Abstract
This study was carried out on 426 samples of raw meats collected from butcheries and supermarkets in Casablanca, Morocco. The samples were examined for the occurrence of Listeria species. Strains of Listeria monocytogenes were characterized by several biochemical tests and confirmed by polymerase chain reaction (PCR). β-hemolytic cultures and nonhemolytic isolates were tested for biochemical properties with the Listeria API test. Among the 43 Listeria species isolates; we identified 10 strains for L. monocytogenes (23.3%), 31 strains for L. innocua (72.1%) and 2 strains for L. welshimeri (4.6%). Strains of L. monocytogenes were separated by multiplex PCR; two serogroups IIb and IVb were thus differentiated. Antibiotic susceptibility of L. monocytogenes to 21 antibiotics was determined by the disk diffusion method. All isolates were susceptible to a wide range of the tested antibiotics with the exception of nalidixic acid, colistine and cephalosporins second and third generation for which they were all resistant.
Introduction
Listeria monocytogenes has long been acknowledged as a significant human and animal pathogen (CitationSchuchat et al 1991; CitationNightingale et al 2004) and it is often implicated as sources of human listeriosis cases and outbreaks (CitationAureli et al 2000; CitationDe Valk et al 2001). However, sporadic listeriosis remains the most frequent manifestation of the illness (CitationGilot et al 1996).
The following individuals are at great risk for listeriosis: pregnant women (and their unborn children), new-born, immunocompromised persons (Walter 2000), and the elderly. This infection is regularly reported in Europe and North America, but in Africa and other developing countries (where the food industry is not very developed) only a few sporadic cases have been reported (CitationBoukadidda et al 1994). In Morocco, the incidence of human listeriosis is rare (CitationBenomar et al 2000).
L. monocytogenes often lives in the cold and moist environment found in refrigerators and it is present in all categories of food (CitationFarber and Peterkin 1991; CitationAzevedo et al 2005). Meat, poultry, and meat products have frequently been shown to be contaminated with this pathogenic bacterium (CitationLawrence and Gilmour 1994; CitationJay 1996). A number of antibiotics have been suggested for the treatment of L. monocytogenes. Unfortunately, failures have been reported for all therapeutic programs because there is no consensus among various authors as to which antibiotic regimen is the most effective (CitationMcLauchlin et al 1991). The mains goals of our work were: i) to detect L. monocytogenes by conventional method and application of a chromogenic medium for the rapid confirmation ii), to confirm and to serotype the strains isolated by polymerase chain reaction (PCR) and multiplex PCR, respectively, iii) and to determine in vitro the activity of various antibiotics against strains of L. monocytogenes isolated from red meat and poultry.
Materials and methods
Sample collecting
A total of 426 samples: (a) raw meat (n = 112), (b) meat product (n = 240), and (c) poultry (n = 74) were collected from butcheries and supermarkets in Casablanca, Morocco. The samples were transported in iceboxes to our laboratory and analyzed immediately upon arrival using the ISO 11290-1 method.
Bacteriological analysis
Twenty-five grams of each sample were weighed into sterile stomacher bags, diluted with 225 ml of Listeria pre-enrichment Frazer broth (Biokar Diagnostics, BK115HA) homogenized and incubated at 30 °C for 24 h. A 1 ml portion from this pre-enrichment culture was transferred to 9 ml enrichment broth (Complete Fraser broth, BIO-RAD), and incubated at 37 °C for 24 h. A loopful of the enrichment culture was streaked onto Palcam, Oxford (AES Laboratory) and RAPID’L. mono (BIO-RAD, France). Plates were incubated at 37 °C for 24 h to 48 h. A great advantage of the chromogenic agar, RAPID’L. mono, that it allows for the differentiation L .monocytogenes from other L. spp. by a simple color change reaction even after 24 h; colonies appear blue. For identification of L. monocytogenes suspect colonies selected from Oxford, Palcam, and RAPID’L. mono agars were streaked on trypticase soya agar plate with 0.6% yeast extract (TSAYE; Biokar Diagnostics) and tested for Gram coloration, motility, oxydase, CAMP test, and hemolysis test. Typical colonies were characterized biochemically by the Listeria API commercial kit (Biomerieux, Mercy l’Etoile, France).
Molecular analysis
Total genomic DNA and PCR amplifications were used as described previously (CitationHoloko et al 2002). For L. monocytogenes-specific identification, two primers pairs derived from the L. monocytogenes gene: hly1: 5′-CGGAGGTTCCGCAAAAGATG-3′ and hly2: 5′-CCTCCAGAGTGATCGATGTT-3′ were used. PCRs were carried out in a thermocycler (GeneAmp PCR system 2700, Applied Biosystems) in a 50 μl reaction volume containing: 5 μl target DNA, 5 μL 10 × Tp PCR, 200 μM dNTP, 1.5 mM MgCl2; 0.4 μM of each primer, and 1 Unite of Taq DNA polymerase.
Amplification started with an initial denaturation step at 94 °C for 5 min, followed by 30 cycles (94 °C for 1 min, 56 °C for 45 s, and 72 °C for 45s). Final extension was performed at 72 °C for 10 min. The PCR products were separated in a 1.2% agarose gel and stained with ethidium bromide at 0.5 μg/ml.
Serotype identification by multiplex PCR
Serogroups and serovars determinations were performed by multiplex PCR, according to the method described by CitationDoumith and colleagues (2004). These analyses were practiced in the Centre National de Référence des Listeria, Institut Pasteur, Paris.
Antibiotics susceptibility
Fresh bacterial colonies of L. monocytogenes isolates were separately grown at 37 °C in brain heart infusion broth (BHI) (Merk V4324393-947) for 24 hours and each inoculum was applied on Muller Hinton (MH) agar (BIO-RAD 2M2121). Antibiotic susceptibility was determined by the disk diffusion method. Standard discs were applied using a disc dispenser and the plates were incubated at 37 °C for 24 h to 48 h. Then the size of inhibition zone was determined as previously described (CitationSoussy 2005). The Antibiotics tested were selected considering i: the most frequently used in treatment, of farm animals (CitationJohnston 1998). ii: those used to prevent and control infectious diseases in poultry production (CitationAbiola et al 2005). iii: the most important drugs used for the treatment of human infections (CitationWiggins et al 1978; CitationWinslow and Pankey 1982).
Results
Occurrence of L. monocytogenes in meats
Out of 426 samples examined, 43 (10%) were found to be contaminated with L. spp. presents the types, numbers, and source of the samples analyzed in this study. Among the 43 strains of L. species isolates, 10 strains for L. monocytogenes (23%), 31 strains for L. innocua (72.1%), and 2 strains for L. welshimeri (4.6%) were identified.
Table 1 Occurrence of Listeria monocytogenes in red meat and poultry
Confirmation by PCR
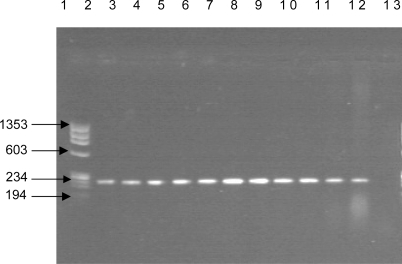
To confirm the identification of the ten strains characterized by biochemical tests, we have done PCR analyses. We used two primers derived from the virulence associated gene hly specifically those that amplifying the 234 bp region of the listeriolysin O gene. PCR amplification gives all amplicons with same molecular size as shown in .
Serotyping
Analysis of serogroups and serovars distribution showed that L. monocytogenes was represented by two serogroups. The first (IIb) comprised strains of serovars 1/2b, 3b, and 7 and the second (IVb) comprised strains of serovars 4b, 4d, and 4e. The results are shown in .
Table 2 Origin of isolates strains and identification of serogroups with multiplex PCR
Antibiotics susceptibility
summarizes activities of the 21 antibiotics tested against the 10 strains of L. monocytogenes isolated from meat and poultry. In general, all isolates are susceptible to a wide range of antibiotics especially for amoxicillin, ticarcillin, gentamicin, tobramycin, amikacin, chloramphenicol, penicillin, and ampicillin. These strains showed different resistance levels of certain antibiotics and were completely resistant to nalidixic acid, colistine, and cephalosprins second and third generations.
Table 3 Activities of antimicrobial agents tested against the strains of Listeria monocytogenes isolated from meat and poultry
Discussion
In our study the incidence of L. monocytogenes was 2.4% and the rate of contamination in ground meat was 3.3%. Compared to that published by other authors, nearly the same incidences were found in raw minced meat 4.7% (CitationYucel et al 2005) and moderate incidences 10.6% have been reported in raw sausages (CitationCordano and Rocourt 2001). On the contrary, in Tunis the presence of L. monocytogenes was lower; out of a total of ground meat samples analyzed only one strain has been contaminated (CitationEtttriqui et al 1995). In the similar study done in Morocco, the prevalence of L. monocytogenes was 16.4% in ground bovine meat, and 32% in raw bovine sausages (CitationKriem et al 1998). This difference regarding the occurrence between our results realized in Casablanca and those effected in Rabat, Morocco, can be explained by the fact that this study was carried out after the inauguration of the new slaughterhouse installed in Casablanca, on the level of which; legislatives texts governing the inspection of the meats and hygiene have been applied. It relates a whole of a very rigorous control at each key point of the meat production. For the raw meat; from all the samples analysed (112), L. monocytogenes was found in only 1 sample (0.9%). According to other studies, the incidence was variable; CitationYucel and colleagues (2005) and CitationHilbert and colleagues (2004) detected this organism in 5.2% and 12% in beef, respectively.
In Morocco, white meat is an important source of protein. In 2004, the population of Casablanca alone consumed 338,000 tons. Two kinds of poultry slaughtering are used in Morocco. One is an automated poultry slaughtering process established recently, whereby automated systems are used for scalding, plucking, eviscerating, rinsing, and packaging carcasses. Carcasses are then stored at 4 °C before sale to supermarkets. The second is traditional slaughtering which is commonly practiced in shops under poor hygienic conditions. More than 90% of poultry slaughtering in Morocco is done by traditional procedures (CitationCohen et al 2007). For this reason, we have also examined poultry for the occurrence of L. monocytogenes and the incidence rate was (1.3%). Other data on the prevalence of this bacterium have been reported by others authors in different countries: 11.5% (CitationYucel et al 2005) and 26% (CitationHilbert et al 2004) in chicken meat, and 32% in poultry meat (CitationCapita et al 2001).
Concerning the antibiotic susceptibility, for gentamicin, all our isolates were susceptible to this antibiotic; our data are similar to those of CitationAureli and colleagues (2003). Likewise, both penicillin and ampicillin showed good activity against L. monocytogenes; these results are in agreement with those reported previously by CitationWong and colleagues (1990) who found 98.3% and 99.4% of susceptibility, respectively. The isolates were all susceptible to chloramphenicol, similarly, CitationYucel and colleagues (2005) indicated that L. monocytogenes isolated from meat products were highly sensitive with a percentage of (100%). While CitationAureli and colleagues (2003), showed in their study that the various strains seem to be moderately susceptible (49.1%) in poultry and totally (100%) susceptible in meat. Of the other antibiotics, trimethoprim and trimethoprim in combination with sulfamethoxazole, except for one strain (meat) was resistant to both antimicrobial agents. In previous other findings, CitationYucel and colleagues (2005) indicated that the percentage of resistant strains of L. monocytogenes in meats was 66%. Finally, for the activity of the first generation cephalosporins tested, only one strain (meat) was resistant to cephalotin and cefazolin and one other strain had showed moderate susceptibility to cefazolin. Comparing to others studies realized in various foods (including meats), CitationWong and colleagues (1990) indicated highly susceptibility to cephalotin (99.4%). Similarly, CitationAbiun and colleagues (1994) and CitationNavratilova and colleagues (2004) showed that cephalotin displayed good activity. For the second and third generation cephalosporins, we have found resistance to cefotaxime, cefoxitin, cefixim, and ceftazidime. Because of the poor activity or complete resistance of the second and third generation cephalosporins against L. monocytogenes, CitationCormican and Jones (1995) confirmed that recent cephalosporins should not be used clinically for treating listeriosis.
In conclusion, in this study we have demonstrated that almost all strains of L. monocytogenes were susceptible to a wide range of antibiotics effective against Gram-positive bacteria, belonging to amino-glycosides group and glycopeptides and some strains among the isolates were resistant to one or more antimicrobial agent. Therefore veterinarians should be able to control the use of antimicrobial drugs in veterinary practice. On the other hand, the survey of this virulent bacterium in meat appears to be critically important from the viewpoint public health. Consistent with other countries, Morocco is experiencing newer consumer trends in the culinary traditions with an obvious tendency to adopt international practice of consuming processed foods, some of which are uncooked or undercooked. Such a tendency combined with incidence of L. monocytogenes contamination reported in meat, thereby increasing the risk of listeriosis for consumers (CitationCohen et al 2006). Where a suspect sample of poultry will be well cooked before consumption, the risks to the consumer are considered minimal. This is probably the reason why poultry and poultry products are rarely associated with listeriosis (CitationVan Schothorst 1994). Results obtained in this study provide evidence that may be used by the Moroccan government to adopt regulations enforcing the application of the hazard analysis critical control points (HACCP) system as a means to identify and control the hazards in foods and especially in meat products. Furthermore, these results may promote the acceptance of programs such as HACCP by the meat industry in an attempt to provide safer and more wholesome products.
Acknowledgements
The authors are grateful to Dr Alban Le Monnier and Dr Alexandre LeClercq from Centre National de Référence des Listeria, Institut Pasteur Paris for their collaboration of the molecular serotyping. We also would like to thank the Casablanca authorities for their help.
References
- AbiolaFADiopMMTeko-AgboA2005Antimicrobial agents residues in liver and gizzard of broilers in the areas of Dakar and Thiès (Sénégal)Rev Med Vet1562648
- AbuinCMFFernandezEJQSampayoCF1994Susceptibilities of Listeria species isolated from food to nine antimicrobial agentsAntimicrob Agents Chemother38165577979303
- AureliPFiorucciGCCaroliD2000An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenesN Engl J Med34212364110781619
- AureliPFerriniAMMannoniV2003Susceptibility of Listeria monocytogenes isolated from food in Italy to antibioticsInt J Food Microbiol833253012745237
- AzevedoIRegaloMMenaC2005Incidence of Listeria spp. in domestic refrigerators in PortugalFood Control161214
- BenomarSNejjariNLahbabiMS2000Listériose néonatale: une infection exceptionnelle au MarocArch Pediatr742810793936
- BoukadiddaJSbouiHMonastiriH1994La listériose humaine en Tunisie: deux nouveaux cas chez le nouveau- néMed Mal Infect2411718
- CapitaRAlonso-CallejaCMorenoB2001Occurrence of Listeria species in retail poultry meat and comparison of a cultural/immunoassay for their detectionInt J Food Microbiol65758211322703
- CohenNEnnajiHHassarM2006The bacterial quality of red meat and offal in Casablanca (Morocco)Mol Nutr Food Res50467590
- CohenNEnnajiHBouchrifB2007Comparative study of microbiological quality of raw poultry meat at various seasons and different slaughtering process in Casablanca (Morocco)J Appl Poultry Res165028
- CordanoAMRocourtJ2001Occurrence of Listeria monocytogenes in food in ChileInt J Food Microbiol70175811759755
- CormicanMGJonesRN1995Antimicrobial activity of cefotaxime tested against infrequently isolated pathogenic species (Unusual Pathogens)Diagn Microbiol Infect Dis224387587049
- De ValkHVaillantVJacquetC2001Two consecutive nationwide outbreaks of listeriosis in France, October 1999–FebruaryAm J Epidemiol1549445011700249
- DoumithMBuchrieserCGlaserP2004Differentiation of the major Listeria monocytogenes serovars by Multiplex PCRJ Clin Microbiol4238192215297538
- EtttriquiADhaouMAFlissI1995Research of Listeria monocytogenes in meat products in region of TunisMHA7813
- FarberJMPeterkinPI1991Listeria monocytogenes, a food-borne pathogenMicrobiol Rev554765111943998
- GilotPGenicotAAndréP1996Serotype and esterase typing for analysis of Listeria monocytogenes populations recovered from foodstuffs and from human patients with listeriosis in BelgiumJ Clin Microbiol341007108815071
- HilbertFMayrhoferSPaulsenP2004Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultryInt J Food Microbiol9723915527915
- HolokoIUrbanovaJKantikovaM2002PCR Detection of Listeria monocytogenes in milk products and differentiation of suspect isolatesActa Vet Brno7112531
- JayJM1996Prevalence of Listeria spp. in meat and poultry productsFood Control720914
- JohnstonAM1998Use of antimicrobial drugs in veterinary practiceBr Med J31766579728002
- KriemMREL-MarrakchiAHammamaA1998Prevalence of Listeria spp. on a variety of meat products in MoroccoMicrobiol Aliment Nutr1617987
- LawrenceLMGilmourA1994Incidence of Listeria spp. and Listeria monocytogenes in a poultry processing environment and in poultry products and their rapid confirmation by multiplex PCRAppl Environ Microbiol60460047811096
- McLauchlinJAudurierATaylorAG1991Treatment failure and recurrent human listeriosisJ Antimicrob Chemother2785171938692
- NightingaleKKSchukkenYHNightingaleCR2004Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environmentAppl Environ Microbiol7044586715294773
- NavratilovaPSchlegelovaJSustackovaA2004Prevalence of Listeria monocytogenes in milk, meat and foodstuff of animal origin and the phenotype of antibiotic resistance of isolated strainsVet Med-Czech4924352
- SchlechWFIII2000Food-borne listeriosisClin Infect Dis31770511017828
- SchuchatASwaminathanBBroomeCV1991Epidemiology of human listeriosisClin Microbiol Rev4169831906370
- SoussyCJ2005Edition de Janvier 2005. Comite de L’antibiogramme de La Société Française de MicrobiologieCommuniqué2005
- Van SchothorstM1994Choice of sampling plan and criteria for Listeria monocytogenes, International Commission on Microbiological Specifications for FoodsInt J Food Microbiol2289968074975
- WigginsGLAlbrittonWLFeeleyJC1978Antibiotic susceptibility of clinical isolates of Listeria monocytogenesAntimicrob Agents Chemother138546096737
- WinslowDLPankeyGA1982In vitro activities of trimethoprim and sulfamethoxazole against Listeria monocytogenesAntimicrob Agents Chemother225146812496
- WongHCChaoWLLeeSJ1990Incidence and characterization of Listeria monocytogenes in foods available in TaiwanAppl Environ Microbiol56310142126699
- YucelNÇitakSÖnderM2005Prevalence and antibiotic resistance of Listeria species in meat products in Ankara, TurkeyFood Microbiol222415