS199595335347639986
- MaenzaJRKerulyJCMooreRDChaissonREMerzWGGallantJERisk factors for fluconazole-resistant candidiasis in human immunodeficiency virus-infected patientsJ Infect Dis19961732192258537662
- McCarthyGMMackieIDKovalJSandhuHSDaleyTDFactors associated with increased frequency of HIV-related oral candidiasisJ Oral Pathol Med1991203323361680189
- ParvingHFluconazole suspension for oropharyngeal candidiasis unresponsive to tabletsAnn Intern Med1997126332333
- PhilipsPZemcovJMahmoodWMontanerJSGCraibKClarkeAMItraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibilityAIDS199610136913768902066
- EichelMJust-NüblingGHelmEBStilleWItraconazole suspension in the treatment of HIV-infected patients with fluconazole-resistant oropharyngeal candidiasis and esophagitisMycoses199639 Suppl 11021068767280
- CartledgeJDMidgleyJGazzardBGItraconazole cyclodextrin solution: the role of in 6 vitro susceptibility testing in predicting successful treatment of HIV-related fluconazole-resistant and fluconazole- susceptible oral candidosisAIDS1997111631689030362
- CoppolaSAngaranoGMontagnaMTEfficacy of itraconazole in treating AIDS-associated infections due to Candida kruseiEur J Epidemiol1995112432447672085
- SkiestDJVazquezJAAnsteadGMPosaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infectionClin Infect Dis20074460761417243069
- RuhnkeMSchmidt-WesthausenATrautmannMIn vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infectionAntimicrob Agents Chemother1997415755779055995
- ZingmanBSTreatment of mucosal candidiasis in HIV-infected patientsJ Mycol Med19966311
- ZingmanBResolution of refractory AIDS-related mucosal candidiasis after initiation of didanosine plus saquinavir. [Letter]N Engl J Med1996334167416758628377
- VecchiarelliAMonariCBaldelliFBeneficial effect of recombinant human granulocyte colony-stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDSJ Infect Dis1995171144814547539473
- SwindellsSKleinschmidtDRHayesFAPilot study of adjunctive GM-CSF (yeast-derived) for fluconazole-resistant oral candidiasis in HIV-infectionInfect Dis Clin Practice19976278279
- VazquezJAGuptaSVillanuevaARole of rhu-GM-CSF as adjunctive treatment of antifungal refractory oropharyngeal candidiasis in patients with AIDSEur J Clin Microbiol199817781783
- VazquezJAVaishampayanJArganozaMTRichardsSBoikovDSobelJDUse of an over the counter product, Breathaway® (melaleuca oral solution), as an alternative agent for refractory oropharyngeal candidiasis in AIDS patientsAIDS199812103310379662200
- SmithDEMidgleyJAllanMConnollyGMGazzardBGItraconazole versus ketoconazole in the treatment of oral and oesophageal candidosis in patients infected with HIVAIDS19915136713711662959
- BarchiesiFGiacomettiAArzeniDFluconazole and ketoconazole in the treatment of oral and esophageal candidiasis in AIDS patientsJ Chemother199243813861287140
- de RepentignyLRatelleJComparison of itraconazole and ketoconazole in HIV-positive patients with oropharyngeal or esophageal candidiasisChemotherapy1996423743838874977