Abstract
Human immunodeficiency virus-1 infection of the central nervous system is an early event after primary infection, resulting in motor and cognitive defects in a significant number of individuals despite successful antiretroviral therapy. The pathology of the infected brain is characterized by enhanced leukocyte infiltration, microglial activation and nodules, aberrant expression of inflammatory factors, neuronal dysregulation and loss, and blood–brain barrier disruption. Months to years following the primary infection, these central nervous system insults result in a spectrum of motor and cognitive dysfunction, ranging from mild impairment to frank dementia. The mechanisms that mediate impairment are still not fully defined. In this review we discuss the cellular and molecular mechanisms that facilitate impairment and new data that implicate intercellular communication systems, gap junctions and tunneling nanotubes, as mediators of human immunodeficiency virus-1 toxicity and infection within the central nervous system. These data suggest potential targets for novel therapeutics.
Introduction
Human immunodeficiency virus-1 (HIV) infection has expanded into a global health pandemic since the first cases were described in 1981, having infected an estimated 33 million people worldwide (UNAIDS, 2008). In the early 1990s, the development of antiretroviral therapy changed the face of the disease. In the developed world, HIV went from causing a fatal disease to being a chronic infection because viral loads are frequently undetectable and CD4 counts are restored to near normal levels in most individuals on antiretroviral therapy. However, the viral reservoirs that persist in infected individuals are not eradicated and, because people live longer with HIV, the virus continues to cause damage despite low viral replication. A predominant clinical consequence of HIV that persists despite treatment is its neurological effects, which are collectively referred to as HIV-associated neurocognitive disorders (HAND) and occur in approximately 50% of infected individuals.Citation1 Because HIV infected people on antiretroviral therapy live longer, the prevalence of cognitive and motor dysfunction is increasing.Citation2,Citation3 Thus, the need for effective central nervous system (CNS)-targeted therapeutics is becoming even more important. The mechanisms of HIV infection in the CNS remain incompletely characterized and it is likely that many therapeutic targets have yet to be identified.
Clinical consequences of HIV infection in the CNS
The clinical features of HAND are both subcortical and cortical, and individuals can develop cognitive impairment in a number of different domains. The clinical manifestations are divided into three major groups: asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HIV-associated dementia.Citation4 Asymptomatic neurocognitive impairment is defined by performance at least one standard deviation below the mean in at least two cognitive areas with no impairment in activities of daily living, mild neurocognitive disorder is performance at least one standard deviation below the mean in at least two cognitive areas with mild to moderate impairment in activities of daily living, and HIV-associated dementia is performance at least two standard deviations below the mean in at least two cognitive areas with severe impairment in activities of daily living.Citation4 Before antiretroviral therapy, over 15% of late stage HIV-infected individuals developed HIV-associated dementia and 50% of infected individuals developed some form of HAND.Citation1 Now, the number with HIV-associated dementia has decreased to less than 5%, but the percentage of individuals with mild neurocognitive disorder has remained at 20%, and the percentage with asymptomatic neurocognitive impairment has increased to 25%.Citation1 Thus, 50% of HIV-infected individuals still develop HAND.Citation1
HAND results in a triad of cognitive, behavioral and motor dysfunction and its progression is often not unidirectional. HIV-infected individuals may progress to asymptomatic neurocognitive impairment, then mild neurocognitive disorder, then HIV-associated dementia, but they can also fluctuate between these forms or go into remission. Diagnosis of HAND is based on neuropsychological testing, neuroimaging, and exclusion of delirium or any CNS opportunistic infections or other cause of dementia as the reason for the neurocognitive impairment.Citation4,Citation5 For diagnosis, neuropsychological testing must include the following domains: verbal/language, attention/working memory, abstraction/executive, memory (learning/recall), speed of information processing, sensory-perceptual, and motor skills.Citation4 The standard of neuroimaging is magnetic resonance imaging, which demonstrates cerebral atrophy and ventricular enlargement in impaired individuals, but there is ongoing work to develop better imaging tools for diagnosis of HAND.Citation5–Citation7
HIV neuropathology
Soon after infection, as early as 15 days,Citation8 HIV enters the CNS in the majority of, if not all, infected individuals. Viral entry into the CNS is believed to be mediated through a “Trojan horse” mechanism, whereby HIV-infected monocytes cross the blood–brain barrier and then release virus into the CNS. HIV released from these infected cells then causes infection in the brain that results in significant inflammation and eventually neuronal damage and loss.
HIV primarily infects CNS macrophages and microglia and can infect astrocytes at low levels.Citation9–Citation13 There is little evidence for HIV infection of oligodendrocytes and neurons. However, significant neuronal dysfunction, including axonal and dendritic pruning, is prevalent throughout the CNS.Citation14–Citation18 This results from the inflammatory factors and neurotoxic substances released by infected as well as activated, uninfected cells. HAND pathology is characterized by multinucleated giant cells, microglial nodules, gliosis, myelin pallor, and neuronal loss, demonstrating the significant role of immune cells and subsequent damage to neurons in the pathogenesis of this disease.
Mechanisms of HIV entry into the CNS and infection of CNS cells
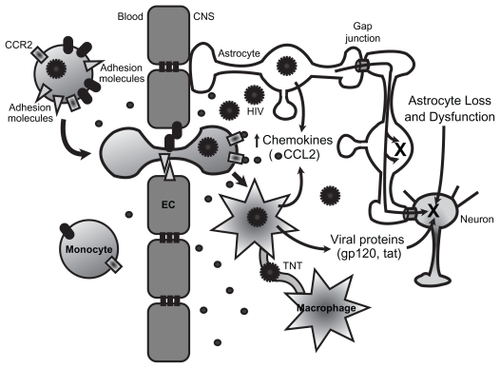
As discussed above, the predominant means of HIV entry into the CNS is through a “Trojan horse” mechanism by which infected monocytes cross the blood–brain barrier and release virus within the CNS that can then infect resident cells (). Other proposed mechanisms include direct infection of cells that comprise the blood–brain barrier, endothelial cells and astrocytes, and transfer via these cells from the periphery to the CNS,Citation19–Citation21 or direct extracellular movement of the virus across a disrupted blood–brain barrier.Citation22 While it is important to consider these mechanisms, they are less well characterized, and are likely minor contributors to CNS infection.
Figure 1 Mechanisms of HIV-mediated CNS damage. HIV infection of peripheral blood mononuclear cells, particularly monocytes, leads to enhanced expression of adhesion molecules and chemokine receptors such as CCR2. This results in increased transmigration of infected cells. HIV enters the CNS through a “Trojan horse” mechanism, crossing the blood–brain barrier, formed by endothelial cells and astrocyte end feet, within infected monocytes. Once inside the CNS, infected monocytes can differentiate into macrophages and secrete a number of inflammatory mediators, particularly chemokines such as CCL2 that further enhance transmigration of immune cells across the blood–brain barrier. HIV infection of macrophages increases the numbers of TNT that connect with other macrophages. HIV or HIV proteins may travel within or on TNT, facilitating viral spread. Infected cells also secrete viral proteins such as gp120 and tat that are toxic to neurons. HIV can infect astrocytes at low levels. Infected astrocytes transfer signals to neighboring uninfected astrocytes and neurons. These signals are transferred through gap junctions and result in apoptosis (X) in both astrocytes and neurons. Astrocytes are necessary for the metabolic maintenance of neurons. Thus, the astrocyte loss and dysfunction that occurs with HIV infection also results in metabolic dysregulation and neuronal toxicity.
Under physiological conditions, leukocyte transmigration across the blood–brain barrier into the CNS parenchyma is a restricted and highly regulated process that requires cell- and tissue-specific mechanisms of leukocyte-endothelial interactions. These interactions involve a variety of intracellular and extracellular molecules, including chemokines, cytokines and their receptors, cell adhesion molecules, tight junctions, and adherens junction proteins on brain microvascular endothelial cells and leukocytes. Aberrant expression of chemokines and their receptors, adhesion molecules, and matrix metalloproteinases in the context of HIV contribute to the transmigration of leukocytes across the blood–brain barrier, exacerbated CNS inflammation, and HIV replication within the brain (see ).Citation22
Chemokines are important mediators of transmigration of leukocytes across the blood–brain barrier. Chemokines play a crucial role in the pathogenesis of HAND because they facilitate leukocyte transmigration and direct movement of resident cells, such as microglia/macrophages. Expression of chemokines and their receptors is dysregulated in HIV and simian immunodeficiency virus encephalitic brains in comparison with normal brains, as well as microglia/ macrophages, astrocytes, endothelial cells, and neurons exposed to HIV or soluble viral proteins.Citation23–Citation31 Chemokine (C-C motif) ligand 2 (CCL2) is the most potent chemoattractant chemokine for monocytes and activated T-cells.Citation32–Citation34 CCL2 levels are elevated in the cerebrospinal fluid and brain tissue of individuals with HIV-associated dementia.Citation28 Our group demonstrated that CCL2 plays a major role in inflammation as well as neuroprotection (see below) in the context of HAND. CCL2 is a critical chemokine in the transmigration of HIV-infected monocytes and lymphocytes across the blood–brain barrier.Citation31 We demonstrated that HIV infection of peripheral blood mononuclear cells (PBMCs) enhanced their transmigration across an in vitro human blood–brain barrier model in response to CCL2.Citation31 HIV-infected PBMCs also had increased expression of chemokine (C-C motif) receptor 2 (CCR2), the receptor for CCL2, which may facilitate this enhanced transmigration (see ).Citation31 The increased transmigration of PBMCs across the blood–brain barrier was associated with blood–brain barrier disruption and was CCL2 specific, given that no increased transmigration of HIV-infected cells was found with CCL3, CCL4, or CCL5.Citation31
In addition to CCR2, previous reports showed enhanced expression of the chemokine receptors CXCR4 and CCR5 on HIV-infected T-cells,Citation35,Citation36 suggesting that HIV infection alters the expression of chemokine receptors to facilitate invasion as well as infection. Another chemokine, fractalkine (CX3CL1), has been associated with enhanced transendothelial migration of CD16+ monocytes under normal and inflammatory conditions and endothelial cells expressing fractalkine trigger CD16+ monocytes to produce CCL2, interleukin-6 (IL-6) and matrix metalloproteinase-9 (MMP-9).Citation37,Citation38 The role of fractalkine in transmigration of HIV-infected monocytes has not yet been examined.
In addition to upregulating chemokine receptors and perhaps enhancing the response to chemotactic agents, HIV infection of leukocytes also alters the expression of a number of adhesion molecules that likely facilitate transmigration of HIV-infected cells across the blood–brain barrier. It has been demonstrated in vivo that HIV infection of human monocytes increases expression of lymphocyte function-associated antigen-1 (LFA-1).Citation39,Citation40 HIV-infected monocytes in contact with endothelial cells induce the expression of E-selectin and vascular cell adhesion molecule-1 (VCAM-1).Citation41 An immunohistochemical examination of HIV encephalitic tissue showed increased expression of intercellular adhesion molecule-1 (ICAM-1) and VCAM-1 on endothelial cells and astrocytes.Citation42 We found that platelet/endothelial cell adhesion molecule-1 (PECAM-1) expression is dysregulated in HIV-infected primary human PBMCs.Citation43 Normally, PECAM-1 is concentrated at sites of cell contact and antibodies blocking the extracellular portion of PECAM-1 selectively reduce diapedesis, but not adhesion or cell activation, of uninfected monocytes.Citation44 Thus, homophilic interactions between PECAM-1 proteins expressed on monocytes and on endothelial cells are critical for diapedesis through interendothelial junctions. We showed that HIV-infected PBMCs shed soluble PECAM-1 (sPECAM-1) in the presence of the chemokine CCL2.Citation43 Using post mortem tissue from individuals with HIV-associated dementia, we found an accumulation of sPECAM-1 within the CNS.Citation43 We also showed that CCL2 increases PECAM-1 on the surface of brain microvascular endothelial cells (unpublished data from Roberts et al). Increased serum levels of sPECAM-1 were detected in individuals with multiple sclerosis and HIV, Citation43,Citation45 suggesting a role for the soluble form of this adhesion molecule in CNS inflammation. We propose that sPECAM-1 competes for the homotypic PECAM-1 interaction between two endothelial cells, which results in destabilization of these interactions with subsequent blood–brain barrier disruption and enhanced transmigration.
These findings support the hypothesis that HIV enters the brain by the transmigration of HIV-infected monocytes across the blood–brain barrier in response to chemokine gradients. HIV infection enhances the expression of specific chemokine receptors on the surface of infected leukocytes, enabling the detection of lower amounts of these chemokines and resulting in leukocyte activation and transmigration into the brain. HIV also increases the expression of a number of adhesion molecules, which facilitate binding and diapedesis across the blood–brain barrier.
CNS damage by viral and immune factors
While HIV is able to infect CNS macrophages, microglia, and astrocytes, as well as causing neuronal dysfunction and loss, there is little evidence to suggest that the virus infects neurons. Therefore, it is believed that infected cells release factors including viral proteins, particularly gp120 and tat, which are toxic to neurons ().
Gp120 is the envelope surface protein of HIV and can bind CXCR4 and CCR5, even in the absence of CD4, on the surface of neurons and trigger neuronal apoptosis.Citation46–Citation48 The majority of brain-derived viruses are R5 (binding to CCR5),Citation49 however, X4 (binding to CXCR4) viruses, which are found in the brain late in the course of disease, or X4 gp120, induce the highest levels of neuronal cell death.Citation50 This may contribute to the more severe forms of HAND that manifest late in disease progression. Specific mutations in the V1 and V3 gp120 loops have been identified in individuals with or without HIV-associated dementia, suggesting adaptation of the virus to the brain through changes in gp120.Citation51–Citation53 Gp120 can be detected in the brain of HIV-infected individuals, colocalizing with microglia/macrophages, microglial nodules, and multinucleated giant cells.Citation54 Post-transcriptional changes in the V3 or V4 regions of gp120 that alter sequence or glycosylation may determine the course of disease as they have been correlated with the incidence of HIV-associated dementia.Citation55,Citation56 Treatment of macrophages or microglia with recombinant gp120 results in the release of many cytokines and chemokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, CCL2, macrophage inflammatory proteins (MIPs), and CCL5, all of which may participate in the neuroinflammation that is also damaging to neurons.Citation57–Citation60
Tat, the transactivator of transcription of HIV, is released from infected cells and can be found in the serum and brain tissue of individuals with HIV encephalitis.Citation61–Citation67 Tat is transcribed and released from cells even when HIV replication is controlled by antiretroviral therapy because HIV integrates into the host genome.Citation63 Tat interacts with a number of cell surface receptors, including integrins, a vascular endothelial growth factor (VEGF) receptor, and low density lipoprotein receptor-related protein-1 (LRP-1).Citation68–Citation70 Tat induces release of cytokines and chemokines from microglia, macrophages, neurons, and astrocytes in the CNS and causes disruption of the blood–brain barrier.Citation71–Citation73 In addition to these inflammatory effects, tat causes significant neurotoxicity in a variety of systems.Citation74–Citation83
The mechanisms that result in tat-induced neuronal apoptosis are still under investigation, but it is clear that glutamate receptors, such as the N-methyl-D-aspartate receptor (NMDAR), participate in the process.Citation72,Citation82–Citation86 Our group demonstrated that tat treatment of mixed cultures of primary human fetal neurons and astrocytes causes apoptosis in both cell types.Citation82,Citation83 This apoptosis is mediated by early formation of a complex involving the NMDAR, LRP-1, post-synaptic density protein of 95 kDa (PSD-95), and neuronal nitric oxide synthase.Citation83 This complex results in activation of the NMDAR and subsequent Ca2+ influx. Increased intracellular Ca2+ activates neuronal nitric oxide synthase and results in nitric oxide production and diffusion.Citation83 Blocking neuronal nitric oxide synthase activity inhibits apoptosis in neurons, and blocking all forms of nitric oxide synthase inhibits apoptosis in neurons and astrocytes, which suggests that nitric oxide is the key mediator of tat-induced apoptosis.Citation83 In addition, we showed that two components of the complex, NMDAR and LRP, are critical to tat-induced apoptosis. Treatment with MK801, a noncompetitive NMDAR antagonist, or with receptor associated protein (RAP), a competitive antagonist of LRP, inhibits tat-induced apoptosis in all neurons and astrocytes.Citation82,Citation83
LRP and its ligands, particularly apolipoprotein E4 (ApoE4), are known to be involved in Alzheimer’s disease, suggesting a potential link between the mechanisms of Alzheimer’s disease and HAND. Polymorphisms in ApoE4 are associated with poor prognoses in a number of neurological disorders, including Alzheimer’s disease, stroke, hemorrhage, and trauma.Citation87–Citation90 It is possible that tat toxicity and ApoE may have similar or overlapping signaling pathways involving activation of LRP, NMDAR, synaptic scaffold proteins, glutamate, and nitric oxide production. Consistent with this hypothesis, it has been demonstrated that ApoE alleles are important predictors for accelerated dementia in HIV-infected individuals and may help predict their disease course.Citation91,Citation92 Thus, we suggest that LRP may play a significant role in the development of HAND and may be a potential target for therapeutics.
Inflammation is a hallmark of HAND and mediates the CNS damage in neurocognitively impaired HIV-infected individuals; however, some aspects of this inflammation may actually be neuroprotective. Several inflammatory cytokines have been reported to be elevated in the CNS and/or cerebrospinal fluid of individuals with HAND, including TNF-α, IL-1β, IL-6, and granulocyte macrophage-colony stimulating factor.Citation93–Citation95 These cytokines may be produced by HIV-infected perivascular macrophages, infiltrated leukocytes, resident microglial cells, endothelial cells, and astrocytes.Citation96 TNF-α upregulates HIV replication and also synergizes with IL-6 and IL-2 to cause reactivation of latent virus.Citation97 However, TNF-α also appears to have neuroprotective properties by increasing NF-κB (nuclear factor kappa-light-chain enhancer of activated B-cells) activity and altering calcium and potassium levels and bcl-2 expression.Citation98 CCL2 can also be neuroprotective. We showed that when mixed cultures of neurons and astrocytes were treated with tat and CCL2 concomitantly, CCL2 inhibits tat-induced apoptosis.Citation82 When we treat with CCL2 two hours after tat, this inhibitory effect is lost, indicating that CCL2 interferes with the early effects of tat. In fact, CCL2 inhibits formation of the complex between NMDAR, LRP-1, PSD-95, and neuronal nitric oxide synthase and inhibits nitric oxide production.Citation83 These data indicate that TNF-α and CCL2 have a dual role within the CNS, suggesting that the actions of these molecules depend on the time frame of their secretion and their microenvironment. Furthermore, due to this dual role, therapies targeting either molecule should be approached cautiously.
Intercellular communication systems amplify CNS damage
Our laboratory was one of the first to examine the role of intercellular communication in the context of HAND. We demonstrated that gap junctions and tunneling nanotubes (TNT) each play a role in neuroinflammation through direct transfer of mediators between the cytoplasm of connected cells.Citation9,Citation99
TNT and gap junctions are the only described communication systems that facilitate exchange of cytoplasmic factors through direct contact between the cytoplasm of connected cells. TNT and gap junctions coordinate important biological processes such as development, metabolism, homeostasis, and the immune response.Citation100–Citation105 The major difference between TNT and gap junctions is the distance between connected cells and the sizes of the molecules transferred. TNT facilitate long-range communication through extended processes, while gap junctions require direct cell-to-cell contact for their formation. Gap junctions and TNT both traffic small molecules, but gap junctions have a size limit of 1.2 kDa, while TNT can also transfer organelles and vesicles.Citation101,Citation105
Gap junctions are channels formed by connexins, a family of proteins with over 21 members in humans.Citation106 Six connexins form a single connexon (hemichannel) and two connexons from apposing cells form a gap junction.Citation105,Citation107 Connexons can be formed by one (homotypic) or several (heterotypic) types of connexins, and gap junctions are formed by two identical (homotypic) or different (heterotypic) connexons. This variation generates connexons and gap junctions that differ in their biophysical properties and permeability.Citation108 The pore that is formed has a large internal diameter, approximately 12 Å, that enables ions and intracellular messengers less than 1.2 kDa to diffuse between connected cells. Molecules that are transferred through these channels include inositol triphosphate (IP3), calcium, cyclic nucleotides, metabolites, toxic substances such as cytochrome c, neurotransmitters, and viral peptides.Citation105,Citation109 Through the diffusion of these second messengers, gap junctions coordinate physiological processes such as development, differentiation, neuronal synchrony, inflammation, survival, apoptosis, metabolism, cell contraction, and signaling.Citation105 In pathologic conditions, it has been proposed that gap junctions participate in cell survival and/or apoptosis and facilitate clearance of pathogens, but the extent of gap junction involvement is not fully characterized.
We demonstrated that gap junctions in astrocytes are critical for the transfer of an apoptotic signal from HIV-infected astrocytes to uninfected astrocytes and neurons.Citation9 Astrocytes are key cells in the CNS that regulate CNS differentiation, neuronal excitation, extracellular metabolites, synaptic plasticity, formation of scar tissue following neuronal loss, immune activation, blood–brain barrier integrity, and glycogen storage.Citation110,Citation111 Our data indicate that, although only a small percentage of cultured primary human fetal astrocytes are infected by HIV, these cells transmit toxic signals through gap junctions to neighboring cells, particularly uninfected astrocytes and neurons, that result in apoptosis ().Citation9 We also demonstrated that gap junctions control glutamate metabolism and secretion of CCL2, suggesting a critical role in maintenance of neuronal function and in CNS inflammation. Citation9 Taken in combination, these results indicate that further investigation of HIV infection of astrocytes and its role in HAND is required despite the low level of productive infection.
We demonstrated a role for gap junctions in the transmigration of PBMCs across the blood–brain barrier.Citation112 PBMCs normally do not express connexins. However, in inflammatory conditions, connexin 43 (Cx43) expression can be induced.Citation112,Citation113 Our data indicate that lipopolysaccharide (LPS) or TNF-α and interferon-γ (IFN-γ) treatment induce Cx43 expression in monocytes.Citation112 These cells migrate across an in vitro model of the blood–brain barrier in response to CCL2 and we observed Cx43 staining in monocytes, astrocytes, and endothelial cells that localized to the sites of heterocellular contact.Citation112 Gap junctions were important for the transmigration of treated monocytes because the gap junction blockers octanol and 18-α-glycerrhetinic acid significantly decreased transmigration. Citation112 Based on our findings, we propose that gap junctions may play an important role in the pathogenesis of HAND by mediating infected astrocyte-induced toxicity as well as transmigration of monocytes across the blood–brain barrier.
TNT form an active communication system that enables neighboring cells to exchange organelles and vesicles, and to coordinate signaling over relatively long distances. In pathologic conditions, these cytoplasmic bridges allow bacteria and viruses to travel between connected cells without being exposed to the immune cell surfaces or extracellular antimicrobial factors.Citation102–Citation104,Citation114–Citation118 TNT formation has been described in immune cells, including B-cells, T-cells, natural killer cells, neutrophils and monocytes, as well as neuronal and glial cells.Citation101 The mechanisms that facilitate TNT formation remain unclear, however a recent study demonstrated that a mammalian protein, M-Sec, induces formation of membrane protrusions that attach to neighboring cells and result in formation of TNT-like structures.Citation119 Our laboratory demonstrated that primary human macrophages infected with HIV have greater numbers of TNT, suggesting that HIV induces the expression or stability of these processes.Citation99 Furthermore, the HIV capsid protein p24 colocalizes with TNT, suggesting that viral spread may occur through this mechanism ().Citation99 Recently, it was demonstrated that HIV evades IgG2 and IgA responses by transferring the viral immunosuppressive protein Nef from infected macrophages to B-cells via TNT, decreasing B-cell response to IgG2 and IgA inducing signals from CD4+ T-cells.Citation120
We propose that intercellular communication mediated by gap junctions and TNT enhances cellular dysfunction and toxicity, inflammation, and HIV infection. An understanding of their role in HAND may indicate useful therapeutic targets to limit the neuropathogenesis of HIV.
Potential therapeutics
The development of successful antiretroviral therapy has significantly reduced the percentage of HIV-infected individuals who develop HIV-associated dementia; however, the percentage of individuals who are neurocognitively impaired has remained unchanged. This is due, in large part, to the fact that many of the antiretrovirals in use do not have adequate CNS penetration. Thus, although the systemic infection is well controlled, CNS infection persists. In addition to promoting an emphasis on CNS-penetrating antiretroviral therapy, a number of ongoing studies are working to develop successful adjunctive therapies to combat HAND. Some have examined ways to improve drug delivery to the CNS, including the use of nanoparticlesCitation121,Citation122 and intranasal delivery of medications.Citation123 Other studies have largely targeted the immune response or neuronal damage.
Oxidizing agents are a well demonstrated component of neurotoxicity and inflammation in HIV-associated CNS disease and a number of studies focused on using antioxidant agents to reduce neuronal damage and loss. One therapy that has been tested in clinical trials is selegiline, which inhibits monoamine oxidase B, resulting in decreased free radicals, increased superoxide dismutase and catalase, and increasing the synthesis of neurotrophic factors.Citation124–Citation128 A selegiline transdermal system was developed and has been tested in a number of Phase II clinical trials. While two trials found improvement in psychomotor speed,Citation129,Citation130 two more recent trials did not find any difference in cognitive or functional outcome and no effect on brain metabolites or levels of markers of oxidative stress, suggesting that the selegiline transdermal system may not be clinically useful for the treatment of HAND.Citation131,Citation132 Another potential antioxidant therapy is a gene therapy system to deliver superoxide dismutase or glutathione peroxidase to neurons, which has been shown to decrease gp120-induced neuronal apoptosis in culture and in the caudate putamen and substantia nigra of mice.Citation133–Citation135 When both superoxide dismutase and glutathione peroxidase were delivered together, this also decreased tat-induced neuronal apoptosis.Citation136
Inflammation is one of the hallmarks of HAND, and therapies to target this inflammation could potentially ameliorate the CNS consequences of HIV. Minocycline is a tetracycline derivative that has been in use since the 1960s for the treatment of bacterial infections. Minocycline can also be used for the treatment of inflammatory conditions, and recent studies showed that it can cross the blood–brain barrier and be neuroprotective.Citation137–Citation139 There are a number of ongoing clinical trials testing the efficacy of minocycline in the treatment of neurological diseases. In the context of HAND, minocycline was demonstrated to reduce the incidence and severity of encephalitis in simian immuno-deficiency virus-infected macaques and a Phase II clinical trial is underway at the National Institute of Allergy and Infectious Diseases in the US, as well as a clinical trial in Uganda to determine whether it reduces cognitive deficits in HIV-infected individuals.Citation140,Citation141
Our group and others demonstrated the importance of the NMDAR in HIV-induced neurotoxicity. Memantine, a non-competitive NMDAR antagonist, is in use in Europe for the treatment of Alzheimer’s disease and Parkinson’s disease and has recently been approved in the US for Alzheimer’s disease treatment.Citation142–Citation147 Memantine prevents gp120, tat, and platelet-activating factor neurotoxicity in cell culture and is neuroprotective in mouse models of HAND.Citation148–Citation151 A recent Phase II clinical trial of memantine in cognitively impaired HIV-infected adults found no significant improvement in cognitive dysfunction over a period of 16 weeks, but individuals treated with memantine did have an improvement in brain metabolism, as measured by N-acetyl aspartate to creatinine ratio using proton magnetic resonance spectroscopy.Citation152 This study suggested that memantine may be an effective treatment for HAND, and that longer term trials are necessary to determine its efficacy.
While there are some promising therapies currently in trial targeting some of the mechanisms of HIV-mediated CNS damage discussed in this review, there is still no definitive treatment for the neurocognitive dysfunction that occurs in up to 50% of infected individuals. It is important to continue investigation of new avenues for potential therapies.
Our recent work with gap junctions and TNT suggests a role for intercellular communication in the neuropathogenesis of HIV and may provide novel targets for therapeutics. We propose that TNT may provide an alternate route for HIV spread in addition to the well characterized receptor-mediated entry. This pathway may not be affected by extracellular antiretroviral therapy and may contribute to the persistence of viral reservoirs. Thus, an understanding of the mechanisms that participate in the formation of TNT and transport of virus or viral proteins through TNT may lead to identification of therapeutic targets to block intracellular viral spread.
We also demonstrated that gap junctions facilitate transfer of an apoptotic signal from astrocytes to neighboring cells, which likely contributes to the cellular dysfunction and cell death in the CNS of HIV infected individuals. Gap junctions play vital roles in electrical and metabolic coordination within the CNS. Thus, it is unlikely that blocking gap junction channels would be a useful therapeutic approach. However, this work demonstrates the importance of the small population of infected astrocytes in the development of HAND. Thus, by understanding the mechanisms of this infection, we can begin to determine how to target this component of viral CNS infection therapeutically to eradicate this significant viral reservoir.
Acknowledgements
We are grateful to Dr. Brad Poulos and the Human Fetal Tissue Repository and the Analytical Imaging Facilities at the Albert Einstein College of Medicine. This work was supported by the National Institutes of Mental Health grants MH070297, MH075679, and MH083497 to JWB, NIH Centers for AIDS Research Grant AI-051519, a KO1 grant from the National Institutes of Mental Health (MH076679) to EAE, MSTP Training Grant 5 T32 GM007288 and the HIV-AIDS and Opportunistic Infections Institutional Training Grant T32 AI-007501 to JEH.
Disclosures
The authors report no conflicts of interest in this work.
References
- EllisRLangfordDMasliahEHIV and antiretroviral therapy in the brain: neuronal injury and repairNature Reviews2007813344
- AnthonyICRamageSNCarnieFWSimmondsPBellJEInfluence of HAART on HIV-related CNS disease and neuroinflammationJ Neuropathol Exp Neurol200564652953615977645
- McArthurJCHaugheyNGartnerSHuman immunodeficiency virus-associated dementia: an evolving diseaseJ Neurovirol20039220522112707851
- AntinoriAArendtGBeckerJTUpdated research nosology for HIV-associated neurocognitive disordersNeurology200769181789179917914061
- AncesBMEllisRJDementia and neurocognitive disorders due to HIV-1 infectionSemin Neurol2007271869217226745
- ChangLTomasiDYakupovRAdaptation of the attention network in human immunodeficiency virus brain injuryAnn Neurol200456225927215293278
- AncesBMRocACWangJCaudate blood flow and volume are reduced in HIV+ neurocognitively impaired patientsNeurology200666686286616567703
- DavisLEHjelleBLMillerVEEarly viral brain invasion in iatrogenic human immunodeficiency virus infectionNeurology1992429173617391513462
- EugeninEABermanJWGap junctions mediate human immunodeficiency virus-bystander killing in astrocytesJ Neurosci20072747128441285018032656
- WileyCASchrierRDNelsonJALampertPWOldstoneMBCellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patientsProc Natl Acad Sci U S A19868318708970933018755
- ConantKTornatoreCAtwoodWMeyersKTraubRMajorEOIn vivo and in vitro infection of the astrocyte by HIV-1Adv Neuroimmunol1994432872897874397
- GorryPROngCThorpeJAstrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementiaCurr HIV Res20031446347315049431
- ChurchillMJWesselinghSLCowleyDExtensive astrocyte infection is prominent in human immunodeficiency virus-associated dementiaAnn Neurol200966225325819743454
- JamesHJSharerLRZhangQExpression of caspase-3 in brains from paediatric patients with HIV-1 encephalitisNeuropathol Appl Neurobiol199925538038610564527
- Adle-BiassetteHChretienFWingertsmannLNeuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damageNeuropathol Appl Neurobiol199925212313310216000
- GiomettoBAnSFGrovesMAccumulation of beta-amyloid precursor protein in HIV encephalitis: relationship with neuropsychological abnormalitiesAnn Neurol199742134409225683
- AnSFGiomettoBGrovesMAxonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDSJ Neuropathol Exp Neurol19975611126212689370237
- MasliahEHeatonRKMarcotteTDDendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research CenterAnn Neurol19974269639729403489
- MankowskiJLSpelmanJPRessetarHGNeurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitroJ Virol19946812820282087966612
- BobardtMDSalmonPWangLContribution of proteoglycans to human immunodeficiency virus type 1 brain invasionJ Virol200478126567658415163749
- ArgyrisEGAcheampongENunnariGMukhtarMWilliamsKJPomerantzRJHuman immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid raftsJ Virol20037722121401215114581551
- BucknerCMLuersAJCalderonTMEugeninEABermanJWNeuroimmunity and the blood–brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDSJ Neuroimmune Pharmacol20061216018118040782
- AlbrightAVShiehJTItohTMicroglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolatesJ Virol19997312052139847323
- LaviEKolsonDLUlrichAMFuLGonzalez-ScaranoFChemokine receptors in the human brain and their relationship to HIV infectionJ Neurovirol1998433013119639073
- GabuzdaDHeJOhagenAVallatAVChemokine receptors in HIV-1 infection of the central nervous systemSemin Immunol19981032032139653047
- McManusCMWeidenheimKWoodmanSEChemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulationAmer J Pathol200015641441145310751368
- ConantKGarzino-DemoANathAInduction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementiaProc Natl Acad Sci U S A1998956311731219501225
- KelderWMcArthurJCNance-SprosonTMcClernonDGriffinDEBeta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementiaAnn Neurol19984458318359818943
- SandersVJPittmanCAWhiteMGWangGWileyCAAchimCLChemokines and receptors in HIV encephalitisAIDS (London, England)199812910211026
- SassevilleVGSmithMMMackayCRChemokine expression in simian immunodeficiency virus-induced AIDS encephalitisAm J Pathol19961495145914678909235
- EugeninEAOsieckiKLopezLGoldsteinHCalderonTMBermanJWCCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDSJ Neurosci20062641098110616436595
- Yla-HerttualaSLiptonBARosenfeldMEExpression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesionsProc Natl Acad Sci U S A19918812525252562052604
- SozzaniSIntronaMBernasconiSMCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expressionJ Leukoc Biol199762130339225989
- VilligerPMTerkeltaubRLotzMMonocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage. Induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acidJ Clin Invest19929024884961365641
- KitchenSGKorinYDRothMDLandayAZackJACostimulation of naive CD8(+) lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infectionJ Virol19987211905490609765450
- ZlozaASullivanYBConnickELandayALAl-HarthiLCD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIVBlood200310262156216412791668
- AncutaPMosesAGabuzdaDTransendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditionsImmunobiology20042091–2112015481136
- AncutaPWangJGabuzdaDCD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cellsJ Leuko Biol20068051156116417056766
- DhawanSWeeksBSSoderlandCHIV-1 infection alters monocyte interactions with human microvascular endothelial cellsJ Immunol199515414224327527819
- StentGCroweSMEffects of HIV-1 on the surface expression of LFA-1 on cultured monocytesJ Acquir Immune Defic Syndr Hum Retrovirol1997152951039241107
- NottetHSPersidskyYSassevilleVGMechanisms for the transendothelial migration of HIV-1-infected monocytes into brainJ Immunol19961563128412958558009
- SeilheanDDzia-LepfoundzouASazdovitchVAstrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complexNeuropathol Appl Neurobiol199723283929160893
- EugeninEAGamssRBucknerCShedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDSJ Leukoc Biol200679344445216507710
- MullerWAWeiglSADengXPhillipsDMPECAM-1 is required for transendothelial migration of leukocytesJ Exp Med199317824494608340753
- LosyJNiezgodaAWenderMIncreased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesionsJ Neuroimmunol199999216917210505971
- LaBrancheCCHoffmanTLRomanoJDeterminants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificityJ Virol19997312103101031910559349
- Aggoun-ZouaouiDCharriaut-MarlangueCRiveraSJorqueraIBen-AriYRepresaAThe HIV-1 envelope protein gp120 induces neuronal apoptosis in hippocampal slicesNeuroreport1996724334368730799
- MeucciOMillerRJgp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1J Neurosci19961613408040888753870
- OhagenADevittAKunstmanKJGenetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDSJournal Virol200377221233612345
- OhagenAGhoshSHeJApoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelopeJ Virol19997328979069882290
- PowerCMcArthurJCJohnsonRTDemented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequencesJ Virol1994687464346498207838
- PowerCMcArthurJCNathANeuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patientsJ Virol19987211904590539765449
- Van MarleGRourkeSBZhangKHIV dementia patients exhibit reduced viral neutralization and increased envelope sequence diversity in blood and brainAIDS (London, England)2002161419051914
- JonesMVBellJENathAImmunolocalization of HIV envelope gp120 in HIV encephalitis with dementiaAIDS (London, England)2000141727092713
- GorryPRDunfeeRLMeffordMEChanges in the V3 region of gp120 contribute to unusually broad coreceptor usage of an HIV-1 isolate from a CCR5 Delta32 heterozygoteVirology2007362116317817239419
- DunfeeRLThomasERWangJKunstmanKWolinskySMGabuzdaDLoss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementiaVirology2007367122223417599380
- CorasanitiMTBagettaGRotirotiDNisticoGThe HIV envelope protein gp120 in the nervous system: interactions with nitric oxide, interleukin-1beta and nerve growth factor signalling, with pathological implications in vivo and in vitroBiochem Pharmacol19985621531569698067
- LeeCTomkowiczBFreedmanBDCollmanRGHIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathwaysJ Leukoc Biol20057841016102316081599
- FantuzziLCaniniIBelardelliFGessaniSHIV-1 gp120 stimulates the production of beta-chemokines in human peripheral blood monocytes through a CD4-independent mechanismJ Immunol200116695381538711313374
- D’AversaTGEugeninEABermanJWNeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activationJ Neurosci Res200581343644615954144
- ChangHCSamaniegoFNairBCBuonaguroLEnsoliBHIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic regionAIDS (London, England)1997111214211431
- EnsoliBBarillariGSalahuddinSZGalloRCWong-StaalFTat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patientsNature1990345627084862184372
- EnsoliBBuonaguroLBarillariGRelease, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivationJ Virol19936712772878416373
- FrankelADPaboCOCellular uptake of the tat protein from human immunodeficiency virusCell1988556118911932849510
- HudsonLLiuJNathADetection of the human immunodeficiency virus regulatory protein tat in CNS tissuesJ Neurovirol20006214515510822328
- WielandUKuhnJEJassoyCRubsamen-WaigmannHWolberVBraunRWAntibodies to recombinant HIV-1 vif, tat, and nef proteins in human seraMed Microbiol Immunol199017911112184337
- WestendorpMOFrankROchsenbauerCSensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120Nature199537565314975007539892
- VogelBELeeSJHildebrandAA novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectinJ Cell Biol199312124614687682219
- AlbiniASoldiRGiunciuglioDThe angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cellsNat Med1996212137113758946838
- LiuYJonesMHingtgenCMUptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligandsNat Med20006121380138711100124
- PuHTianJFloraGHIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brainMol Cell Neurosci200324122423714550782
- KingJEEugeninEABucknerCMBermanJWHIV tat and neurotoxicityMicrobes Infect2006851347135716697675
- RappaportJJosephJCroulSMolecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, TatJ Leukoc Biol199965445846510204574
- JonesMOlafsonKDel BigioMRPeelingJNathAIntraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargementJ Neuropathol Exp Neurol19985765635709630236
- MagnusonDSKnudsenBEGeigerJDBrownstoneRMNathAHuman immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicityAnn Neurol19953733733807695237
- NathAPsooyKMartinCIdentification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxicJ Virol1996703147514808627665
- NewDRMaMEpsteinLGNathAGelbardHAHuman immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron culturesJ Neurovirol1997321681739111179
- SabatierJMVivesEMabroukKEvidence for neurotoxic activity of tat from human immunodeficiency virus type 1J Virol19916529619671898974
- ShiBRainaJLorenzoABusciglioJGabuzdaDNeuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementiaJ Neurovirol1998432812909639071
- StrijbosPJZamaniMRRothwellNJArbuthnottGHarkissGNeurotoxic mechanisms of transactivating protein Tat of Maedi-Visna virusNeurosci Lett199519732152188552302
- WeeksBSLiebermanDMJohnsonBNeurotoxicity of the human immunodeficiency virus type 1 tat transactivator to PC12 cells requires the Tat amino acid 49–58 basic domainJ Neurosci Res199542134408531224
- EugeninEAD’AversaTGLopezLCalderonTMBermanJWMCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosisJ Neurochem20038551299131112753088
- EugeninEAKingJENathAHIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytesProc Natl Acad Sci U S A200710493438344317360663
- WangPBarksJDSilversteinFSTat, a human immunodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal ratsNeuroscience199988258559710197777
- HaugheyNJNathAMattsonMPSlevinJTGeigerJDHIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicityJ Neurochem200178345746711483648
- BonaviaRBajettoABarberoSAlbiniANoonanDMSchettiniGHIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neuronsBiochem Biophys Res Commun2001288230130811606043
- StrittmatterWJSaundersAMSchmechelDApolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer diseaseProc Natl Acad Sci U S A1993905197719818446617
- SlooterAJTangMXvan DuijnCMApolipoprotein E epsilon4 and the risk of dementia with stroke. A population-based investigationJ Amer Med Assoc199727710818821
- AlbertsMJGraffagninoCMcClennyCApoE genotype and survival from intracerebral haemorrhageLancet199534689745757658797
- TardiffBENewmanMFSaundersAMPreliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart CenterAnn Thorac Surg19976437157209307463
- CorderEHLannfeltLBogdanovicNFratiglioniLMoriHThe role of APOE polymorphisms in late-onset dementiasCell Mol Life Sci19985499289349791536
- BurtTDAganBKMarconiVCApolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progressionProc Natl Acad Sci U S A2008105258718872318562290
- ShariefMKCiardiMThompsonEJTumour necrosis factor-alpha mediates blood–brain barrier damage in HIV-1 infection of the central nervous systemMediators Inflamm19921319119618475460
- AchimCLHeyesMPWileyCAQuantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brainsJ Clin Invest1993916276927758514884
- PerrellaOGuerrieroMIzzoESosciaMCarrieriPBInterleukin-6 and granulocyte macrophage-CSF in the cerebrospinal fluid from HIV infected subjects with involvement of the central nervous systemArquivos de neuro-psiquiatria19925021801821308387
- Gonzalez-ScaranoFMartin-GarciaJThe neuropathogenesis of AIDSNat Rev Immunol200551698115630430
- ChunTWEngelDMizellSBEhlerLAFauciASInduction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokinesJ Exp Med1998188183919653086
- SriramKO’CallaghanJPDivergent roles for tumor necrosis factor-alpha in the brainJ Neuroimmune Pharmacol20072214015318040839
- EugeninEAGaskillPJBermanJWTunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV traffickingCell Immunol2009254214214818835599
- EugeninEAGaskillPJBermanJWTunneling nanotubes (TNT): A potential mechanism for intercellular HIV traffickingCommunicative and Integrative Biology20092324324419641744
- GerdesHHBukoreshtlievNVBarrosoJFTunneling nanotubes: a new route for the exchange of components between animal cellsFEBS Letters2007581112194220117433307
- OnfeltBDavisDMCan membrane nanotubes facilitate communication between immune cells?Biochem Soc Trans200432Pt 567667815493985
- OnfeltBPurbhooMANedvetzkiSSowinskiSDavisDMLongdistance calls between cells connected by tunneling nanotubulesSci STKE20052005313pe5516333019
- RustomASaffrichRMarkovicIWaltherPGerdesHHNanotubular highways for intercellular organelle transportScience (New York, N.)2004303566010071010
- SaezJCBerthoudVMBranesMCMartinezADBeyerECPlasma membrane channels formed by connexins: their regulation and functionsPhysiol Rev20038341359140014506308
- WilleckeKEibergerJDegenJStructural and functional diversity of connexin genes in the mouse and human genomeBiol Chem2002383572573712108537
- BennettMVRubinJBBargielloTAVerselisVKStructure-function studies of voltage sensitivity of connexins, the family of gap junction forming proteinsJpn J Physiol199343 Suppl 1S301S3108271512
- HarrisALBevansCGExploring hemichannel permeability in vitroMethods Mol Biol200115435737711218659
- BennettMVZukinRSElectrical coupling and neuronal synchronization in the Mammalian brainNeuron200441449551114980200
- RouachNAvignoneEMemeWGap junctions and connexin expression in the normal and pathological central nervous systemBiol Cell2002947–845747512566220
- KielianTEsenNEffects of neuroinflammation on glia-glia gap junctional intercellular communication: a perspectiveNeurochem Int2004452–342943615145557
- EugeninEABranesMCBermanJWSaezJCTNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responsesJ Immunol200317031320132812538692
- El-SabbanMEMerhiRAHaidarHAHuman T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cellsBlood20029993383338911964307
- OnfeltBNedvetzkiSBenningerRKStructurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteriaJ Immunol2006177128476848317142745
- OnfeltBNedvetzkiSYanagiKDavisDMCutting edge: Membrane nanotubes connect immune cellsJ Immunol200417331511151315265877
- MagalhaesACBaronGSLeeKSUptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cellsJ Neurosci200525215207521615917460
- WatkinsSCSalterRDFunctional connectivity between immune cells mediated by tunneling nanotubulesImmunity200523330931816169503
- ShererNMLehmannMJJimenez-SotoLFHorensavitzCPypaertMMothesWRetroviruses can establish filopodial bridges for efficient cell-to-cell transmissionNat Cell Biol20079331031517293854
- HaseKKimuraSTakatsuHM-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complexNat Cell Biol200911121427143219935652
- XuWSantiniPASullivanJSHIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduitsNat Immunol20091091008101719648924
- DouHGrotepasCBMcMillanJMMacrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDSJ Immunol2009183166166919535632
- DouHMoreheadJDestacheCJLaboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophagesVirology2007358114815816997345
- HansonLRFreyWH2ndStrategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDSJ Neuroimmune Pharmacol200721818618040829
- AnsariKSYuPHKruckTPTattonWGRescue of axotomized immature rat facial motoneurons by R(-)-deprenyl: stereospecificity and independence from monoamine oxidase inhibitionJ Neurosci1993139404240538366359
- CarrilloMCKitaniKKanaiSSatoYMiyasakaKIvyGO(−) Deprenyl increases activities of superoxide dismutase and catalase in certain brain regions in old male miceLife Sci199454149759818139387
- MatsuiYKumagaeYMonoamine oxidase inhibitors prevent striatal neuronal necrosis induced by transient forebrain ischemiaNeurosci Lett199112621751781922929
- SaloPTTattonWGDeprenyl reduces the death of motoneurons caused by axotomyJ Neurosci Res19923123944001374134
- TattonWGGreenwoodCERescue of dying neurons: a new action for deprenyl in MPTP parkinsonismJ Neurosci Res19913046666721686284
- A randomized, double-blind, placebo-controlled trial of deprenyl and thioctic acid in human immunodeficiency virus-associated cognitive impairmentDana Consortium on the Therapy of HIV Dementia and Related Cognitive DisordersNeurology19985036456519521250
- SacktorNSchifittoGMcDermottMPMarderKMcArthurJCKieburtzKTransdermal selegiline in HIV-associated cognitive impairment: pilot, placebo-controlled studyNeurology200054123323510636157
- SchifittoGYiannoutsosCTErnstTSelegiline and oxidative stress in HIV-associated cognitive impairmentNeurology2009
- SchifittoGZhangJEvansSRA multicenter trial of selegiline transdermal system for HIV-associated cognitive impairmentNeurology200769131314132117652642
- AgrawalLLouboutinJPReyesBAVan BockstaeleEJStrayerDSAntioxidant enzyme gene delivery to protect from HIV-1 gp120-induced neuronal apoptosisGene Ther200613231645165616871233
- LouboutinJPAgrawalLReyesBAVan BockstaeleEJStrayerDSProtecting neurons from HIV-1 gp120-induced oxidant stress using both localized intracerebral and generalized intraventricular administration of antioxidant enzymes delivered by SV40-derived vectorsGene Ther200714231650166117914406
- LouboutinJPAgrawalLReyesBAVan BockstaeleEJStrayerDSHIV-1 gp120 neurotoxicity proximally and at a distance from the point of exposure: protection by rSV40 delivery of antioxidant enzymesNeurobiol Dis200934346247619327399
- AgrawalLLouboutinJPStrayerDSPreventing HIV-1 Tat-induced neuronal apoptosis using antioxidant enzymes: mechanistic and therapeutic implicationsVirology2007363246247217336361
- SapadinANFleischmajerRTetracyclines: nonantibiotic properties and their clinical implicationsJ Am Acad Dermatol200654225826516443056
- ElewaHFHilaliHHessDCMachadoLSFaganSCMinocycline for short-term neuroprotectionPharmacotherapy200626451552116553511
- StirlingDPKoochesfahaniKMSteevesJDTetzlaffWMinocycline as a neuroprotective agentNeuroscientist200511430832216061518
- FollstaedtSCBarberSAZinkMCMechanisms of minocycline-induced suppression of simian immunodeficiency virus encephalitis: inhibition of apoptosis signal-regulating kinase 1J Neurovirol200814537638819003592
- ZinkMCUhrlaubJDeWittJNeuroprotective and anti-human immunodeficiency virus activity of minocyclineJ Amer Med Assoc20052931620032011
- LiptonSAParadigm shift in neuroprotection by NMDA receptor blockade: memantine and beyondNat Rev Drug Discov20065216017016424917
- FleischhackerWWBuchgeherASchubertHMemantine in the treatment of senile dementia of the Alzheimer typeProg Neuropsychopharmacol Biol Psychiatry198610187933517967
- WinbladBPoritisNMemantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine)Int J Geriatr Psych1999142135146
- RabeyJMNissipeanuPKorczynADEfficacy of memantine, an NMDA receptor antagonist, in the treatment of Parkinson’s diseaseJ Neural Transm19924277282
- ReisbergBDoodyRStofflerASchmittFFerrisSMobiusHJMemantine in moderate-to-severe Alzheimer’s diseaseNew Engl J Med2003348141333134112672860
- TariotPNFarlowMRGrossbergGTGrahamSMMcDonaldSGergelIMemantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trialJ Amer Med Assoc20042913317324
- ToggasSMMasliahEMuckeLPrevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantineBrain Research199670623033078822372
- LiptonSAMemantine prevents HIV coat protein-induced neuronal injury in vitroNeurology1992427140314051620355
- NathAHaugheyNJJonesMAndersonCBellJEGeigerJDSynergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantineAnn Neurol200047218619410665489
- AndersonERGendelmanHEXiongHMemantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitisJ Neurosci200424327194719815306653
- SchifittoGNaviaBAYiannoutsosCTMemantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy studyAIDS (London, England)2007211418771886