Abstract
Although reducing blood pressure is the most important approach to reduce cardiovascular outcomes in the hypertensive population, the majority of patients fail to attain the targets. Most patients with hypertension need at least 2 antihypertensive agents to achieve blood pressure goals. The 2007 European hypertension guidelines state that combined therapy is needed when monotherapy does not attain blood pressure objectives and as a first-line treatment in high-risk patients. This point has been reinforced in the 2009 update of the European guidelines. The advantages of combination therapy are well documented with the potential for increased antihypertensive efficacy as a result of different mechanisms of action, and a lower incidence of adverse effects because of the lower doses used and the possible compensatory responses. Moreover, the use of fixed dose combinations are specially recommended as they facilitate treatment compliance. The inhibition of the renin-angiotensin system appears to be very beneficial in the treatment of patients with hypertension along the cardiovascular continuum and the combination of a renin-angiotensin system inhibitor and a diuretic is particularly recommended. Many clinical trials have demonstrated the benefits of the fixed combination perindopril/indapamide in the treatment of hypertension. The aim of this manuscript is to update the published data on the efficacy and safety of this fixed combination.
Introduction
Arterial hypertension, a major risk factor for the establishment and development of cerebrovascular, cardiovascular and renal diseases, is very prevalent worldwide. It has been estimated that about a quarter of the general population is hypertensive, a proportion that increases with age.Citation1–Citation3 In Spain, 44% of the middle-aged population and 68% of patients aged 60 years or older exhibit hypertension.Citation1 In United States about 65 million people are hypertensive.Citation2,Citation3 It has been calculated that hypertension is responsible for 1 of every 14 deaths for any reason and for 1 of every 2.5 cardiovascular deaths.Citation4
Even small elevations above optimal systolic or diastolic blood pressure (BP) values increase the probability of cardiovascular outcomes.Citation5 Thus, in 18,876 healthy subjects, an increased risk of new onset heart failure in individuals with systolic BP 130–139 mmHg compared with those with optimal BP (<120 mmHg) has recently been reported, with a linear trend in heart failure risk across the normal range of systolic BP.Citation6 Similar findings have been reported in patients with ischemic heart disease.Citation7 A post hoc analysis of INVEST (International Verapamil SR-Trandolapril Study) trial, performed in 22,576 patients with hypertension and coronary artery disease, showed there was a steep reduction in cardiovascular risk in parallel to the proportion of visits with controlled BP, independent of baseline characteristics and mean on-treatment BP.Citation7 In the classical systematic review of Collins et alCitation8 a 42% stroke risk reduction (P < 0.0001) and a 14% coronary heart disease risk reduction in those hypertensives who attained BP goals, when compared to those treated but not adequately controlled, was reported. As a result, it is crucial not only to reduce BP values but to achieve BP goals in order to improve cardiovascular prognosis.Citation5
Although in the last decades BP control rates have progressively improved (ie, in Spain, BP control has increased from <20% in 1990s to the current 40%),Citation9 they are far from optimal and this occurs everywhere (Italy about 31%, United Kingdom 36%, Germany 40% and France 46%).Citation2 However, after the results of EUROASPIRE III, it seems that this improvement has stopped or at least slowed.Citation10 EUROASPIRE surveys analyzed rates of modifiable cardiovascular risk factors in patients with coronary heart disease. EUROASPIRE I, II, and III were designed as cross-sectional studies and included the same selected geographical areas and hospitals in the Czech Republic, Finland, France, Germany, Hungary, Italy, the Netherlands, and Slovenia. These studies showed that although the proportion with raised total cholesterol has markedly decreased, from 94.5% in EUROASPIRE I to 76.7% in II, and 46.2% in III (P < 0.0001), the proportion of patients with raised BP (≥140/90 mmHg in patients without diabetes or ≥130/80 mmHg in patients with diabetes) remained unchanged (58.1% in EUROASPIRE I, 58.3% in II, and 60.9% in III; P =0.49).Citation10
These data suggest that, although in the general hypertensive population BP control rates are rising, this does not occur in those hypertensive patients at higher risk such as those with coronary heart disease. In fact, as cardiovascular risk increases, a lesser proportion of patients attain BP goals.Citation10,Citation11 This is very relevant, since nowadays the majority of patients attended by specialists or general practitioners, belong to high- or very high-risk groups.Citation12,Citation13 Furthermore, since the prevalence of diabetes, obesity and sedentary life style is growing, it is likely that the number of high risk hypertensive patients will rise in the future.Citation14
Although it is well known that the majority of hypertensive patients will need more than 1 antihypertensive drug to attain BP objectives (particularly those at higher risk),Citation15,Citation16 several surveys have reported that combined therapy is actually underused.Citation9–Citation12 The 2007 European guidelines for the management of arterial hypertension, indicate that combined therapy is required when monotherapy fails to attain BP goals. They also show that a combination of 2 drugs at low doses as first line treatment, can be prescribed when total cardiovascular risk is high or very high, or when initial BP values are in the range of grade 2 or 3.Citation5 The evidence that in the vast majority of hypertensives effective BP control can only be achieved by combination of at least 2 antihypertensive agents continues to grow, as a last update of European guidelines shows. Moreover, the combination of 2 antihypertensive drugs may offer advantages also for treatment initiation, particularly in patients at high cardiovascular risk in which early BP control may be desirable.Citation17 Fortunately, although the use of combined therapy is still low and far from optimal, its prescribing has improved in the last decade.Citation18,Citation19
The use of a combination of 2 antihypertensive agents at fixed doses in a single tablet should be preferred, since decreasing the number of pills that have to be taken daily has been associated with an improvement in compliance, and consequently, better BP control rates during follow-up.Citation20 As current recommendations report, there are several 2-drug fixed combinations suitable for clinical use. However, trial evidence of outcome reduction has been obtained particularly for the combination of a diuretic or a calcium channel blocker, with an angiotensin-converting enzyme (ACE) inhibitor, or a diuretic with an angiotensin receptor blocker. Importantly, the use of the angiotensin receptor blocker/calcium channel blocker combination also appears to be rational and effective.Citation17 As a result, these combinations should be recommended for priority use. This manuscript aims to update the published data on the efficacy and safety of the fixed combination perindopril plus indapamide.
Renin-angiotensin system and organ damage
Although the renin-angiotensin aldosterone system (RAAS) is important for the cardiovascular system homeostasis, the BP control, and the sodium and water balance, its excessive activation promotes the development and worsening of cardiovascular disease.Citation21 Angiotensin II is associated with all phases of cardiovascular disease, from the early (hypertension), to the mid (left ventricular hypertrophy and microalbuminuria), to the late stages (myocardial infarction, heart failure stroke, and renal disease).
Left ventricular hypertrophy is one of the most relevant subclinical organ damage in patients with hypertension.Citation5 Although many factors have been involved in the establishment and development of left ventricular hypertrophy in hypertension, it is likely that the RAAS activity and the increased afterload are the main ones.Citation22 Its presence increases 2- to 5-fold the risk of major cardiovascular events.Citation23 However, left ventricular hypertrophy regression, or at least reduction, is associated with a better prognosis.Citation5 Although the most important point in the treatment of hypertensive population with left ventricular hypertrophy is BP reduction, several trials have reported that RAAS inhibitors could be recommended as first-line therapy in this setting.Citation5,Citation24,Citation25
It is well known that renal disease and hypertension are closely related. Hypertension is one of the most frequent causes of new-onset renal disease and their progression toward end-stage renal failure and conversely, chronic kidney disease promotes the development of hypertension.Citation26 Microalbuminuria is an early manifestation of renal involvement in patients with hypertension, particularly in diabetic population. The presence of microalbuminuria in this context has been related to an increase of mortality, and its reduction with a better prognosis.Citation5 Many clinical trials have shown that RAAS inhibition is a very effective therapeutic strategy in hypertensive patients with renal impairment.Citation27,Citation28 RAAS inhibition promotes a decrease of glomerular pressure, a decline of albumin excretion rate due to the dilatation of efferent arterioles, and a reduction of local inflammation and growth in the glomerulus. This translates into a reduced vascular trophic remodelling and results in different and additive beneficial effects on renal function and structure.Citation26
Endothelial dysfunction is a predictor of cardiovascular events in hypertensive patients.Citation29,Citation30 Endothelial dysfunction as well as vascular endothelial cell apoptosis occurs in the early atherosclerotic lesions, but also as cardiovascular disease progresses.Citation31 This endothelial impairment damages the functioning of endothelium, affecting nitric oxide bioavailability, promoting vasoconstriction, inflammation, thrombosis and platelet activation what finally provokes the development of atherosclerotic disease.Citation32 By contrast, ACE inhibitors improve endothelium-dependent vasodilation in hypertensive patients, protecting them from ischemic heart disease.Citation33
Pharmacology and rationale for the combination of perindopril and indapamide
Perindopril is a prodrug that is rapidly absorbed in the gastrointestinal tract after oral administration. Bioavailability of perindopril is 61%–85%. The biotransformation of perindopril to perindoprilat, the active metabolite, is approximately 20%. Notably, food intake may reduce hepatic biotransformation to perindoprilat. The peak plasma concentration and the peak pharmacological activity of perindoprilat occur at 3 to 4 hours and 4 to 6 hours, respectively, after oral administration of perindopril. The rates of protein binding of perindoprilat are low (<30%). Free perindoprilat is eliminated via the urine. Although the elimination half-life of the free fraction of perindoprilat is between 3 and 5 hours, the terminal half-life of the dissociation of perindoprilat from plasma and tissue ACE is about 25 to 30 hours. The steady-state concentration of perindoprilat is reached within 4 days when chronically administered.Citation34–Citation36
Indapamide is an oral diuretic with natriuretic properties that acts in the proximal segment of the distal tubule. Interestingly, the main effect of indapamide is on sodium and chloride excretion, but with less effect on potassium or uric acid urine excretion. Nevertheless, there is an appreciable increase in urinary volume only at doses greater than 2.5 mg/day. Despite these renal effects, it has been suggested that the reduction in vascular reactivity to pressor amines caused by indapamide has a more important role in its antihypertensive effect.Citation34,Citation37,Citation38
Indapamide has high lipid solubility and as a consequence, its absorption from the gastrointestinal tract is fast (30 to 60 minutes after oral administration), and complete. Indapamide is bound to plasma proteins in 79% and has a relatively low apparent volume of distribution of approximately 60 L. Plasma elimination half life is biphasic and between 14 and 25 hours. The steady-state concentration of indapamide is reached within 3 to 4 days when chronically administered. Indapamide is widely metabolized in the Liver, principally by CYP2C9 and CYP3A4 isozymes, and by cytosolic hydrolysis enzymes. The main route of elimination is the urine, and 20 to 23% in the feces. In contrast to hydrochlorothiazide, indapamide does not adversely affect lipid profile or glucose tolerance either in hypertensive patients with diabetes.Citation34,Citation37,Citation38
The combination of perindopril, an ACE inhibitor, and indapamide, a chlorosulphamoyl diuretic, is recommended as one of the antihypertensive combinations of priority use by the last update of European hypertension guidelines.Citation17 Due to their synergistic mechanisms of action, the doses at which this combination is given is up to 2 times lower than the usual dose used for monotherapy, showing a higher antihypertensive effect with lesser side effects. On the one hand, as indapamide depletes the cell of sodium and of calcium, this reduces the vascular response to angiotensin II and on the other hand, perindopril blocks the activation of RAAS and sympathetic nervous system induced by indapamide. Moreover, the potassium depletion caused by indapamide is buffered by perindopril due to its potassium-sparing effect. Notably, the co-administration of perindopril and indapamide does not change their pharmacokinetic properties when compared to both drugs in monotherapy, and this facilitates its administration.Citation34,Citation39
Efficacy and safety of the combination perindopril/indapamide
Hypertension
Several randomized clinical trials and observational studies have analyzed the benefits of the fixed combination perindopril/indapamide in the treatment of hypertensive population. In a study performed in stable hypertensive patients with systolic BP >130 mmHg and/or diastolic BP > 85 mmHg, even with up to 2 antihypertensive drugs, excluding ACE inhibitors, angiotensin II receptor blockers or a diuretic, patients were randomized to receive perindopril 2 mg/indapamide 0.625 mg or cilazapril 2.5 mg once daily for a period of 12 weeks after a 2-week placebo run-in phase.Citation40 Although systolic BP was significantly reduced by both groups, diastolic BP was significantly reduced only by the combination perindopril/indapamide. Notably, the response rate, defined as systolic BP ≤ 140 mmHg and diastolic BP ≤ 90 mmHg at the last visit or a >20 mmHg reduction in systolic BP and/or >10 mmHg reduction in diastolic BP, was significantly higher with the combination (100%) than with cilazapril (70%) (P = 0.0086). Interestingly, there was no difference in the number of adverse events between the 2 groups.
In the STRATHE trial, the efficacy and the tolerability of three different strategies in the treatment of hypertension (low-dose combination, sequential monotherapy and stepped-care) were compared.Citation41,Citation42 Hypertensive patients were randomized to a 9-month treatment. In the ‘low-dose combination’ group (n = 180), perindopril (2 mg) and indapamide (0.625 mg) were first administered with the possibility of increasing the doses in 2 steps up to 4 and 1.25 mg respectively. In the ‘sequential monotherapy’ group (n = 176), the treatment was initiated with atenolol (50 mg), replaced if necessary by losartan (50 mg), and afterwards by amlodipine (5 mg). In the ‘stepped-care’ group (n = 177), valsartan, was given first at a 40 mg dose, then at a 80 mg dose, to be finally co-administered with hydrochlorothiazide 12.5 mg if required. The main results of this study showed that the proportion of patients that achieved BP goals, was significantly higher in the ‘low-dose combination’ group (62%) than in the ‘sequential monotherapy’ (49%, P = 0.02) and the ‘stepped-care’ group (47%, P = 0.005). Moreover, the percentage of patients that normalized their BP was significantly greater in the ‘low-dose combination’ group (56%) than in the ‘sequential monotherapy’ (42%, P = 0.002) or in the ‘stepped-care’ group (42%, P = 0.004). Interestingly, these better BP results were not obtained at the expense of a worsening tolerability.
The Optimax II study was performed to assess whether the pre-existence of metabolic syndrome defined by the NCEP-ATP III criteria, had any impact on BP control in hypertensive patients receiving a fixed perindopril/indapamide combination therapy.Citation43 A total of 24,069 hypertensive patients were prospectively included and the follow-up lasted 6 months. About 30% of patients exhibited metabolic syndrome. Patients were divided in 3 groups: previously untreated, who received the combination therapy as initial treatment; previously treated but with unsatisfactory results and/or treatment intolerance, they had its previous treatment switched to perindopril/indapamide; and previously treated, with good treatment tolerance but uncontrolled BP, who received the study treatment in adjunction to the previous one. The normalization rates were 70.3%, 68.4%, and 64.1%, respectively, (P < 0.0001). Interestingly, the pre-existence of metabolic syndrome did not show any significant influence on these figures.
A meta-analysis was performed to assess the efficacy and safety profiles (through review of randomized, controlled trials) of the fixed, low-dose combination perindopril 2 mg and indapamide 0.625 mg given as first-line antihypertensive therapy in patients with mild to moderate hypertension.Citation44 In this systematic review, a total of 11 trials (5,936 individuals) were reviewed. In the 5 studies that compared perindopril indapamide versus placebo, the combination significantly reduced both systolic and diastolic BP values. In the other 6 studies, perindopril indapamide was compared to other antihypertensive therapies (perindopril 4 mg/day in monotherapy, losartan 50 mg/day, irbesartan 150 mg/day, enalapril 40 mg/day), showing significantly higher reductions in BP values with the combination perindopril/indapamide. Adverse events and withdrawals were not significantly different between perindopril indapamide and control groups.
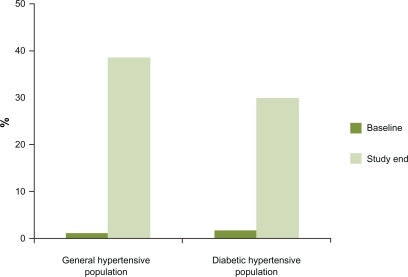
Although the results of controlled randomized trials are very important, they are selective and significant differences may remain between them and the ‘real world’ of general practice. Therefore, it is not always reliable to translate these results to clinical practice.Citation45,Citation46 In this context, observational studies may be useful to determine the impact of compliance, tolerability and BP control in daily clinical activity.Citation47,Citation48 In a descriptive, multicenter survey carried out in primary care setting across Spain, general practitioners were asked about their own experience in the use of the fixed combination perindopril 2 mg plus indapamide 0.625 mg in hypertensive patients for a minimum of 6 weeks.Citation47 They found in 3,198 patients, that BP control rates increased from 1.1% at baseline, to 38.7% with the combination (). Moreover, the great majority of physicians considered the efficacy and tolerability of the combination perindopril and indapamide as good or very good (88.8% and 96.2%, respectively). Furthermore, most patients (92%) were satisfied or very satisfied with the therapy. Another study with a similar design, but including specialists, was performed including a total of 5,126 patients with hypertension and diabetes.Citation48 At baseline, 1.7% of the general practitioners’ patients and 1.3% of the specialists’ patients had their BP controlled, and with the combined therapy, BP control rates increased to 30.7% and 29.8%, respectively (P < 0.001 vs baseline and not significantly different between groups) (). Approximately 85% of physicians considered the efficacy and tolerability of combined therapy as ‘good’ or ‘very good’ and 93% of the patients were ‘satisfied’ or ‘very satisfied’ with the combined therapy.
Figure 1 Changes in blood pressure control rates (%) during the study in PRETEND and PRETENDIABETES studies with the fixed combination perindopril 2 mg plus indapamide 0.625 mg. Drawn from data of.Citation47,Citation48
Organ damage
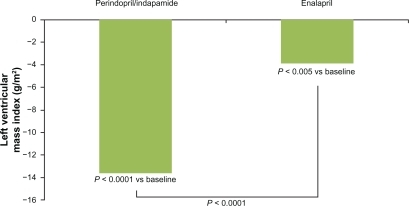
The fixed combination perindopril/indapamide has been shown to be an effective therapy for the treatment of patients with hypertension and subclinical organ damage.Citation49–Citation52 In the PICXEL study,Citation49 the efficacy of a strategy based on first-line combination with perindopril/indapamide versus monotherapy with enalapril in reducing echocardioghraphic left ventricular hypertrophy in hypertensive patients was compared. After 1 year, treatment systolic and diastolic BP decreased significantly more in the perindopril/indapamide than in the enalapril group (P < 0.0001 and P = 0.003, respectively). Moreover, the left ventricular mass index decreased by 13.6 ± 23.9 g/m2 with perindopril/indapamide (P < 0.0001 vs baseline) and 3.9 ± 23.9 g/m2 with enalapril (P < 0.005 vs baseline and P < 0.0001 between groups) (). Both treatments were well tolerated. In an ancillary study of the PICXEL trial, the fixed combination perindopril/indapamide reduced 24-hour and daytime systolic BP as well as pulse pressure significantly more than enalapril treatment (P < 0.01). No significant between-group differences were noted for diastolic BP or for night-time measurements. Trough/peak ratios were higher with perindopril/indapamide than with enalapril. Moreover, more patients required dose increases with enalapril (87%) than with perindopril/indapamide (71%).Citation50
Figure 2 Effect of the combination perindopril/indapamide (2 mg/0.625 mg up to 8 mg/2.5 mg) and enalapril (10 mg up to 40 mg/daily) on left ventricular mass index (g/m2). Data from data of the PICXEL study.Citation49
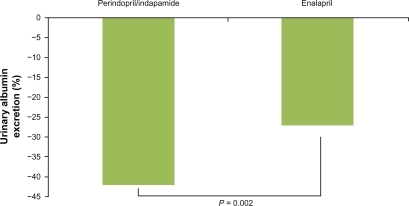
The effects of the combination perindopril/indapamide on kidney disease have also been assessed.Citation51 For this purpose, the combination of perindopril/indapamide was compared with enalapril monotherapy on albumin excretion rate (AER) in patients with type 2 diabetes, albuminuria, and hypertension in a 12-month, randomized study. After a 4-week placebo period, patients with albuminuria >20 and <500 μg/min, were randomized to a combination of 2 mg perindopril/0.625 mg indapamide or to 10 mg daily enalapril. After a 12-week period, doses were adjusted on the basis of BP to a maximum of 8 mg perindopril/2.5 mg indapamide or 40 mg enalapril. Combined therapy exhibited higher systolic and diastolic BP reductions than enalapril (−3.0, P = 0.012 and −1.5, P = 0.019, respectively) and higher AER reduction (−42% vs −27%, P = 0.002) (). The greater AER reduction remained significant after adjustment for mean BP. Adverse events were similar in the 2 groups.
Figure 3 Effect of the combination perindopril/indapamide (2 mg/0.625 mg up to 8 mg/2.5 mg) and enalapril (10 mg up to 40 mg/daily) on urinary albumin excretion (% of change from baseline). Data from data of the PREMIER study.Citation51
In a post hoc analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial, the effects of BP lowering and intensive glucose control on the incidence and progression of retinopathy in type 2 diabetes patients were analyzed.Citation52 The main results of this study showed that although BP lowering or intensive glucose control did not significantly reduce the incidence and progression of retinopathy, consistent trends towards a benefit were observed, with significant reductions in some lesions observed with both interventions. These effects of the 2 treatments were independent and additive.
Cardiovascular events
Several and important trials have specifically studied the efficacy of the combination perindopril/indapamide on cardiovascular events.Citation53–Citation63 The PROGRESS (perindopril protection against recurrent stroke study) trial, was designed to determine the effects of a BP-lowering regimen in hypertensive and non-hypertensive patients with a history of stroke or transient ischemic attack.Citation54–Citation56 A total of 6,105 subjects from 172 centers in Asia, Australasia, and Europe were randomized to active treatment consisting of a flexible regimen based on perindopril (4 mg daily), with the addition of indapamide at the discretion of treating physicians (n = 3051) or placebo (n = 3054). The primary end point of the study was total stroke (fatal or non-fatal). After a 4-year follow-up, perindopril/indapamide reduced BP by 9/4 mmHg. Those treated with perindopril/indapamide exhibited a 28% relative risk reduction (95% CI 17–38, P < 0.0001) in the primary outcome, and a 26% risk reduction for total major vascular events. There were similar reductions in the risk of stroke in hypertensive and non-hypertensive subgroups (all P < 0.01). The combination perindopril/indapamide reduced BP by 12/5 mmHg and stroke risk by 43%, whereas perindopril in monotherapy reduced BP by 5/3 mmHg, without a discernable reduction in the risk of stroke.
In the ADVANCE trial,Citation57–Citation61 the effects of the routine administration of the combination perindopril/indapamide on serious vascular events in patients with diabetes, irrespective of initial BP levels or the use of other BP-lowering drugs were assessed. After a 6-week active run-in period, 11,140 patients with type 2 diabetes were randomized to the combination perindopril/indapamide or placebo, in addition to current therapy. The primary endpoints were a composite of major macrovascular and microvascular events, defined as death from cardiovascular disease, non-fatal stroke or non-fatal myocardial infarction, and new or worsening renal or diabetic eye disease. After a mean of 4.3 years of follow-up, those assigned to perindopril/indapamide had a mean reduction in systolic BP of 5.6 mmHg and diastolic BP of 2.2 mmHg. The relative risk of a major macrovascular or microvascular event was reduced by 9% (P = 0.04). The relative risk of death from cardiovascular disease was reduced by 18% (P = 0.03) and death from any cause by 14% (P = 0.03) (). The fixed combination of perindopril and indapamide was well tolerated. The authors concluded that the results of the ADVANCE trial suggest that over 5 years, 1 death of any cause would be averted among every 79 patients assigned to active therapy.
Table 1 Main results of ADVANCE trialCitation57
A recent combined analysis using individual data from ADVANCE, EUROPA, and PROGRESS studies was performed to determine the consistency of the treatment effect of a perindopril-based regimen in patients with vascular disease or at high risk of vascular disease.Citation62 All-cause mortality and major cardiovascular outcomes during a follow-up of about 4 years in 29,463 patients randomly assigned to a perindopril-based treatment regimen or placebo were analyzed. The perindopril-based regimens were associated with a significant reduction in all-cause mortality (HR 0.89; P = 0.006), cardiovascular mortality (HR 0.85; P = 0.004), non-fatal myocardial infarction (HR 0.80; P < 0.001), stroke (HR 0.82; P = 0.002), and heart failure (HR 0.84; P = 0.015).
The results of the HYVET (Hypertension in the Very Elderly Trial) study have been very important to clarify how the management of the hypertensive population aged 80 years or older should be.Citation63 In this study, 3,845 patients from Europe, China, Australasia, and Tunisia, who were ≥80 years and had a sustained systolic BP ≥ 160 mmHg, were randomized to receive either indapamide (sustained release, 1.5 mg) or matching placebo. Perindopril (2 or 4 mg), or matching placebo, was added if necessary to achieve the target BP of 150/80 mmHg. The primary end point was fatal or nonfatal stroke. After 2 years of treatment, mean BP was 15.0/6.1 mmHg lower in the active-treatment group than in the placebo group. Active treatment was associated with a 30% reduction in the rate of fatal or nonfatal stroke (P = 0.06), a 39% reduction in the rate of death from stroke (P = 0.05), a 21% reduction in the rate of death from any cause (P = 0.02), a 23% reduction in the rate of death from cardiovascular causes (P =0.06), and a 64% reduction in the rate of heart failure (P < 0.001) ().
Table 2 Main results of HYVET trialCitation63
Safety and tolerability
The fixed combination perindopril plus indapamide is a safe and well-tolerated drug, with a low incidence of adverse events. In general, drug-related adverse events are mild and transient with a very low discontinuation rate (about 2%). The most frequent adverse events reported with the fixed combination perindopril (2–4 mg) plus indapamide (0.625–1.25 mg) are cough (4.4%), headache (3.1%), asthenia (1.6%), dizziness (1.4%) and flu-like symptoms (1.2%). Due to their complementary mechanisms of action, hyponatremia and hypokalemia are uncommon with perindopril/indapamide therapy. This antihypertensive combination does not adversely affect lipid profile or glucose tolerance even in hypertensive patients at risk.Citation34
The combination perindopril and indapamide is contraindicated in patients with a history of previous hypersensitivity to either of the active compounds, perindopril or indapamide, in subjects with bilateral renal artery stenosis (or unilateral in subjects with only one kidney), in patients with severe renal insufficiency (creatinine clearance below 30 mL/min), as well as during pregnancy and for lactating women.Citation34 It should be noted that these contraindications are the same for all RAAS blockers.
Conclusions and place in therapy
The majority of patients with hypertension often require more than one drug to achieve BP goals. The last update of the European guidelines for the management of arterial hypertension recommends the use of fixed combinations in those patients that require more than one antihypertensive drug to attain BP objectives. The combination of an ACE inhibitor with a diuretic is highly recommended in this context. Many trials have demonstrated the beneficial effects of perindopril on the whole spectrum of the cardiovascular continuum.
Clinical trials have shown that perindopril/indapamide is an effective and well-tolerated fixed-dose antihypertensive combination. As expected, it provides greater antihypertensive efficacy than either component taken as monotherapy. This combination has been demonstrated to reduce left ventricular mass index as well as albumin excretion rate, probably beyond its antihypertensive effect. But, moreover, relevant controlled randomized clinical trials such as ADVANCE, PROGRESS or HYVET have importantly shown that treatment with perindopril/indapamide reduces cardiovascular outcomes in different contexts, such as the diabetic population, a history of cerebrovascular disease or the elderly.
The ACCOMPLISH trial showed that not all antihypertensive fixed combinations have the same impact on cardiovascular outcomes. In this trial, the benazepril–amlodipine combination was superior to benazepril–hydrochlorothiazide in reducing cardiovascular events in a hypertensive population with a high proportion of patients with diabetes and obesity.Citation64 It should be kept in mind that in this situation, a thiazide may worsen glucose and lipid profiles and this could influence outcomes. However, indapamide does not have these deleterious effects on lipid and glucose profiles. Although both indapamide and thiazides are diuretics, their mechanisms of action differ, as well as their clinical benefits on vascular protection. Moreover, the evidence on the benefits of perindopril in outcome trials is much more robust than that with benazepril. As a result, the results of ACCOMPLISH should not be directly applied to the fixed combination perindopril-indapamide.
As a result, as 2007 ESH/ESC guidelines recommend, the fixed combination perindopril/indapamide at low doses could be suitable in the treatment of hypertensive patients at high or very high risk, as initial therapy.
Acknowledgments/disclosures
The authors have no conflicts of interests directly related with this manuscript.
References
- BanegasJRRodríguez-ArtalejoFde la Cruz TrocaJJBlood Pressure in Spain: distribution, awareness, control and benefits of a reduction in average pressureHypertension19983299810029856963
- WangYRAlexanderGCStaffordRSOutpatient hypertension treatment, treatment intensification, and control in Western Europe and the United StatesArch Intern Med200716714114717242314
- OngKLCheungBMManYBPrevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004Hypertension200749697517159087
- BanegasJRRodriguez-ArtalejoFGracianiAMortality attributable to cardiovascular risk factors in SpainEur J Clin Nutr200357Suppl 1S18S2112947446
- ManciaGDe BackerGDominiczakA2007 Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens2007251105118717563527
- BrittonKAGazianoJMDjousséLNormal systolic blood pressure and risk of heart failure in US male physiciansEur J Heart Fail2009111129113419861382
- ManciaGMesserliFBakrisGBlood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril StudyHypertension20075029930517606861
- CollinsRPetoRMacMahonSBlood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological contextLancet19903358278381969567
- BarriosVBanegasJRRuilopeLMEvolution of blood pressure control in SpainJ Hypertens2007251975197717762665
- KotsevaKWoodDDe BackerGEUROASPIRE Study GroupCardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countriesLancet200937392994019286092
- BarriosVEscobarCCalderonABlood pressure and lipid goal attainment in the hypertensive population in the primary care setting in SpainJ Clin Hypertens (Greenwich)2007932432917485967
- BarriosVEscobarCCalderonACONTROLRISK Investigators Cardiovascular risk profile and risk stratification of the hypertensive population attended by general practitioners and specialists in Spain. The CONTROLRISK studyJ Hum Hypertens20072147948517314997
- Marquez-ContrerasECocaAde la FigueraMCardiovascular risk profile of uncontrolled hypertensive patients. The Control-Project studyMed Clin (Barc)2007128869117288921
- RydenLStandlEBartnikMGuidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD)Eur Heart J2007288813617220161
- SicaDARationale for fixed-dose combinations in the treatment of hypertension: the cycle repeatsDrugs2002624462
- MotwaniJGCombining renin-angiotensin-aldosterone system blockade with diuretic therapy for treatment of hypertensionJ Renin Angiotensin Aldosterone Syst20023727712228846
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentJ Hypertens20091015 [Epub ahead of print]
- AmarJVaurLPerretMPRATIK study investigators. Hypertension in high-risk patients: beware of the underuse of effective combination therapy (results of the PRATIK study)J Hypertens20022077978411910316
- CocaABlood pressure control among treated hypertensive patients by Primary Care in Spain. The 2003 Controlpres studyHipertension200522514
- BangaloreSKamalakkannanGParkarSFixed-dose combinations improve medication compliance: a meta-analysisAm J Med200712071371917679131
- VolpeMAngiotensin II: an amplifier of cardiovascular riskCurr Hypertens Rep2004624724815257857
- SchmiederREThe role of non-haemodynamic factors of the genesis of LVHNephrol Dial Transplant2005202610261216221701
- LevyDGarrisonRJSavageDDPrognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart StudyN Engl J Med1990322156115662139921
- KlingbeilAUSchneiderMMartusPA meta-analysis of the effects of treatment on left ventricular mass in essential hypertensionAm J Med2003115414612867233
- EscobarCBarriosVCalderónAElectrocardiographic left ventricular hypertrophy regression induced by an angiotensin receptor blocker-based regimen in hypertensive patients with the metabolic syndrome: data from the SARA StudyJ Clin Hypertens (Greenwich)20081020821418326961
- BarriosVEscobarCEcharriRNew therapeutic progress in the cardio-renal protection of the hypertensive patient with a focus on olmesartanHot Topics in Hypertension20098716
- YusufSSleightPPogueJfor the Hearth Outcomes Prevention Evaluation (HOPE) Study GroupEffects of angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patientsN Engl J Med200034214515310639539
- WachtellKOlsenMHDahlofBMicroalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: the Losartan Intervention For Endpoint reduction in hyper tension (LIFE) studyJ Hypertension200220405412
- SchachingerVBrittenMBZeiherAMPrognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart diseaseCirculation20001011899190610779454
- TardiffJCAngiotensin-converting enzyme inhibitors and atherosclerotic plaque: a key role in the cardiovascular protection of patients with coronary artery diseaseEur Heart J Supple200911Suppl EE9E16
- BonettiPOLermanLOLermanAEndothelial dysfunction: a marker of atherosclerotic riskArterioscler Thromb Vasc Biol20032316817512588755
- BehrendtDGanzPEndothelial function. From vascular biology to clinical applicationsAm J Cardiol20029040L48L
- BuusNHJorgensenCGMulvanyMJLarge and small artery endothelial function in patients with essential hypertension – effect of ACE inhibition and beta-blockadeBlood Press20071610611317612909
- COVERSYL PLUS® Data sheetIn Information for health professionals http://www.medsafe.govt.nz/profs/datasheet/c/CoversylPlustab.htm. Last update 2006 March 03. Accessed January 2010.
- SongJCWhiteCMClinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors: an updateClin Pharmacokinet20024120722411929321
- BrugtsJJFerrariRSimoonsMLAngiotensin-converting enzyme inhibition by perindopril in the treatment of cardiovascular diseaseExpert Rev Cardiovasc Ther2009734536019379059
- RobinsonDMWellingtonKIndapamide sustained release: a review of its use in the treatment of hypertensionDrugs20066625727116451099
- SchiaviPJochemsenRGuezDPharmacokinetics of sustained and immediate release formulations of indapamide after single and repeated oral administration in healthy volunteersFundam Clin Pharmacol20001413914610796061
- MathesonAJCheerSMGoaKLPerindopril/indapamide 2/0.625 mg/day: a review of its place in the management of hypertensionDrugs2001611211122911465878
- SungSHWuTCLinSJChenJWEfficacy of a very-low-dose combination of perindopril and indapamide – preterax compared with cilazapril monotherapy in patients with inadequate blood pressure control – a randomized, double-blind, add-on studyJ Chin Med Assoc20087124725318490229
- MouradJJWaeberBZannadFComparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approachJ Hypertens2004222379238615614033
- WaeberBMouradJJApplication in the STRATHE trial of a score system to compare the efficacy and the tolerability of different therapeutic strategies in the management of hypertensionVasc Health Risk Manag2008424925218629368
- MouradJJLameiraDGuillausseauPJBlood pressure normalization by fixed perindopril/indapamide combination in hypertensive patients with or without associate metabolic syndrome: results of the OPTIMAX 2 studyVasc Health Risk Manag2008444345118561520
- KangSWuYFAnNRenMA systematic review and meta-analysis of the efficacy and safety of a fixed, low-dose perindopril-indapamide combination as first-line treatment of hypertensionClin Ther20042625727015038948
- ConcatoJShahNHorwitzRIRandomized, controlled trials, observational studies, and the hierarchy of research designsN Engl J Med20003421887189210861325
- StegPGLopez-SendonJLopez de SaEExternal validity of clinical trials in acute myocardial infarctionArch Intern Med2007167687317210880
- BarriosVEscobarCDivisonJAMedialdeaFClinical experience with a low-dose fixed combination of perindopril plus indapamide in a primary-care setting: the PRETEND studyTherapy20074677683
- BarriosVEscobarCDivisonJAMedialdeaFLow-dose fixed combination of perindopril plus indapamide in the diabetic hypertensive populationExpert Rev Cardiovasc Ther200861063106918793109
- DahlöfBGossePGuéretPPerindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL studyJ Hypertens2005232063207016208150
- AsmarRGarcia-PuigJGossePAmbulatory blood pressure in hypertensive patients with left ventricular hypertrophy: efficacy of first-line combination perindopril/indapamide therapyVasc Health Risk Manag2007337138017969366
- MogensenCEVibertiGHalimiSEffect of low-dose perindopril/indapamide on albuminuria in diabetes: preterax in albuminuria regression: PREMIERHypertension2003411063107112654706
- BeulensJWPatelAVingerlingJREffects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trialDiabetologia2009522027203619633827
- CampbellDJA review of Perindopril in the reduction of cardiovascular eventsVasc Health Risk Manag2006211712417319455
- PROGRESS Collaborative GroupRandomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attackLancet20013581033104111589932
- PROGRESS Collaborative GroupEffects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular diseaseEur Heart J20032447548412633548
- HisatomiACraigATeruoOfor the PROGRESS Collaborative GroupPerindopril-based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trialJ Hypertens20102839540019864958
- PatelAADVANCE Collaborative GroupMacMahonSEffects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trialLancet200737082984017765963
- ChalmersJJoshiRKengneAPADVANCE Collaborative GroupEfficacy and safety of fixed combination of perindopril and indapamide in type 2 diabetes: results from ADVANCE in context of available evidenceJ Hypertens200826Suppl 3S23S30
- WaeberBde la SierraARuilopeLMThe ADVANCE trial: clarifying the role of perindopril/indapamide fixed-dose combination in the reduction of cardiovascular and renal events in patients with diabetes mellitusAm J Cardiovasc Drugs2009928329119791837
- ZoungasSde GalanBENinomiyaTCombined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trialDiabetes Care2009322068207419651921
- DuXNinomiyaTde GalanBADVANCE Collaborative GroupRisks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE studyEur Heart J2009301128113519282274
- BrugtsJJNinomiyaTBoersmaEThe consistency of the treatment effect of an ACE-inhibitor based treatment regimen in patients with vascular disease or high risk of vascular disease: a combined analysis of individual data of ADVANCE, EUROPA, and PROGRESS trialsEur Heart J2009301385139419346520
- BeckettNSPetersRFletcherAEHYVET Study GroupTreatment of hypertension in patients 80 years of age or olderN Engl J Med20083581887189818378519
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med20083592417242819052124