Abstract
In hypertensive subjects, cardiovascular risk reduction is critically related to the decrease of systolic blood pressure (SBP). De-stiffening therapy means that, in a controlled therapeutic trial of long duration, a selective reduction of SBP has been obtained in the studied group by comparison with the control group, and that this SBP reduction is due to a decrease of either arterial stiffness, or wave reflections, or both. Central SBP reduction and cardiovascular remodeling are specifically involved. Most protocols require the presence of an angiotensin II blocker, potentially associated with a diuretic compound and/or a calcium-channel blocker. Cardiovascular outcomes are significantly reduced by comparison with the control group, particularly when this latter group involves administration of a beta-blocking agent.
Introduction
A reduction in SBP has explained most of the treatment benefit in outcome trials in patients with hypertension.Citation1–Citation6 This benefit has been obtained mostly using ANG-II inhibition. Such findings focused attention on the factors that modulate SBP and PP levels in hypertensive individuals, and therefore on the role of increased arterial stiffness and/or wave reflections in the mechanism of hypertension.
This review has four parts: 1) Mechanisms of propagation of the pressure wave along the vascular circuit; 2) Pulsatile arterial hemodynamics as independent predictors of CV risk; 3) Relationship between pulsatile arterial hemodynamics and renin-angiotensin system; and 4) Principal strategies for lowering large artery stiffness in the treatment of hypertension and CV prevention.
Mechanisms of pressure wave propagation along the vascular tree
There are two components of pressure and flow: a steady component and a pulsatile component. The former is represented by MAP, the product of blood flow by vascular resistance, an index of the “caliber” of small arteries. The latter is represented by PP, which is determined by stroke volume, arterial stiffness and wave reflections. The second two factors, but not stroke volume, are influenced by the ability to change the cyclic flow coming from the heart into a continuous flow at the peripheral level in order to obtain an adequate oxygenation of tissue.
Blood pressure propagation, arterial stiffness and wave reflections
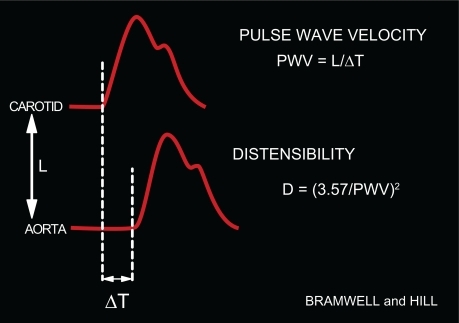
Following ventricular contraction, the pressure pulse generated by the heart travels along the aorta as a wave (). It is possible to calculate the velocity of propagation of this wave (ie, PWV) along the aorta from the interval between two BP curves located at two different sites in the arterial tree (). Because of the fundamental principle is that pulse waves travel faster in stiffer arteries, PWV measurement is considered the best surrogate to evaluate arterial stiffness. Its value is 3 to 5 m/s in young persons at rest, but increases considerably with age (). Given that peripheral arteries are markedly stiffer than central arteries, an important feature of PWV determinations is the large heterogeneity of the arterial tree.
Figure 1 Clinical determination of PWV. PWV is the ratio between: 1) the distance between the carotid and femoral transducers (L), and 2) the time delay (ΔT) between the foot of the carotid and femoral BP curves simultaneously measured. From PWV, distensibility may be deduced.Citation1,Citation2
When BP measurements are made simultaneously at different points along the aorta, the pressure wave changes shape as it travels down the aorta (). Whereas SBP actually rises with distance from the heart, the DBP and MAP fall slightly (about 4 mm Hg) during the same course along the aortic trajectory. Thus, pressure-oscillation amplitude between systole and diastole, which is represented by PP () nearly doubles. This SBP and PP amplification () is a physiological finding, approximately 14 mm Hg between the thoracic aorta root and the brachial artery, and continuing in aortic ramifications out to about the third-generation level of branches. Thereafter, both PP and MAP drop sharply to the levels found in the microcirculation, a vascular area in which steady flow is nearly achieved.
Figure 2 Progression of the pressure wave along the aortic tree. Three steps are involved: propagation, reflection, and summation of the incident and the reflected waves.
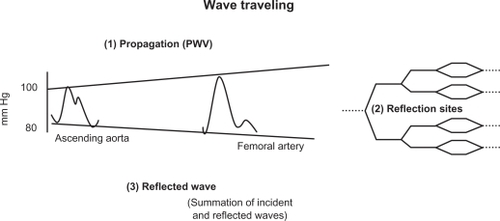
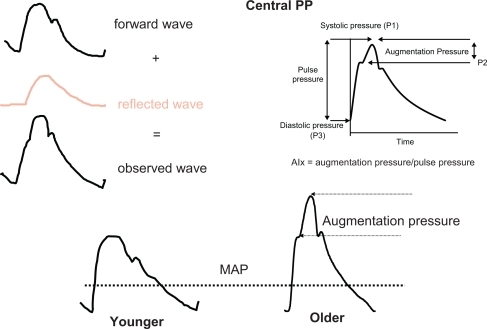
Figure 3 The upper part gives a schematic representation of the BP curve on the right, and the forward and reflected wave on the left. In the lower part, the BP curve is represented in younger (on the left) and in older (on the right) subjects. Augmentation index (AIx) is the ratio between: 1) the difference between peak SBP and the shoulder of the ascending part of the BP curve, and 2) pulse pressure. AIx, measured in %, represents the supplementary increase in SBP due to wave reflections. This hemodynamic profile is observed in the elderly, not in young people. MAP is mean arterial pressure, corresponding to the steady pressure necessary to a continuous cardiac pump.
If an individual’s body length is about 2 m at most, and aortic PWV is approximately 5 m/s, something must happen to the shape of the BP curve within each beat if heart rate is 60/min, which is the generation of wave reflections and their summation with the incident wave, as summarized in . The incident wave is driven away from the heart through the highly conductive arteries. However, it encounters an impedance mismatch at the junction of the highly conductive artery and highly resistant arterioles, blocking its entry into the arterioles, and it is reflected, traveling backwards towards the heart (). Thus, the shape of every pulse wave results from the summation of the incident (forward-traveling) and reflected (backward-traveling) pressure waves ().
Role of age on BP curve and wave reflections
Reflected waves may be initiated from any discontinuity of the arterial or arteriolar wall, but are mainly issued from high resistance vessels.Citation1,Citation2 Pulse-wave propagation and reflection vary considerably according to age. In young adults at their maximum height and maximum elasticity of their central arteries (low PWV), the summation of the incident arterial pressure wave and the reflected wave results in progressive PP amplification, so that SBP is higher in the brachial artery than the ascending aorta (). This hemodynamic profile contrasts with MAP and DBP, which decline minimally in vessels at increasing distance from the heart at all ages (). Note that, in the thoracic aorta, because PWV is relatively low, the reflected wave comes back during diastole, thereby maintaining DBP and boosting coronary perfusion (, lower part). Hence, an optimal arterial function is maintained, along with adequate coronary perfusion.
The pattern of wave reflections and the pulse wave shape are directly dependent on aging and arterial stiffness. The development of increasing arterial stiffness (high PWV) and altered wave reflections with aging and hypertension completely abolishes the differences between central and peripheral PP by age 50 to 60 years, with major consequences for ventricular load and coronary perfusion. The increased PWV means that the reflected waves return to the aortic root earlier, during late systole. In this situation, the reflected waves combine with the forward-traveling wave to create an increased “augmentation” of the central SBP and ventricular load (, lower part). In elderly persons with isolated systolic hypertension, aortic SBP can be elevated by a much as 30 to 40 mm Hg as a result of the early return of the wave reflection.Citation1,Citation2 Furthermore, because the backward pressure returns in systole, and not in diastole, as a consequence of enhanced PWV, DBP and coronary blood flow tend to be reduced, a situation favoring coronary ischemia. Finally, the classical phenotype of systolic hypertension in the elderly is observed (). It is worth noting that reduced heart rate shifts wave reflection from diastole in systole, thus increasing central SBP. Inversely, angiotensin and calcium blockade as well as insulin reduce wave reflection and central SBP. Insulin resistance has an opposite effect.
Mechanical forces and vascular remodeling
It is important to note that an arterial wall is a complex tissue composed of different cell populations capable of structural and functional changes, in response to direct injury and athero-genic factors, or to modifications of long-term hemodynamic conditions. The principal geometric modifications induced by hemodynamic alterations are changes of the arterial lumen and/or arterial wall thickness due to activation, proliferation and migration of VSM cells, and rearrangements of cellular elements and ECM.Citation1,Citation3–Citation8 Chronic alterations of mechanical forces lead to modifications of the geometry and composition of the vessel walls, as observed in hypertension, particularly in the elderly.Citation1,Citation2,Citation7 To maintain tensile stress within physiological limits, arteries respond by thickening their walls (Laplace’s law). On the other hand experimental and clinical data indicate that acute and chronic augmentations of arterial blood flow induce proportional increases of the vessel lumen, whereas diminished flow leads to reduction of the inner arterial diameter.Citation1,Citation2,Citation9 The presence of the endothelium is a major prerequisite for normal vascular adaptation to chronic changes of blood flow and pressure.
Hypertensive remodeling is characterized by the increased wall/lumen ratio of arterioles, which represent the site of vascular resistance and also the origin of wave reflections ().Citation9–Citation12 Regression of arteriolar hypertrophy is associated with diminution of vascular resistance and of reflection coefficients, thereby causing a lower SBP, PP and AIx, a classical marker of wave reflections (, lower side).Citation10–Citation12 This process occurs approximately after 1-year of treatment in hypertensive subjects under angiotensin or calcium blockade, but not under thiazide diuretics and/or beta-blocking agents.Citation11 Endothelial dysfunction may sometimes participate to this process, mainly through NO deficiency and development of oxidative stress.Citation1,Citation2,Citation9–Citation13
Pulsatile arterial hemodynamics as independent predictors of CV risk
This section will show how brachial PP, aortic PWV, and to a greater extent central PP and wave reflections, are independent predictors of CV risk, implicating the possibility of specific drug treatments, called de-stiffening therapy, in relation with pulsatile arterial hemodynamics.
Brachial PP
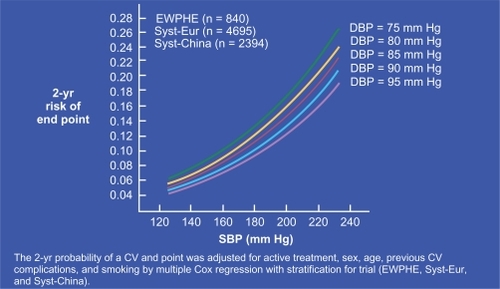
Following the seminal works by Darné et al and Madhavan et al,Citation14,Citation15 several authors showed almost simultaneouslyCitation16–Citation18 that, after 50 to 60 years of age, brachial PP was a strong CV risk factor for myocardial infarction in populations of hypertensive individuals. The best predictor function of all possible linear combinations of SBP and DBP was shown to be similar to that of PP, indicating that their association was not a statistical artefact caused by the correlation between SBP and PP.Citation18 The result was independent of MAP and stronger than for SBP. As shown in , CV risk rises sharply with SBP. However, at any given SBP value, CV risk is higher when DBP is lower, ie, when PP is increased.Citation17 This important finding was confirmed by the result of a longitudinal study,Citation19 which indicated that, during a 20 years follow-up, subjects with higher CV mortality were those whose SBP rose and DBP declined, and their CV mortality rate was significantly higher than that for those individuals whose SBP and DBP increased.Citation19 Finally, it was demonstrated that, in the elderly, neither SBP nor DBP was superior to PP for predicting coronary risk and PP was found to be an independent predictor of CV mortality, even under drug treatment of hypertension.Citation20,Citation21 Similar results were obtained for individuals with recurrent myocardial infarction, congestive heart failure or myocardial dysfunction. Finally, brachial PP was shown to be predictor of CV risk in subjects with ESRD, diabetes mellitus or even systemic vasculitis.Citation22–Citation25
Figure 4 Curves plotting the 2-year risk of CV events in the elderly as a function of increasing SBP. Note that, at each given SBP value, the CV risk was higher when baseline DBP was lower. Reproduced with permission from Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089.Citation17 Copyright © 2000 American Medical Association. All rights reserved.
Aortic PWV
More recently, aortic PWV, a classic index of arterial stiffness, was shown to be an independent predictor of CV mortality in hypertensive individuals.
Based on the characteristics of patientsCitation26 with ESRD, logistic-regression and Cox analyses identified for the first time that the odds ratios for PWV (>12 m/s) were 5.6 (95% CI 2.4 to 11.9 for all-cause mortality, and 5.9 (95% CI 2.3 to 15.5) for CV mortality. Furthermore, while the carotid wall/lumen ratio did not predict CV risk, carotid stiffness itself and carotid wave reflections (and not wall thickness) were a significant predictor of CV mortality.Citation27,Citation28 Similar observations were obtained for ESRD patients with diabetes mellitusCitation29 and for kidney-transplant recipients.Citation30
In subjects with essential hypertension, Blacher et alCitation31 showed that CV risk assessed using Framingham equations was linearly associated with the PWV increase. Furthermore, the odds ratio of being at high risk of CV mortality (>5% for 10 years) for patients with PWV > 13.5 m/s was 7.1 (95% CI 4.5 to 11.3). That studyCitation32 provided the first evidence that a single aortic PWV measurement could be a strong independent predictor of CV risk for hypertensive patients. The results of longitudinal studiesCitation32–Citation36 confirmed that aortic PWV is a significant and independent predictor of CV risk, more potent than PP itself.
Central PP
Studies of pulsatile arterial hemodynamics showed that, while MAP remains nearly constant along the arterial tree, PP rises markedly from central (thoracic aorta and carotid artery) to peripheral (brachial) arteries. This physiological amplification can be explained because the pressure-wave propagation along arterial vessels is associated with a progressive artery-diameter decline and arterial stiffness increase, resulting in modifications of wave-reflections (timing and/or amplitude). Therefore, aortic PP is expected to be more relevant to the investigation of CV risk than brachial PP, because it is closer to the heart, coronary arteries and carotid arteries, which are the most important sites of CV events.Citation1,Citation2 Aortic, but not brachial, pulsatility has been shown to be independently associated with CAD in patients undergoing coronary angiography before or after angioplasty.Citation32,Citation37,Citation38 Furthermore, in 409 subjects followed for 4 to 5 years by Jankowski et al,Citation39 a 10-mm Hg aortic PP increase was associated with a corresponding 13% increase of CV events. In atherosclerotic subjects, central wave reflections were shown to be independent predictors of CAD.Citation39 In ESRD patients, aortic PWV and carotid wave reflections (and/or central PP) were shown to predict independently CV mortality.Citation1,Citation2,Citation40 Finally, in the same ESRD patients and in elderly subjects with essential hypertension, central PP was demonstrated to be an independent predictor of mortality.Citation41 Taken together, all these findings suggest that central PP was superior to brachial PP for prediction of coronary risk and indicate that, during long-term antihypertensive drug therapy, serial central BP determinations are required to predict CV complications and justify the development of new de-stiffening strategies enabling to prevent CV risk.Citation2
Pulsatile arterial hemodynamics and renin-angiotensin system
ANG-II blockade is classically associated with reduction of vascular resistance and MAP. In contrast, the effects on PWV and central and peripheral PP have been poorly investigated until recently. Studies on animal models and in humans suggest that ANG-II blockade is associated with reverse remodeling of both small and large arteries via specific mechanisms including anti-inflammatory and antifibrotic effects as well as changes of arterial attachments linking α5β1-integrin to its specific ligand fibronectin.Citation42–Citation44 Such effects are very important to consider in order tp obtain a significant and selective reduction of central PP and arterial stiffness under ANG-II blockade. They affect both small and large arteries and are acting through the MAP-kinase system.Citation10,Citation44
In hypertensive rats under low-salt diet (but not under high-salt diet), ANG-II blockade by the ARB valsartan normalizes central PP (<50 mm Hg) but not MAP for the same drug dosage.Citation42,Citation43 In hypertensive subjects under ANG-II blockade, not only PWV is decreased independently of MAP but also central wave reflections are attenuated and carotid-brachial SBP and PP amplification are increased. ANG-II blockade improves, or even normalizes, the wall thickness of small resistance arteries, and at the same time, reduces pressure wave reflections, suggesting a cause/effect relationship between the two factors.Citation1,Citation5 The arterial properties do not differ consistently whether ACEI or ARB are used and are the basis of all new strategies using de-stiffening therapy.
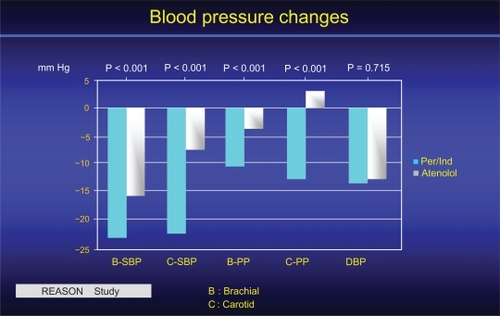
The Reason studyCitation10,Citation45 was the first to investigate the long-term interactions among PP, arterial stiffness and wave reflections in relationship to drug treatment and end-organ damage (cardiac mass) in hypertensive subjects of the middle age. The ACEI Per, associated with low-dose Ind, was compared for 1 year of treatment with the beta-blocking agent atenolol. For the same DBP and MAP decreases, Per/Ind lowered SBP and PP more than atenolol (). The reduction was more pronounced centrally (carotid artery) than peripherally (brachial artery). While the two drug regimens lowered PWV equally, only Per/Ind reduced central PP and AIx.Citation10,Citation45 In addition, Per/Ind decreased cardiac hypertrophy more than atenolol, and this diminution was attributed to the AIx decrease, indicating that the reduction of cardiac end-organ damage reflected mainly an effect on central wave reflections.Citation10,Citation45 Under drug treatment, the lowering of SBP was significantly predicted by baseline PWV.
Figure 5 Central (C) and brachial (B) BP of the REASON study before and after one year treatment 10. Whereas the DBP reduction was similar for the 2 groups of subjects, the reduction of SBP and mostly of PP was more pronounced on central than brachial arteries, in favour of Per/Ind when compared to atenolol. Reproduced with permission from Asmar et al 2001. Hypertension. 2001;38:922–926.Citation52 Copyright © 2001 American Heart Association.
De-stiffening strategy and BP control
ANG-II blockade and diuretics
The main therapeutic trial demonstrating the predictive role of aortic stiffness in hypertensive subjects was conducted in ESRD patients on hemodialysis.Citation46 The objective of that trial was to lower CV morbidity and mortality through a therapeutic regimen involving successively: salt and water depletion by dialysis; then, after randomization, ACEI or CCB; and, finally, the combination of the two agents and/or their association with a beta-blocker. Using that protocol, it was possible to evaluate, over long-term follow-up (51 months), whether or not the drug-induced MAP reduction was associated with a parallel diminution of PWV impacting on CV risk. During follow-up, it was evident that survivors’ MAP, brachial PP and aortic PWV were lowered in parallel. In contrast, for patients who died from CV events, MAP had been reduced to the same extent as in survivors, but drug treatment had not significantly modified PWV or brachial PP. Thus, survival of ESRD patients was significantly better when aortic PWV declined in response to BP lowering. The adjusted relative risks for all-cause and CV mortality rates in those with unchanged PWV in response to BP changes were respectively: 2.59 (95% CI, 1.51 to 4.43) and 2.35 (95% CI, 1.23 to 4.51) (P < 0.01). The prognosis value of PWV sensitivity to BP reduction on survival was independent of age, BP changes and blood-chemistry abnormalities ().The results indicated that arterial stiffness was not only a risk factor contributing to the development of CV disease but also that it was a marker of established, more advanced and less reversible arterial lesions. This interpretation was supported by the loss of aortic PWV sensitivity to BP lowering for nonsurvivors, compared to survivors whose arterial stiffness remained responsive to BP reduction. Finally, in that trial, prolonged survival seemed to be more closely associated with the use of an ACEI than other drugs or the number of drugs per se. The use of beta-blockers and/or CCB had no direct impact on the outcomes.Citation46
In diabetes subjects, the Per/Ind combination was studied in double-blind vs placebo with or without intensive glucose therapy. The BP control was followed for 4.3 years in 11,340 subjects. Reduction of BP was associated with a reduction of overall and CV mortality. The prediction of CV mortality was significant for brachial artery SBP and PP but not for MAP and DBP. It is worth noting that only diabetes mellitus, and not BP, was the criterion of selection of ADVANCE.Citation47 However, the predictive values were in agreement with those of REASON.Citation53
ANG-II blockade and CCB blockade
The CAFE study, a subanalysis of the ASCOT trial 48, conducted on 2073 subjects, showed that aortic PP, recorded noninvasively by radial tonometry and the application of generalized transfer functions, was a determinant of clinical outcomes, independently of age, other traditional CV risk factors and even peripheral PP. In agreement with the REASON study,Citation10,Citation45 the results of the CAFE study showed that treating subjects with a regimen based on the beta-blocker atenolol and a diuretic versus one based on the CCB amlodipine and an ACEI had similar effects on brachial SBP and PP but different effects on central aortic pressures.Citation48 Even though brachial pressure reductions were similar for the two arms of the study, central SBP and PP decreases were greater for the CCB amlodipine and the ACEI Per arm. That study’s results not only demonstrated that brachial PP does not always reflect the effect of different pressure-lowering treatments on central aortic pressures, but also suggested that central pressure changes might better predict clinical outcomes other than brachial pressures.Citation48 Therefore, antihypertensive drug therapy should selectively lower SBP and PP through complex interactions between small and large artery effects, thereby opening the way for the development of new long-term CV-treatment strategies involving both small and large arteries.
The comparison effects between a CCB and a diuretic in combination with the same ARB OLM were studied using aortic SBP and brachial ambulatory SBP.Citation49 This was a prospective, randomized, open-label, blinded end-point study in 207 hypertensive patients (mean age: 68.4 years). Patients received OLM monotherapy for 12 weeks, followed by additional use of AZE (n = 103) or hydrochlorothiazide (n = 104) for 24 weeks after randomization. After adjustment for baseline covariates, the extent of reduction in central SBP of the OLM/AZE group was significantly greater than that in the OLM/DIURETIC group (between-group difference 95% CI 5.2 0.3 to 10.2 mm Hg, P = 0.039), while the difference in the reduction in brachial SBP between the groups was not significant (2.6 to 2.2 to 7.5 mm Hg, P = 0.29. The aortic PWV showed a significantly greater reduction for the OLM/AZE combination than for the OLM/DIURETIC combination (08 0.5 to 1.1 m/s, P < 0.001) after adjustment for covariates. The extent of reduction in brachial ambulatory SBP was similar between the groups. These data showed that the combination of OLM 20 mg/AZE 16 mg had a more beneficial effect on central SBP and arterial stiffness than the combination of OLM 20 mg/hydrochlorothiazide 12.5 mg, despite the lack of a significant difference in brachial SBP reduction between the two treatments.
In the present study, Matsui et alCitation49 have provided evidence on the mechanism of reduction of SBP and PP amplification by CCB, namely by reducing PWV and AIx. CCB is a powerful vasodilating agent, increasing the large artery diameter independently of BP reduction and of any endothelium-dependent effect. Reduction of pressure wave reflections is the second important mechanism of central SBP reduction by CCB. Although heart rate was decreased in the OLM/AZE arm, thus favoring an earlier timing of wave reflections in systole, AI was reduced. This may be explained partly by the prolonged time of return of the reflected wave, due to lower PWV. Another possibility is that the long-term drug treatment causes regression of arteriolar hypertrophy as usually observed under CCB treatment. This might cause distal shift of reflections sites or decrease of reflection coefficients, thus lowering amplitude of wave reflections.Citation5 The same possibility may be observed with ANG-II blockade, but not with diuretic or traditional beta-blocking agents given alone.Citation50 Whether AZE has such effects, most likely via arteriolar vasodilatation and structural changes, or by altering baro-reflex sensitivity, or even acting synergistically with ANG-II blockade,Citation51 are possibilities that merit further exploration.
In conclusion, this report has shown that it is possible to obtain in the long term a selective reduction of brachial and mostly central PP through a decrease of arterial stiffness and wave reflections. Most of the therapeutic protocols involve angiotensin blockade. Because CV reduction is dominantly related to the control of SBP, the de-stiffening strategy should be now extensively developed.
Abbreviations
| ACEI | = | angiotensin-converting-enzyme inhibitor; |
| AIx | = | augmentation index; |
| AI | = | augmentation pressure; |
| ANG-II | = | angiotensin II; |
| ARB | = | angiotensin AT1-receptor blocker; |
| AZE | = | azelnidipine; |
| BP | = | blood pressure; |
| CAD | = | coronary artery disease; |
| CCB | = | calcium channel blocker; |
| CI | = | confidence interval; |
| CV | = | cardiovascular; |
| DBP | = | diastolic blood pressure; |
| ECM | = | extracellular matrix; |
| ESRD | = | end-stage renal disease; |
| Ind | = | indapamide; |
| MAP | = | mean arterial pressure; |
| NO | = | nitrite oxide; |
| OLM | = | olmesartan; |
| Per | = | perindopril; |
| PP | = | pulse pressure; |
| PWV | = | pulse wave velocity; |
| BP | = | systolic blood pressure; |
| VSM | = | vascular smooth muscle. |
Acknowledgements
This work was performed with the help of INSERM (Institut de la Santé et de la Recherche Médicale) and GPH-CV (Groupe de Pharmacologie et d’Hémodynamique Cardio-vasculaire), Paris. We thank Dr Anne Safar for helpful and stimulating discussions.
Disclosure
The author declares no conflicts of interest.
References
- NicholsWWO’RourkeMFMcDonald’s Blood Flow in Arteries Theoretical, Experimental and Clinical Principles4th edEdward ArnoldLondon20064994
- SafarMEO’RourkeMFArterial stiffness in hypertensionHandbook of HypertensionElsevier2006362
- BlackHRThe paradigm has shifted, to systolic blood pressureHypertension19993438638710489381
- KannelWBGordonTSchwartzMJSystolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham studyAm J Cardiol1971273353465572576
- KannelWBHypertension as a cardiovascular risk factorBulpittCJHandbook of Hypertension Epidemiology of HypertensionElsevier ScienceAmsterdam19851534
- ManciaGGrassiGSystolic and diastolic blood pressure control in antihypertensive drug trialsJ Hypertens2002201461146412172300
- LangilleBLRemodeling of developing and mature arteries: endothelium, smooth muscle, and matrixJ Cardiovasc Pharmacol199321Suppl 1S11S1177681126
- LevyBIAmbrosioGPriesARStruijker-BoudierHAMicrocirculation in hypertension: a new target for treatmentCirculation200110473574011489784
- GibbonsGHDzauVJThe emerging concept of vascular remodelingN Engl J Med1994330143114388159199
- LondonGMAsmarRGO’RourkeMFSafarMEAnd on behalf of the REASON project. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenololJ Am Coll Cardiol200443929914715189
- SafarMERizzoniDBlacherJMuiesanMLAgabiti-RoseiEMacro and microvasculature in hypertension: therapeutic aspectsJ Hum Hypertens20082259059518509346
- WilliamsBMechanical influences on vascular smooth muscle cell functionJ Hypertens199816192119299886878
- GlagovSHemodynamic risk factors: mechanical stress, mural architecture, medial nutrition and vulnearbility of arteries to atherosclerosisWisslerRWGeerJCThe Pathogenesis of AtherosclerosisWilliams & WilkinsBaltimore1972164199
- DarnéBGirerdXSafarMCambienFGuizeLPulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortalityHypertension1989133924002522417
- MadhavanSOoiWLCohenHAldermanMHRelation of pulse pressure and blood pressure reduction to the incidence of myocardial infarctionHypertension1994233954018125567
- FranklinSSKhanSAWongNDLarsonMGLevyDIs pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart studyCirculation199910035436010421594
- BlacherJStaessenJAGirerdXPulse pressure not mean pressure determines cardiovascular risk in older hypertensive patientsArch Intern Med20001601085108910789600
- MillarJALeverAFBurkeVPulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension TrialJ Hypertens1999171065107210466460
- BenetosAZureikMMorcetJA decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in menJ Am Coll Cardiol20003567368010716470
- DomanskiMJDavisBRPfefferMAKastantinMMitchellGFIsolated systolic hypertension: prognostic information provided by pulse pressureHypertension19993437538010489379
- MitchellGFMoyeLABraunwaldESphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular EnlargementCirculation199796425442609416890
- ChaeCUPfefferMAGlynnRJMitchellGFTaylorJOHennekensCHIncreased pulse pressure and risk of heart failure in the elderlyJAMA199928163463910029125
- KlassenPSLowrieEGReddanDNAssociation between pulse pressure and mortality in patients undergoing maintenance hemodialysisJAMA20022871548155511911757
- SchramMTKostensePJVan DijkRADiabetes, pulse pressure and cardiovascular mortality: the Hoorn StudyJ Hypertens2002201743175112195114
- BenetosASafarMRudnichiAPulse pressure: a predictor of long-term cardiovascular mortality in a French male populationHypertension199730141014159403561
- BlacherJGuerinAPPannierBMarchaisSJSafarMELondonGMImpact of aortic stiffness on survival in end-stage renal diseaseCirculation1999992434243910318666
- BlacherJPannierBGuerinAPMarchaisSJSafarMELondonGMCarotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal diseaseHypertension1998325705749740628
- LondonGMBlacherJPannierBGuerinAPMarchaisSJSafarMEArterial wave reflections and survival in end-stage renal failureHypertension20013843443811566918
- ShojiTEmotoMShinoharaKDiabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal diseaseJ Am Soc Nephrol2001122117212411562410
- BarenbrockMKoschMJosterEKistersKRahnKHHausbergMReduced arterial distensibility is a predictor of cardiovascular disease in patients after renal transplantationJ Hypertens200220798411791029
- BlacherJAsmarRDjaneSLondonGMSafarMEAortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patientsHypertension1999331111111710334796
- StefanadisCWooleyCFBushCAKolibashAJBoudoulasHAortic distensibility abnormalities in coronary artery diseaseAm J Cardiol198759130013043591683
- LaurentSBoutouyriePAsmarRAortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patientsHypertension2001371236124111358934
- MeaumeSBenetosAHenryOFRudnichiASafarMEAortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of ageArterioscler Thromb Vasc Biol2001212046205011742883
- Willum-HansenTStaessenJATorp-PedersenCPrognostic value of aortic pulse wave velocity as index of arterial stiffness in the general populationCirculation200611366467016461839
- CruickshankKRisteLAndersonSGWrightJSDunnGGoslingRGAortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular functionCirculation20021062085209012379578
- HiraiTSasayamaSKawasakiTYagiSStiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosisCirculation19898078862610739
- GatzkaCDCameronJDKingwellBADartAMRelation between coronary artery disease, aortic stiffness, and left ventricular structure in a population sampleHypertension1998325755789740629
- JankowskiPKawecka-JaszczKCzarneckaDPulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patientsHypertension20085184885518268136
- WeberTAuerJO’RourkeMFArterial stiffness, wave reflections, and the risk of coronary artery diseaseCirculation200410918418914662706
- SafarMEBlacherJPannierBCentral pulse pressure and mortality in end-stage renal diseaseHypertension20023973573811897754
- KakouABezieYMercierNSelective reduction of central pulse pressure under angiotensin blockage in SHR: role of the fibronectin-alpha5beta1 integrin complexAm J Hypertens20092271171719424161
- LabatCLacolleyPLajemiMde GasparoMSafarMEBenetosAEffects of valsartan on mechanical properties of the carotid artery in spontaneously hypertensive rats under high-salt dietHypertension20013843944311566919
- LouisHKakouARegnaultVRole of alpha1beta1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in miceAm J Physiol Heart Circ Physiol2007293H2597H260417660399
- de LucaNAsmarRGLondonGMO’RourkeMFSafarMESelective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjectsJ Hypertens2004221623163015257187
- GuerinAPBlacherJPannierBMarchaisSJSafarMELondonGMImpact of aortic stiffness attenuation on survival of patients in end-stage renal failureCirculation200110398799211181474
- KengneAPCzernichowSHuxleyRBlood pressure variables and cardiovascular risk: new findings from ADVANCEHypertension20095439940419470869
- WilliamsBLacyPSThomSMDifferential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) studyCirculation20061131213122516476843
- MatsuiYEguchiKO’RourkeMFDifferential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin ii receptor blocker on central aortic pressure in hypertensive patientsHypertension20095471672319667251
- Agabiti-RoseiEHeagertyAMRizzoniDEffects of antihypertensive treatment on small artery remodellingJ Hypertens2009271107111419293726
- JinnoTIwaiMLiZCalcium channel blocker azelnidipine enhances vascular protective effects of AT1 receptor blocker olmesartanHypertension20044326326914707152
- AsmarRGLondonGMO’RourkeMESafarMEREASON Project Coordinators and InvestigatorsImprovement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenololHypertension20013892292611641310
- ADVANCE Collaborative GroupPatelAMacMahonSChalmersJIntensive blood glucose control and vascular outcomes in patients with type 2 diabetesN Engl J Med20083582560257218539916