Abstract
Rigid control of blood pressure (BP) is essential to prevent cardiovascular disease. However, only about 40% of hypertensive patients undergoing pharmacological intervention with a single agent achieve their BP goals in contemporary clinical practice. Combined therapy using currently available agents is effective in maximizing treatment outcome, although it raises medical costs and decreases the drug compliance rate. To overcome such negative consequences, a combination tablet containing an angiotensin II receptor blocker (ARB) with a small dose of hydrochlorothiazide (HCTZ) is now available on the international market, including Japan. This article briefly describes the unique properties of telmisartan, a highly selective ARB for the angiotensin II type 1 receptor, including its long-acting characteristics and recent prospective multicenter randomized clinical trials, followed by a description of a newly-introduced combination tablet in Japan, which contains telmisartan and HCTZ. This article also reviews its safety and efficacy based on currently available evidence. Finally, evidence comparing telmisartan/HCTZ with other combination therapies is presented.
Introduction
The Japanese Society of Hypertension’s Guidelines for the Management of Hypertension 2009 (JSH 2009)Citation1 recommends rigid control of blood pressure (BP) and a target BP level based on a patient’s clinical condition in order to prevent the onset of vascular diseases including stroke and myocardial infarction (MI). However, only 40% of patients with hypertension achieve the target BP goal in contemporary clinical practice, indicating the difficulty of BP management with a single hypertensive drug.Citation2 Therefore, the revised version of JSH 2009 recommends the use of more than one antihypertensive drug with a different mechanism of action for patients who fail to achieve BP goals with a single agent. Among possible antihypertensive agents, the combination of angiotensin II receptor blocker (ARB) with a small dose of diuretic not only offers synergistic antihypertensive effects but also provides possible benefits other than BP control.
Diuretic agents achieve antihypertensive effects by promoting renal Na+ excretion and simultaneously activate the renin-angiotensin system. On the other hand, ARBs inhibit the activated renin-angiotensin system, possibly promoting the synergistic antihypertensive effect with the concomitant use of an ARB and diuretic agent. Adverse effects on metabolic pathways, such as hypokalemia, decreased carbohydrate tolerance, and hyperuricemia, are a common concern with the use of diuretic agents. In contrast, ARBs are known to elevate serum K+ levels and improve insulin sensitivity, thereby possibly balancing out the adverse effects of a diuretic agent on the metabolic pathway. A small dose of the diuretic agent also is considered to be minimized by elevation of uric acid level.
In addition, considering salt-sensitive hypertension is essential for hypertension management, a condition that is common in Japanese. Administration of ARBs is generally recommended for patients with organ damage. The renin-angiotensin system is accelerated with decreasing intake of salt, but inhibited when salt intake is increased. Hence, limiting the intake of salt should be recommended when ARBs are administered for patients with salt-sensitive hypertension, although the concomitant use of a diuretic agent is expected to have the same effect as limiting the intake of salt by promoting Na+ excretion and possibly enhancing the anti-hypertensive effect of ARBs.Citation3
Properties of telmisartan
Angiotensin II type 1 (AT1) receptor blockers, which act by selectively blocking the binding of angiotensin II to the AT1 receptor, are widely used in current antihypertensive therapy.Citation4–Citation6 ARBs are reported to have benefits other than BP management and have demonstrated cardiovascular, cerebral, and renal protective effects by inhibiting renin-angiotensin activation at tissue levels.Citation7–Citation9
Telmisartan is an ARB that is highly selective for the AT1 receptor and has a long duration of action due to its long terminal elimination half-life.Citation10,Citation11 Its longer half-life was demonstrated in the MICADO study (),Citation12 which evaluated two identically designed multinational, randomized, double-blind, forced-titration studies. A total of 930 patients were enrolled in this study. Patients were divided either into the telmisartan group (40–80 mg/day) or into the valsartan group (80–160 mg/day) and followed up for 8 weeks. Uptitration occurred after 2 weeks of low-dose treatment. Following 4 weeks of high-dose therapy, patients underwent either a 1-day double-blind active treatment or placebo treatment. After an additional 2-week active treatment, a crossover was conducted. The result was very impressive in that the last 6-hour mean diastolic BP was decreased by 7.6 ± 7.9 mmHg in the telmisartan group (n = 447) compared with 5.8 ± 7.8 mmHg in the valsartan group (P = 0.0044) after active therapy. Similarly, the telmisartan group showed reduction in the last 6-hour mean systolic BP of 11.1 mmHg, whereas it was only 9.1 mmHg in the valsartan group (P = 0.0066). After the placebo therapy, the telmisartan group (n = 437) showed a reduction in 24-hour mean diastolic BP of 7.2 ± 6.5 mmHg compared with 5.5 ± 6.2 mmHg in the valsartan group (n = 431) (P = 0.0004). Following the placebo therapy, reduction in 24-hour mean systolic BP was 10.7 mmHg in the telmisartan group and 8.7 mmHg in the valsartan group (P = 0.0024). The mean reductions in diastolic and systolic BP were significantly higher in the telmisartan group than in the valsartan group. Therefore, it was concluded that telmisartan offers more sustained BP control because of its long half-life.
Table 1 Summary of clinical trials investigating efficacy of telmisartan
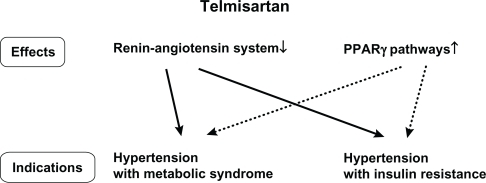
Telmisartan is also known to exhibit peroxisome proliferator-activated receptor gamma (PPAR-γ) activation, and thereby yields a favorable influence on glucose and lipid metabolism by improving insulin resistance.Citation13 Amongst available ARBs in addition to telmisartan, candesartan, irbesartan, and losartan were reported to activate PPAR-γ+Citation14–Citation16 However, telmisartan has the strongest ability to activate PPAR-γ in vitro compared with other ARBs.Citation13 PPAR-γ is an established therapeutic target in the treatment of insulin resistance, diabetes, and the metabolic syndrome.Citation17,Citation18 Telmisartan was also demonstrated to have a selective PPAR-γ activation effect and to ameliorate insulin resistance.Citation19 In fact, replacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes demonstrated improvement of insulin resistance, elevation of adiponectin, and reduction in high-sensitive C-reactive protein (hs-CRP).Citation20 This study, conducted by Miura et al included 18 hypertensive type 2 diabetes patients aged 36–79 years. These patients were treated with valsartan (80 mg/day, n = 11) or candesartan (8 mg/day, n = 7) for more than 6 months. During the therapeutic period, clinical and biochemical changes were not observed in these patients. The patients then received treatment with telmisartan (40 mg/day), instead of the previous ARBs, for 12 weeks. Fasting insulin levels were significantly decreased after telmisartan treatment (10.7 ± 3.8 to 8.6 ± 2.7 mU/L, P < 0.01), although reduction in fasting plasma glucose levels (132.5 ± 55.1 to 126.5 ± 39.3 mg/dL) and glycosylated hemoglobin (HbA1c) showed no significant difference (6.89 ± 0.89 to 6.79 ± 0.96). Significant elevation in serum adiponectin levels (6.95 ± 2.91 to 7.97 ± 3.48 μg/mL, P < 0.005) and a significant reduction in hs-CRP levels (0.154 ± 0.155 to 0.109 ± 0.120 mg/dL, P < 0.05) were observed in these patients. Adiponectin and hs-CRP are closely associated with insulin resistance and development of atherosclerosis.Citation21,Citation22 Thus, this study suggested that telmisartan has beneficial effects on the risk factors for cardiovascular disease, which is a major concern in the treatment of type 2 diabetes. Therefore, it is reasonable to select telmisartan for hypertensive patients with metabolic syndrome or insulin resistance ().
ONTARGET and TRANSCEND trials
Angiotensin-converting enzyme (ACE) inhibitors were the former standard renin-angiotensin system blockers, proven to have benefits other than BP control. Previous randomized controlled trials enrolling approximately 150,000 patients demonstrated that ACE inhibitors decreased incidences of death, myocardial infarction, stroke, and heart failure among patients with heart failure.Citation23 They also showed the reduction of unfavorable events including left ventricular dysfunction, previous vascular disease, and high-risk diabetes.Citation24,Citation25 Although ACE inhibitors and ARBs have been classified as renin-angiotensin system blockers, ARBs have not shown their effectiveness on myocardial infarction. Furthermore, data from 26 large-scale trials comparing an ACE inhibitor against ARB with placebo or another drug class demonstrated that ACE inhibitors, but not ARBs, show evidence of blood pressure-independent effects on the risk of major coronary disease events.Citation26 To evaluate the role of ARBs as an alternative or an addition to ACE inhibitors for preventing cardiovascular events, a group of investigators evaluated whether the ARB telmisartan was inferior to the ACE inhibitor ramipril in a study called Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). They also investigated whether a combination of the two drugs was superior to ramipril alone as a treatment to prevent cardiovascular events in high-risk patients who suffered from cardiovascular disease or diabetes mellitus but did not have heart failure.Citation27 At a median follow-up of 56 months, the primary composite outcomes, including death from cardiovascular diseases, myocardial infarction, stroke, or hospitalization for heart failure, was similar in the ramipril (16.5%) and telmisartan groups (16.7%). As expected, the ramipril group had higher rates of cough (4.2% vs 1.1%, P < 0.001) and edema (0.3% vs 0.1%, P = 0.01). In the combination group, the primary outcome was seen with 16.3% showing no significant difference between the two drugs. Thus, telmisartan was equally effective as ramipril in treating patients with cardiovascular disease or high-risk diabetes. Therefore, the ONTARGET study concluded that ARBs were not inferior to ACE inhibitors with regard to benefit beyond blood pressure control.
A study called Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) was also performed simultaneously in order to evaluate whether telmisartan would be effective for patients with cardiovascular disease or diabetes with end-organ damage who are intolerant to ACE inhibitors.Citation28 In this study, after a 3-week run-in period, 5926 patients were randomly assigned to the telmisartan (n = 2954) and placebo groups (n = 2972). The primary outcome, the composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure, occurred in 15.7% of patients in the telmisartan group and 17.0% of the patients in the placebo group, demonstrating no significant difference between these groups (P = 0.216). The secondary outcome, the composite of cardiovascular death, myocardial infarction, or stroke, occurred in 13% of patients in the telmisartan group and 14.8% of patients in the placebo group (P = 0.048 unadjusted; P = 0.068 after adjustment for multiplicity of comparisons and overlap with primary outcome), indicating a reduction in relative risk by 13%.
As mentioned previously, former studies including LIFE,Citation29 VALUE,Citation30 and CHARM-AlternativeCitation31 demonstrated that ARBs increased the rates of myocardial infarction compared with their opponent drugs. Although no significant difference was observed between the losartan and atenolol groups in the LIFE study, (9.2% vs 8.7%), higher rates of myocardial infarction were observed in those who received the ARB treatment (1.07 [0.88–1.31], P = 0.491). In the VALUE trial, of the patients assigned to the valsartan base regimen, 369 patients (4.8%) suffered from myocardial infarction. On the other hand, 313 patients (4.1%) suffered from myocardial infarction in the amlodipine base regimen (Hazard Ratio 1.19 [1.02–1.38], P = 0.02). The CHARM-Alternative trial also demonstrated that an ARB had a higher incidence of myocardial infarction. The total number of patients who suffered from myocardial infarction was 75 in the candesartan group and 48 in the placebo group (1.52 [1.06–2.18], P = 0.025). The results of these studies raised a concern that the administration of ARBs possibly increases the rate or occurrence of myocardial infarction. The myocardial infarction rate achieved by the TRANSCEND study put an end to this concern. The telmisartan group had a lower myocardial infarction rate than that in the placebo group, despite no significant statistical difference (3.9% vs 5.0%, P = 0.059). TRANSCEND is the only study that demonstrated the effectiveness of telmisartan in reducing the incidences of myocardial infarction. This study therefore helped to identify the class effect among the available ARBs.
Characteristics of telmisartan/HCTZ tablets
Despite the evidence described previously in this paper, many patients with hypertension fail to achieve their BP goals in contemporary clinical practice, thereby motivating pharmaceutical manufacturers to develop a combination tablet. The combination of telmisartan at 40 mg and hydrochlorothiazide (HCTZ) at 12.5 mg (telmisartan 40 mg/HCTZ 12.5 mg combination tablets) or telmisartan 80 mg and HCTZ 12.5 mg (telmisartan 80 mg/HCTZ 12.5 mg combination tablets) was introduced recently and is now available on the international market, including Japan.
As mentioned previously, telmisartan is expected to yield a strong and stable antihypertensive effect over a prolonged period of time due to strong binding to the AT1 receptor and long action. HCTZ is a type of thiazide diuretic, but its diuretic effect is weaker than the loop diuretic agent. Despite its gradual onset of antihypertensive effect, HCTZ has strong and promising effects, especially for patients with salt-sensitive hypertension.
Antihypertensive effects of telmisartan/HCTZ combination tablets
The Japanese phase III clinical trial of the telmisartan 40 mg/HCTZ 12.5 mg combination tablet was conducted on 213 patients with essential hypertension who failed to achieve their BP goals (diastolic BP < 90 mmHg) with 40 mg telmisartan. These patients were randomly assigned to the study (telmisartan 40 mg/HCTZ 12.5 mg combination tablet) and control groups (telmisartan 40 mg). Patients with poor compliance were excluded from the study. The primary endpoint was trough sitting BP after 8 weeks from the onset of the study. At the end of the study period, systolic and diastolic BP were decreased by 23.3 mmHg and 14.1 mmHg in the study group 2 weeks following the wash-out. After randomization, the BP-lowering effect was significantly greater in the study group than in the control group (systolic BP −14.0 mmHg vs −8.4 mmHg, P = 0.001; diastolic BP −9.7 mmHg vs −5.0 mmHg, P < 0.0001).Citation32 Given the fact that the subjects in this study were poor responders who failed to achieve their BP goals with telmisartan at 40 mg, reduction in systolic BP by 23.3 mmHg was significant within the current hypertension management ().
Table 2 Summary of clinical trials investigating efficacy of telmisartan/HCTZ tablets
Furthermore, the safety and efficacy of the telmisartan 80 mg/HCTZ 12.5 mg combination tablet were evaluated by Lacourcière et al.Citation33 In this study, the antihypertensive effects of a fixed-dose combination of telmisartan 80 mg/HCTZ 12.5 mg, and telmisartan 80 mg monotherapy were compared with patients who had a history of mild to moderate essential hypertension and inadequate BP control following 8 weeks of telmisartan monotherapy. At the end of this period, 491 patients whose diastolic BP was ≥90 mmHg were double-blind randomized to once-daily administration of telmisartan 80 mg/HCTZ 12.5 mg (n = 246) or telmisartan 80 mg (n = 245). Following 4 and 8 weeks of double-blind therapy, trough clinic BP was evaluated. At the end of the double-blind treatment, it was found that patients who underwent telmisartan 80 mg/HCTZ 12.5 mg therapy experienced a further significant decrease in clinic systolic/diastolic BP (−5.7 mmHg) compared with those who underwent telmisartan 80 mg monotherapy (−3.1 mmHg) (P < 0.01). In addition, the number of patients with normalized BP was significantly higher in the telmisartan 80 mg/HCTZ 12.5 mg group than in the telmisartan 80 mg group (41.5% vs 26.1%; P < 0.05). The results of this study demonstrated that a fixed-dose combination of telmisartan 80 mg/HCTZ 12.5 mg yields greater reduction in BP in nonresponders than for those who continue telmisartan monotherapy. Although the difference in the reduction of BP was small, it was still considered to be big enough to be economically effective, because decreases in systolic BP as small as 2 mmHg have a great impact on reducing mortality from cardiovascular disease.Citation34
In addition, the dose determination study comparing telmisartan 80 mg/HCTZ 12.5 mg vs telmisartan 40 mg/HCTZ 12.5 mg demonstrated that telmisartan 80 mg/HCTZ 12.5 mg was significantly more effective than telmisartan 40 mg/HCTZ 12.5 mg in reducing mean supine diastolic BP and systolic BP (P < 0.05 for both).Citation35
In the Japanese dose determination study (phase II), responders were defined as patients who achieved their systolic BP goals <140 mmHg, or those whose systolic BP was less than 10 mmHg. Based on this definition, 87.5% of the patients in the telmisartan 40 mg/HCTZ 12.5 mg group and 93.7% of the patients in the telmisartan 80 mg/HCTZ 12.5 mg group achieved their treatment goal.Citation36 Furthermore, the Japanese prolonged-administration study for 184 essential hypertensive patients who failed to achieve their BP goals with telmisartan 40 mg demonstrated a favorable BP control with both telmisartan 40 mg/HCTZ 12.5 mg tablets and telmisartan 80 mg/HCTZ 12.5 mg tablets, with BP-lowering effects maintained for long-term periods.Citation37
Comparison with other combination tablets
To evaluate the effectiveness of fixed-dose combinations of ARBs with HCTZ, a multicenter, randomized, prospective, open-label, blinded-endpoint clinical study involving 805 patients with mild-to-moderate hypertension was conducted.Citation38 The patients were randomly divided into 3 groups: telmisartan 40 mg/HCTZ 12.5 mg, losartan 50 mg plus HCTZ, or telmisartan 80 mg/HCTZ 12.5 mg. The primary endpoint of the mean reductions in the last 6 hours mean diastolic BP for the telmisartan 40 mg/HCTZ 12.5 mg and telmisartan 80 mg/HCTZ 12.5 mg group were significantly higher: −2.0 mmHg (P = 0.0031) and −2.8 mmHg (P = 0.0003), respectively. The study demonstrated that during the last 6 hours of the 24-hour dosing interval, telmisartan 40 mg/HCTZ 12.5 mg produced a significantly reduced BP than losartan 50 mg/HCTZ 12.5 mg, which corresponds to the high-risk early-morning hours. (This study also showed further BP reduction by the administration of telmisartan 80 mg/HCTZ 12.5 mg). Therefore, telmisartan 40 mg/HCTZ 12.5 mg is considered to inhibit the early morning BP elevation, and is thereby expected to prevent the onset of cardiovascular events.
The San Marino Observational Outlooking Trial on Hypertension study (SMOOTH), a prospective, randomized, open-label, blinded-endpoint, multicenter trial, compared the BP-lowering effect between telmisartan 80 mg/HCTZ 12.5 mg and valsartan 160 mg/HCTZ 12.5 mg for 840 overweight/obese hypertensive patients.Citation39 After 10 weeks, the telmisartan 80 mg/HCTZ 12.5 mg group showed significantly greater reductions in BP than the valsartan 160 mg/HCTZ 12.5 mg group in the last 6-hour mean ambulatory BP, and differences in favor of telmisartan/HCTZ: systolic BP 3.0 mmHg, P = 0.0002; diastolic BP 1.6 mmHg, P = 0.0006) in the morning as well as during daytime and night-time periods (P < 0.003). Thus, the combination of telmisartan 80 mg plus HCTZ 12.5 mg could be a more preferable treatment alternative than valsartan 160 mg plus HCTZ 12.5 mg, especially for overweight/obese patients with hypertension.
Safety of telmisartan/HCTZ combination tablets
The adverse event rate with the telmisartan 40 mg/HCTZ 12.5 mg combination tablet in the Japanese study was 11.2% (47/421)Citation32: postural vertigo (12), dizziness (10), hyperuricemia (7), light-sensitive response (4), hypotension (3), hepatic dysfunction (3), and pollakiuria (2). In the Japanese phase III study, the patients who received telmisartan 40 mg/HCTZ 12.5 mg combination tablet for 8 weeks showed no significant changes in serum K+, serum total cholesterol, and blood glucose levels. Although elevation in the serum uric acid level was observed in the early phase, it declined gradually.Citation36 In this situation, long-term use of telmisartan/HCTZ combination tablet least affects the metabolic pathway and does not clinically induce adverse effects.
Conclusions
Reduction in the number of drugs enhances the drug compliance rate. For those who poorly responded and failed to achieve their BP goals with a single antihypertensive drug, the combination of ARB/HCTZ possibly offers additional benefits. The telmisartan/HCTZ combination tablet was demonstrated to have a strong and long-acting effect. Therefore, for the management of hypertension as recommended by the revised version of JSH 2009, using the telmisartan/HCTZ combination tablet has enormous promise in contemporary medical treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- OgiharaTKikuchiKMatsuokaHJapanese Society of Hypertension Committee. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009)Hypertens Res2009324107
- ObaraTOhkuboTFunabashiJIsolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME studyJ Hypertens2005231653166016093909
- ItohSSynergic effect mechanisms on ARB/diuretic combination tabletsJ Blood Press20061313041307
- SiragyHMBedigianMMechanism of action of angiotensin-receptor blocking agentCurr Hypertens Rep1999128929510981080
- SiragyHMAT (1) and AT (2) receptors in the kidney: role in disease and treatmentAm J Kidney Dis2000363 Suppl 1S4S910986153
- WeirMRDzauVJThe renin-angiotensin-aldosterone system: a specific target for hypertension managementAm J Hypertens199912S205S213
- SchiffrinELVascular and cardiac benefits of angiotensin receptor blockersAm J Med200211340941812401536
- SchmiederREMechanisms for the clinical benefits of angiotensin II receptor blockersAm J Hypertens20051872073015882557
- MorimotoSYanoYMakiKSawadaKRenal and vascular protective effects of telmisartan in patients with essential hypertensionHypertens Res20062956757217137211
- WienenWEntzerothMvan MeelJCA review on telmisartan: a novel, long-acting angiotensin II-receptor antagonistCardiovasc Drug Rev200018127156
- SharpeMJarvisBGoaKLTelmisartan: a review of its use in hypertensionDrugs2001611501152911558835
- LacourcièreYKrzesinskiJMWhiteWBDavidaiGSchumacherHSustained antihypertensive activity of telmisartan compared with valsartanBlood Press Monit2004920321015311147
- BensonSCPershadsinghHAHoCIIdentification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activityHypertension20043993100215007034
- SchuppMJankeJClasenRAngiotensin type I receptor blockers induce peroxisome proliferators-activated receptor-gamma activityCirculation20041092054205715117841
- JankeJSchuppMEngeliSAngiotensin type 1 receptor antagonists induce human in vitro adipogenesis through peroxisome proliferators-activated receptor-γ activationJ Hypertens2006241809181616915030
- ErbeDVGartrellKZhangYLMolecular activation of PPAR-γ by angiotensin II type 1-receptor antagonistsVasc Pharmaco200645154162
- PerhadsinghHAPeroxisome proliferator-activated receptor-γ therapeutic target of diseases beyond diabetes: quo vadisExpert Opin Invest Drugs200413215228
- SavageDBTanGDAceriniCLHuman metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γDiabetes20035291091712663460
- SchuppMClemenzMGinesteRMolecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activityDiabetes2005543442345216306360
- MiuraYYamamotoNTsunekawaSReplacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetesDiabetes Care20052875775815735228
- YamauchiTKamonJWakiYThe fat-derived hormone adiponectin reverses insulin resistance associated with both lipotrophy and obesityNat Med794194611479627
- RossRAtherosclerosis: An inflammatory diseaseN Engl J Med19993401151269887164
- The SOLVD InvestigatorsEffect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failureN Engl J Med19913252933022057034
- PfefferMABraunwaldEMoyéLAEffect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: Results of the survival and ventricular enlargement trialN Engl J Med19923276696771386652
- Heart Outcomes Prevention Evaluation Study InvestigatorsEffects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE sub-studyLancet2000355253259 [Erratum, Lancet. 2000;356:860.]10675071
- Blood Pressure Lowering Treatment Trialists’ CollaborationBlood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin systemJ Hypertens20072595195817414657
- The ONTARGET InvestigatorsTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med20083581547155918378520
- The Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) InvestigatorsEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trialLancet200937211741183
- LindholmLHIbsenHDahlofBCardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenololLancet2002359995100311937178
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trialLancet20043632022203115207952
- GrangerCBMcMurrayJVYusufSEffects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trialLancet200336277277613678870
- HigakiJThe examination of telmisartan 40 mg/hydrochlorothiazide for patients with essential hypertensionJ New Remedies and Clinic200857308325
- LacourcièreYTytusRO’KeefeDLenisJOrchardRMartinKEfficacy and tolerability of a fixed-dose combination of telmisartan plus hydrochlorothiazide in patients uncontrolled with telmisartan monotherapyJ Hum Hypertens20011576377011687919
- StamlerJRoseGStamlerRElliottPDyerAMarmotMINTERSALT study findings. Public health and medical care implicationHypertension1989145705772807518
- McGillJBReillyPATelmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: A multicenter, randomized, double-blind, placebo-controlled, parallel-group trialClin Ther20012383385011440284
- Internal document for Nippon Boehringer Ingelheim (data submitted to the Japanese Ministry of Health and Labor for the approval of telmisartan/HCTZ combination tablets).
- HigakiJThe examination of long-term usage of telmisartan 40 mg/hydrochlorothiazide tablet and telmisartan 80 mg/hydrochlorothiazide tablet for patients with essential hypertensionJ New Remedies and Clinic200958346361
- NeutelJMLittlejohnTWChrysantSGSinghATelmisartan Study GroupTelmisartan/hydrochlorothiazide in comparison with losartan/hydrochlorothiazide in managing patients with mild-to-moderate hypertensionHypertens Res20052855556316335883
- SharmaAMDavidsonJKovalSLacourcièreYTelmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: The SMOOTH studyCardiovasc Diabetol200762817910747