Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections have increased dramatically over the last two decades. The types of infections can range from complicated skin and skin structure infections (cSSSI) to pneumonia and endocarditis. Oral antimicrobial therapy, such as trimethoprim-sulfamethoxazole, clindamycin, long-acting tetracyclines, or linezolid may provide enhanced benefit to those with uncomplicated cutaneous lesions when used in conjunction with incision and drainage in an outpatient setting. However, resistance, susceptibilities, patient-specific circumstances, and adverse effects can impact a healthcare professional’s choice of antibiotics. In patients with complicated infections requiring hospitalization or parenteral treatment, vancomycin remains the drug of choice, even though increased resistance and decreased efficacy have crept into clinical practice. Linezolid, quinupristin/dalfopristin, daptomycin, and tigecycline are alternative intravenous agents for the treatment of CA-MRSA. Investigational agents such as dalbavancin, telavancin, oritivancin, iclaprim, ceftobiprole, ceftaroline, and others may expand our therapeutic armamentarium for the treatment of infections caused by CA-MRSA in the future.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) was first described in 1961 soon after the introduction of methicillin, the first beta-lactamase resistant penicillin.Citation1 MRSA soon emerged as a common nosocomial organism infecting patients in hospitals and intensive care units around the world.Citation2 Prior to the 1990s, MRSA was almost exclusively a nosocomial organism. During the 1990s, MRSA began to infect patients with no known contact to healthcare organizations and who were otherwise healthy. These types of MRSA were noted to be genetically unique and soon began to be referred to as community-associated MRSA (CA-MRSA).Citation3 Understanding this complex pathogen has now become a primary focus for many practitioners as illness related to CA-MRSA can be life-threatening. Despite major medical advances, MRSA continues to cause significant disease.
Epidemiology
CA-MRSA differs from healthcare-associated MRSA (HA-MRSA) genetically and epidemiologically. Methicillin resistance is mediated by the mecA gene which resides on the staphylococcal cassette chromosome mec (SCCmec). SCCmec encodes for the penicillin-binding protein 2a (PBP2a) resulting in the inability of methicillin to bind to S. aureus.Citation4 CA-MRSA strains predominantly carry SCCmec type IV; however, SCCmec type V has also been identified in these strains.Citation3 These SCCmec genes are generally smaller than those genes found in HA-MRSA strains allowing for more rapid spread. In addition, these genes only confer resistance to methicillin and other beta-lactams while maintaining susceptibility to narrow-spectrum antibiotics such as tetracyclines, trimethoprim-sulfamethoxazole (TMP-SMX), and clindamycin. CA-MRSA, like HA-MRSA, remains susceptible to vancomycin, linezolid, daptomycin, and quinupristin/dalfopristin (Q/D).Citation3,Citation5 One distinct feature of CA-MRSA is its ability to carry the gene encoding for Panton-Valentine leukocidin (PVL), a deadly exotoxin. This exotoxin is the key feature associated with CA-MRSA that causes necrotizing infections of the soft tissue as well as necrotizing pneumonias.Citation2,Citation6 CA-MRSA did not originate from the hospital setting. Rather, it appears as though methicillin-susceptible S. aureus (MSSA) acquired the SCCmec gene producing a new genetic variant with two distinct clones occurring in the United States, USA300 and USA400. USA300 more frequently contains PVL genes and is now considered a major cause of necrotizing soft tissue infections.Citation2,Citation3 The Centers for Disease Control and Prevention (CDC) define CA-MRSA infections as those seen in people who meet the following criteria:Citation7
– Diagnosis of MRSA made in the outpatient setting or by a positive culture for MRSA within 48 hours of admission to the hospital.
– No medical history of MRSA infection or colonization.
– No medical history in the past year of:
Hospitalization
Admission to a nursing home, skilled nursing facility, or hospice
Dialysis
Surgery
– No permanent indwelling catheters or medical devices that pass through the skin into the body.
One important development with CA-MRSA is the observation of these strains among patients in the healthcare setting. Because most patients acquire CA-MRSA from the community and because these infections can be quite serious, many may require hospitalization which can potentially transmit these strains to other inpatients.Citation8 A review of 352 patients within the same institution with HA-MRSA found that CA-MRSA had become the most common cause of HA-MRSA. When MRSA strains were phenotyped, it was noted that the SCCmec IV gene increased from 17% to 56% in a 5-year period.Citation9
Clinical presentation and risk factors
Skin and skin structures are the predominant sites of infection of CA-MRSA, furuncles being the most common type reported. CA-MRSA can also cause cellulitis, deep tissue abscesses, and in more serious cases, necrotizing fasciitis and osteomyelitis. CA-MRSA may be associated with localized necrosis caused by the PVL gene. Often, patients experience sudden onset of a raised red lesion with central necrosis resembling a spider bite.Citation10 This can be confusing to both patients and physicians especially in areas of the United States where spider bites leading to cellulitis are uncommon. Presentations of this sort may be indicative of CA-MRSA infection as necrosis is a common feature of CA-MRSA both in skin and skin structure infections (SSSI) and lung infections. Pneumonia associated with PVL-producing CA-MRSA generally occurs as a necrotizing pneumonia with a high mortality rate.Citation11 More cases of CA-MRSA necrotizing pneumonia have been seen in young, otherwise healthy children and adults.Citation3 CA-MRSA necrotizing pneumonias are often seen following or concomitantly with an influenza-like illness. Patients often present with hemoptysis and a rapid onset of respiratory decompensation. In addition, patients appear septic with symptoms of hypotension, tachycardia, tachypnea, fever, and may often develop leukopenia.Citation12
CA-MRSA has been reported to cause serious outbreaks of infections in certain populations including personnel in competitive team sports, Alaskan natives, Native Americans, correctional facility inmates, children, and military personnel.Citation13 Transmission of CA-MRSA is predominantly through direct contact with an infected person. However, data from CA-MRSA outbreaks indicate that fomites, or inanimate objects, can also facilitate the spread of CA-MRSA.Citation14 One notable incident occurred in 2003 when 8 MRSA infections developed in 5 professional football players. The MRSA clone was identified as a PVL-containing CA-MRSA which appeared to have been transmitted via close contact with infected abrasions and equipment.Citation15
Risk factors for the acquisition of CA-MRSA are difficult to identify. It seems evident that close physical contact plays an important role; however, beyond that there are few well-designed studies that analyze risk factors.Citation16 Risk factors that most healthcare professionals agree on are listed in .Citation2,Citation3,Citation16,Citation17
Table 1 Risk factors for infection with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)Citation2,Citation3,Citation16,Citation17
In vitro activity
The assessment of in vitro activity of a given compound against select clinically significant pathogens has always been an early component in the development of new antimicrobials. Once the in vitro activity has been well defined, additional drug-specific data follow including basic pharmacokinetic parameters, human safety data, and ultimately, human outcome data. Specific to S. aureus, and more specifically MRSA, the differentiation into CA-MRSA and HA-MRSA subsets is a relatively new concept. Consequently, in vitro data for MRSA is not often subdivided into CA-MRSA or HA-MRSA. For the assessment of in vitro activity of various drugs against CA-MRSA, data are currently limited but accumulating. While it is unclear whether the genotypic identification of a given strain is clinically significant once susceptibility testing has been performed, it is clear that the susceptibility patterns differ based on the origin of the strain.
Use of the term CA-MRSA with regard to in vitro susceptibilities in contemporary literature generally refers to one of two issues. First, it may refer to strains which are consistent with the CDC definition as previously described.Citation7 Alternatively, the strain may have been genotyped and specifically identified to be a community-associated strain (ie, USA300, USA400, etc). For the purpose of this review, either determination has been considered sufficient to access in vitro activity. The in vitro activities of several antimicrobial agents have been evaluated against CA-MRSA and are summarized in . Included in this table are enteral and parenteral agents, both traditional and contemporary, which are commonly associated in the treatment of CA-MRSA infections.
Table 2 In vitro activity of select antimicrobial agents against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)
Treatment options
Current guidelines recommend incision and drainage of uncomplicated-SSSI caused by CA-MRSA.Citation18 Many patients can be treated with surgical drainage; however, antimicrobial therapy may provide additional benefits to the patient.Citation19 Factors that may persuade a clinician to incorporate antimicrobial therapy into the treatment plan include: presence of cellulitis, swift enhancement of the SSSI, systemic illness, immunocompromised patient, age of the patient, abscess location that is difficult to access, and inappropriate response to the initial incision and drainage.Citation20 Multiple non-beta-lactam oral antibiotics are available for outpatient therapy including: TMP-SMX, clindamycin, long-acting tetracyclines, and linezolid.
Enteral treatment options
Trimethoprim-sulfamethoxazole (TMP-SMX)
TMP-SMX (Septra®; King Pharmaceuticals, Bristol, TN, USA) is not approved by the Food and Drug Administration (FDA) for the treatment of staphylococcal infections.Citation21 However, TMP-SMX has shown in vitro bactericidal properties against strains of CA-MRSA.Citation22 The evidence to support the efficacy of TMP-SMX came from a randomized, controlled clinical trial comparing it with vancomycin in intravenous drug abusers with various S. aureus infections, including bacteremia, endocarditis, osteomyelitis, and SSSI. Vancomycin demonstrated superiority in the S. aureus treatment arm; however, the authors concluded that TMP-SMX is a viable treatment option in select cases of MRSA.Citation23 In another study conducted in an ambulatory clinic, the increased use of TMP-SMX correlated with improved clinical outcomes in patients with SSSI.Citation24 While data are lacking, TMP-SMX is widely used in clinical practice.
Clindamycin
Clindamycin (Cleocin®; Pfizer, Inc, New York, NY, USA) is another common antibiotic used to treat CA-MRSA.Citation25 It has a potential protective effect against toxins, including the PVL toxin.Citation26 There is a potential for resistance with high-inoculum infections via efflux or ribosomal alterations. A disk diffusion antibiotic assay (D-test) identifies inducible clindamycin resistance in erythromycin-resistant, clindamycin-susceptible S. aureus isolates.Citation27 While the rate of resistance to clindamycin continues to be low in CA-MRSA, the frequency of inducible resistance is variable and unknown.Citation28 Therefore, if confirmatory D-testing is not available, clindamycin should not be used to treat erythromycin-resistant CA-MRSA.
Tetracyclines
Doxycycline (Vibramycin®; Pfizer, Inc, New York, NY, USA) and minocycline (Minocin®; Wyeth Pharmaceuticals, Philadelphia, PA, USA) can also be effective in treating CA-MRSA SSSI.Citation29,Citation30 Although there are limited published data, the long-acting tetracyclines appear to be good treatment options in most tetracycline-susceptible MRSA SSSI.Citation31 Most laboratories in the United States test S. aureus for susceptibility to tetracycline (Sumycin®; Par Pharmaceuticals, Spring Valley, NY, USA) and not doxycycline or minocycline.Citation32 The use of tetracycline as a surrogate may overestimate the prevalence of resistance to doxycycline or minocycline since there are two different genes that confer resistance, tetM and tetK. TetK confers resistance to tetracycline, while tetM confers resistance to all agents in the class. TetK has been the predominant gene associated with resistant isolates of MRSA in the community, which points towards the continued use and effectiveness of doxycycline and minocycline.Citation33
Linezolid
Linezolid (Zyvox®; Pfizer, Inc, New York, NY, USA) may be a reasonable alternative following failure of treatment or allergic reactions.Citation34 It is a bacteriostatic antimicrobial approved to treat MRSA complicated-SSSI (cSSSI). Controlled trials with linezolid versus vancomycin determined them to be comparable in treatment of MRSA cSSSI.Citation35 Though resistance to linezolid is rare, the possibility of resistance has emerged with its increased use. Citation36 Similar to clindamycin, linezolid also suppresses the PVL toxin in CA-MRSA. This may prove beneficial in severe human infections with necrosis.Citation37
Other antimicrobials
Rifampin (Rifadin®; Sanofi-Aventis, Bridgewater, NJ, USA) should never be used as a single agent for the treatment of MRSA SSSI because of the rapid resistance that can develop in S. aureus.Citation38,Citation39 Rifampin can be used in combination with other antimicrobial agents for potential eradication enhancement, but there is lack of evidence through studies, available for the benefit.Citation40
Due to the relative ease of S. aureus in developing resistance with fluoroquinolone usage, they cannot be recommended for use in MRSA SSSI. The fluoroquinolones can cause chromosomal mutations in genes encoding the subunits of the drugs’ target enzymes, DNA gyrase and topoisomerase IV.Citation41
Parenteral treatment options
Several parenteral agents are currently available for the treatment of serious infections caused by MRSA, regardless of the strain, which include vancomycin (or teicoplanin), Q/D, linezolid, daptomycin, and tigecycline (). While vancomycin has long been used for the treatment of MRSA, increasing resistance has begun to limit its use in contemporary clinical practice.Citation85 Vancomycin has long been considered the “gold standard” for the treatment of MRSA, but recent reports of treatment failures are causing concern.Citation42 Since the emergence of CA-MRSA, limited attention has been directed toward the efficacy of vancomycin due to the availability of multiple new compounds. Of the remaining parenteral agents, linezolid is the only agent with an oral formulation and has been previously discussed.
Table 3 Antimicrobials recommended for outpatient treatment of suspected methicillin-resistant (MRSA) skin and skin structure infections
Selection of empiric therapy should be guided by local susceptibility and modified based on results of culture and susceptibility testing. The duration of therapy for most SSSI is 7–10 days, but may vary depending upon the severity of infection and clinical response. Some infections may require a more prolonged treatment course.
Table 4 Parenteral agents for the treatment of severe community-associated methicillin-resistant Staphylococcus aureus infectionsCitation34,Citation43,Citation48,Citation57,Citation85
Quinupristin/dalfopristin (Q/D)
Q/D (Synercid®; Monarch Pharmaceuticals, Bristol, TN, USA) is a semisynthetic streptogramin antibiotic with a potent gram-positive spectrum of activity, including CA-MRSA.Citation43–Citation45 Prospective randomized controlled trials support its use for the treatment of bacteremia caused by vancomycin-resistant Enterococcus faecium and for cSSSI caused by MSSA or Streptococcus pyogenes.Citation43,Citation46,Citation47 Despite previously demonstrated in vitro activity, Q/D lacks an indication in MRSA infections because this pathogen was not isolated in sufficient quantity to be evaluated. Historically the use of Q/D has been limited due to the high incidence of adverse reactions. Use is generally reserved for patients in whom conventional therapy is not tolerated or is otherwise contraindicated.
Daptomycin
Daptomycin (Cubicin®; Cubist Pharmaceuticals, Lexington, MA, USA) is a lipoglycopeptide antibiotic with a potent in vitro gram-positive spectrum of activity, specifically with rapid bactericidal action against MRSA.Citation48 Additional in vitro data have also demonstrated the activity of daptomycin against CA-MRSA.Citation44,Citation49 However, some data suggest a relationship between decreased susceptibility of vancomycin to MRSA and a decreased susceptibility of daptomycin to MRSA.Citation50–Citation53 Randomized controlled trials support the use of daptomycin for the treatment of cSSSI due to susceptible strains of gram-positive pathogens, and for the treatment of bacteremia and right-sided endocarditis caused by S. aureus.Citation54,Citation55 MRSA was the pathogen identified at baseline in 9.3% (n = 40) of daptomycin-treated patients in the cSSSI trials and 37.4% (n = 45) in the right-sided endocarditis trial.Citation54,Citation55 The MRSA strains in these trials were not differentiated into HA-MRSA or CA-MRSA. Outcomes were found to be similar between daptomycin and comparator for infections caused by MRSA. Prospective outcome data, however, are lacking for the treatment of CA-MRSA infections specifically. A retrospective evaluation assessed the use of daptomycin for the treatment of community-phenotype-MRSA (CP-MRSA) infections, defined by the authors as MRSA with susceptibility to both clindamycin and TMP-SMX.Citation56 All other phenotypes were classified by the authors as other-phenotype-MRSA (OP-MRSA). Of the 352 patients included in this evaluation, 100 were classified as CP-MRSA and 252 as OP-MRSA. The CP-MRSA group tended to be younger with fewer underlying diseases. Success rate, time to clinical response, and duration of therapy were similar in both groups, prompting the authors to conclude that daptomycin was equally efficacious for the treatment of CP-MRSA or OP-MRSA infections in this select group of patients. However, these data cannot necessarily be extrapolated to all patient populations.
Tigecycline
Tigecycline (Tygacil®; Wyeth Pharmaceuticals, Philadelphia, PA, USA), the first glycycline antibiotic, has a broad range of in vitro activity against most gram-positive (including MRSA), most gram-negative, and anaerobic bacteria.Citation57 Mendes and colleagues evaluated tigecycline against 1989 human isolates of CA-MRSA collected from the SENTRY Antimicrobial Surveillance Program, 94.7% of which were PVL positive.Citation45 Tigecycline was active against 98.2% of the strains tested, similar to the susceptibility rates for vancomycin, teicoplanin, Q/D and linezolid. Randomized controlled clinical trials have demonstrated its safety and efficacy for the treatment of cSSSI and complicated intra-abdominal infections (cIAI).Citation58,Citation59 While MRSA has been included in the FDA approval for its association to cSSSI, it is lacking for cIAI due to a small sample size in the registration trials, similar to Q/D. A retrospective evaluation of the 173 MRSA isolates obtained from these phase III trials, 85% of which were from the cSSSI trial, found that 76 isolates had characteristics consistent with known CA-MRSA on the basis of genotyping and the presence of both PVL-encoding locus and SCCmec type IV.Citation60 All strains demonstrated in vitro susceptibility to tigecycline.
Drugs under investigation
Multiple antimicrobial agents with potent in vitro MRSA activity are currently in development. These agents are summarized in with regard to drug name, route of administration, dose, indication for which it is under evaluation, and phase of development at this point of time. Drug classes currently in clinical use with additional drugs in development include glycopeptides, oxazolidinones, and a dihydrofolate reductase inhibitor. In addition, for the first time ever, beta-lactam antibiotics which possess MRSA activity, specifically new cephalosporins and carbapenems, are being developed.
Table 5 Investigational agents with in vitro activity against methicillin-resistant Staphylococcus aureus
Glycopeptides
Adding to vancomycin and teicoplanin of the glycopeptide drug class, dalbavancin, telavancin, and oritavancin are currently in the developmental stage. Dalbavancin is a semisynthetic lipoglycopeptide with a long half-life allowing for once-weekly dosing.Citation61 Building on a successful phase II trial evaluating dalbavancin versus standard-of-care, a phase III trial evaluated dalbavancin versus linezolid for the treatment of cSSSI.Citation62,Citation63 MRSA was identified in 51% of patients from whom a pathogen was isolated at baseline. Dalbavancin was well tolerated and efficacy was found to be noninferior. A phase II trial for bacteremia has yielded similar results.Citation64 In an in vitro evaluation of 329 pathogens associated with diabetic foot infections (DFIs), susceptibility to dalbavancin was compared with other antimicrobial agents.Citation65 Of these 329 pathogens, 60 were MRSA, with an estimated 50% suspected CA-MRSA. Though this was not confirmed via pulse field gel electrophoresis (PFGE), >50% of the suspected CA-MRSA isolates were resistant to clindamycin with an MIC >8 μg/mL. The authors observed that dalbavancin was more active than vancomycin, daptomycin, and linezolid against MRSA. They concluded that the in vitro data, coupled with dalbavancin outcome data (versus linezolid for the treatment of SSSI, including DFI), indicate that it may provide an advantage to patients with DFI in emergency departments, inpatient settings, and outpatient settings.
Telavancin, another lipoglycopeptide, possesses potent MRSA activity.Citation66 An in vitro evaluation tested telavancin against 60 strains of CA-MRSA (as defined by the CDC).Citation44 Thirty-two (54%) of the 60 CA-MRSA strains were identified via PFGE to be the USA300 strain which harbors PVL genes. Telavancin demonstrated bactericidal activity against all 60 CA-MRSA isolates, including all USA300 strains. A phase III study found telavancin to be safe and at least as effective as vancomycin for the treatment of cSSSI caused by gram-positive organisms, including MRSA.Citation67 Many of the MRSA isolates were SCCmec type IV and PVL positive.Citation68
Oritavancin, another semisynthetic lipoglycopeptide with MRSA activity, has a long half-life allowing for less frequent dosing compared to contemporary glycopeptides.Citation69 Two phase III trials have been completed evaluating oritavancin versus comparator for the treatment of cSSSI.Citation70,Citation71 Although these data are as yet unpublished, favorable results have been reported in abstract form and a new drug application (NDA) was submitted to the FDA in early 2008 for the treatment of cSSSI. Additional data specific to CA-MRSA are lacking.
Oxazolidinones
Several oxazolidinones are currently in development. While multiple compounds are under investigation, most are in pre-clinical development stage and have not yet been named beyond their chemical number. AZD2563, for example, has demonstrated in vitro activity similar to linezolid against gram-positive pathogens, including MRSA.Citation72 Additional oxazolidinones have been evaluated by McKee and colleagues, but they are far from clinical development.Citation73
Dihydrofolate reductase inhibitor
Iclaprim, a dihydrofolate reductase inhibitor similar to trimethoprim, is currently being evaluated as an intravenous formulation for the treatment of cSSSI in phase III trials. Positive outcomes have resulted in the submission of a NDA to the FDA., though the FDA has denied its approval. Iclaprim is currently under review in Europe and Canada. An intravenous-to-oral switch of iclaprim for the treatment of cSSSI is under evaluation in a phase II trial. Additionally, intravenous iclaprim for the treatment of hospital-acquired pneumonia, including both ventilator-associated pneumonia and healthcare-associated pneumonia is under evaluation in a phase III trial. Data specific to CA-MRSA are lacking.
Cephalosporins
Traditionally, cephalosporins have lacked activity against MRSA. Recently this changed with the introduction of ceftobiprole and ceftaroline which have a high affinity for PBP2a, and therefore exhibit good in vitro activity against MRSA, including CA-MRSA.Citation74–Citation76 When tested against 152 strains of well-characterized CA-MRSA, the MIC50 and MIC90 for ceftobiprole were 0.5 and 0.5 μg/mL, respectively, with a range of 0.25–1 μg/mL. Breakpoints have not yet been established and consequently these results are up for interpretation. Both ceftaroline and ceftobiprole are currently under investigation for safety and efficacy of the treatment of cSSSI; a NDA has since been filed with the FDA for ceftobiprole.
Carbapenems
Similar to the aforementioned cephalosporins, some investigational carbapenems have demonstrated in vitro activity against MRSA. Specifically, ME1036 has demonstrated activity against CA-MRSA.Citation76 When tested against 152 strains of well-characterized CA-MRSA, the MIC50 and MIC90 were 0.12 and 0.25 μg/mL, respectively, with a range of 0.06–0.5 μg/mL. This, and other carbepenems with MRSA activity, are in the early stages of clinical development and may not reach market for several years. A review by Lo and colleagues has examined these agents in more detail.Citation77
Complementary/alternative medicine
Tea tree oil (TTO) has been evaluated as a potential agent for both MRSA decolonization and treatment. It is available in many forms and is widely used in cosmetic preparations. Concentrations of TTO and product integrity are often variable since it is not a regulated drug.
Several studies have evaluated the in vitro activity of TTO versus MRSA and many have shown promise, including one study indicating that TTO may be of benefit for eradication of MRSA biofilms.Citation78 Translating in vitro data to clinical practice has yielded less consistent results. A study by Dryden and colleagues evaluated TTO for MRSA eradication in hospitalized patients.Citation79 A standard 5-day regimen of mupirocin 2% nasal ointment, chlorhexidine gluconate 4% skin cleanser and silver sulfadiazine 1% cream was compared to 10% tea tree cream and 5% tea tree body wash. Results indicated that mupirocin was significantly more effective at eradicating MRSA nasal carriage compared to TTO, 78% and 41% clearance, respectively.
A study by McMahon and colleagues investigated whether the effect of sub-lethal challenge with TTO impacted the antibiotic resistance profiles of significant human pathogens.Citation80 The authors note that TTO products often contain variable concentrations, and previously available data had not evaluated the impact of inappropriate or sublethal TTO concentrations. With respect to the MRSA strains used in the study, TTO caused an increased (2-fold or greater) MIC value for 7 out of 10 antibiotics tested.
In summary, before TTO is widely accepted as a standard-of-care for treating MRSA or for MRSA decolonization, further clinical trial data are needed. If providers recommend TTO for treatment of CA-MRSA, it should be in conjunction with one of the traditional therapies previously discussed in this article.
Resistance
As discussed, most of the USA300 isolates are resistant to beta-lactam and macrolide antibiotics. Given that there are often other oral agents to treat most CA-MRSA strains, the question of development of resistance to these (doxycyline, minocycline, clindamycin, TMP-SMX) drugs is of concern. An epidemiologic study evaluated 123 USA300 isolates collected in an ambulatory health center in Boston, Massachusetts.Citation81 Of all the isolates, 83% had PFGE revealing either USA300–0114 (58% of total isolates) or USA300–0247 (24% of total isolates). There were 12 multidrug-resistant (MDR) isolates reported, all of which contained the tetK and ermC genes. Of the USA300–0114 isolates (n = 73), 72 (99%) were resistant to erythromycin, 36 (49%) were resistant to clindamycin and 10 (14%) were resistant to doxycycline. Two of the strains showed multidrug resistance to erythromycin, levofloxacin, clindamycin and tetracycline. Of the USA 300–0247 isolates (n = 29), 26 (90%) were resistant to erythromycin, 22 (76%) were resistant to clindamycin and 21 (72%) were resistant to tetracycline. Sixteen of the strains showed multidrug resistance to erythromycin, levofloxacin, clindamycin and tetracycline. Twelve isolates were tested for mupirocin susceptibility, and all were mupirocin resistant with MIC ≥ 128 μg/mL. The authors note that their resistance rates were considerably higher than other reported data as well as compared to the local health network.
A second retrospective study describes a USA300 strain that exhibits plasmid-mediated resistance to erythromycin, clindamycin and mupirocin.Citation82 This retrospective study aimed to determine the incidence of an MDR MRSA clone (USA300) in San Francisco and risk factors associated with it. Based on an analysis from nine medical centers, the annual incidence of USA300 infection in San Francisco was estimated to be 275 cases per 100 000 persons (95% CI, 256–295 cases per 100 000 persons). With respect to USA300 infections containing a MDR conjugative plasmid, the annual incidence was 26 per 100 000 persons (95% CI, 16–36 cases per 100 000 persons). When eight zip codes were pooled, the incidence rose to 59 cases per 100 000 persons (95% CI, 36–82 cases per 100 000 persons). The other 18 zip codes evaluated only had incidence or 4 per 100 000 persons (95% CI, 0–8 cases per 100 000 persons). A single zip code had 25.7% of male same-sex couples, and incidence of MDR USA300 was 170 cases per 100 000 persons (95% CI, 41–299 cases per 100 000 persons). The authors conclude that men who have sex with men may be at higher risk for infection with MDR USA300. Data such as these reflect the need for astute monitoring of local epidemiologic patterns for CA-MRSA isolates.
Outpatient treatment approach
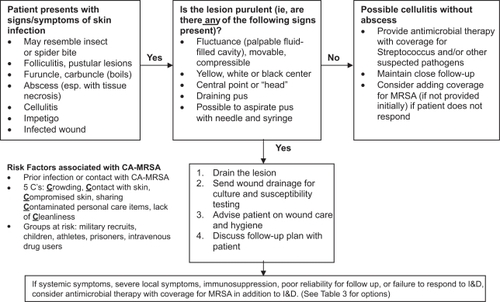
The treatment approach for CA-MRSA infections is variable based upon severity and site of infection. As discussed previously in this article, the majority of CA-MRSA infections are cutaneous infections and many may be managed on an outpatient basis. While the general consensus is that minor infections can be managed by incision and drainage (I&D) alone, many practitioners are opting to also treat with oral antimicrobials when MRSA diagnosis is confirmed by culture. A study by Moran and colleagues evaluated CA-MRSA patients presenting to the Emergency Department.Citation83 Approximately 20% of patients underwent I&D alone, 10% were treated with antibiotics alone, and 66% received both I&D and antibiotic therapy. The 2005 Infectious Diseases Society of America Practice Guideline for the Diagnosis and Management of Skin and Soft-Tissue Infections recommends that if the infection involves inflammation of the surrounding tissue or has manifested in systemic symptoms, I&D with concomitant antimicrobial therapy.Citation84 summarizes a possible treatment approach for the outpatient management of suspected CA-MRSA SSSI.
Figure 1 Outpatient management of suspected community-associated methicillin-resistant Staphylococcus aureus skin and skin structure infections. Adapted from Aurora Health Care MRSA Clinical Guidelines 2008. Kathryn Leonhardt, MD, MPH, Editor.
Abbreviations: I&D, incision and drainage; MRSA, methicillin-resistant S. aureus; CA-MRSA, community-associated MRSA.
Conclusions
Although the changing epidemiology of MRSA has become better understood in recent years, the impact, both in and out of the hospital, still require further research and investigation. Understanding how to treat and prevent CA-MRSA is critical. Risk factors, optimal treatment, and infection prevention strategies need to be better defined and economic outcomes need to be measured. The most effective intervention appears to be prevention although this is one of the most difficult. As clinicians and patients become more educated about CA-MRSA and its spread, common practices will likely require modification.
Disclosures
The authors report no conflicts of interest in this work.
References
- JevonsMPCoeAWParkerMTMethicillin resistance in staphylococciLancet19631904907
- BoucherHWCoreyGREpidemiology of methicillin-resistant Staphylococcus aureusClin Infect Dis200846S344S34918462089
- WallinTRHernHGFrazeeBWCommunity-associated methicillin-resistant Staphylococcus aureusEmerg Med Clin N Am200826431455
- JohnsonLBSaravolatzLDCommunity-acquired MRSA: current epidemiology and management issuesInfect Med20052211620
- FileTMImpact of Community-associated methicillin-resistant Staphylococcus aureus in the hospital settingCleve Clin J Med200774S6S1117847173
- NaimiTSLeDellKHComo-SabettiKComparison of Community- and health care-associated methicillin-resistant Staphylococcus aureus infectionJAMA20032902976298414665659
- Center for Disease Control MRSA definition reference http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca_clinicians.html#4. Accessed October 20, 2008.
- OtterJAFrenchGLNosocomial transmission of community-acquired methicillin-resistant Staphylococcus aureus: an emerging threatLancet Infect Dis2006675317123892
- MareeCLDaumRSBoyle-VavraSCommunity-associated methicillin-resistant Staphylococcus aureus causing healthcare-associated infectionsEmerg Infect Dis20071323624217479885
- DaumRSSkin and soft tissue infections caused by methicillin-resistant Staphylococcus aureusN Engl J Med200735738039017652653
- GilletYVanhemsPLinaGFactors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidinClin Infect Dis20074531532117599308
- GilletYIssartelBVanhemsPAssociation between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patientsLancet200235975375911888586
- WeberJTCommunity-associated methicillin-resistant Staphylococcus aureusClin Infect Dis200541101373140616231249
- MillerLGDiepBAColonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infectionsClin Infect Dis20084675276018220477
- KazakovaSVHagemanJCMatavaMA clone of methicillin-resistant Staphylococcus aureus among professional football playersN Engl J Med200535246847515689585
- SalgadoCDFarrBMCalfeeDPCommunity-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factorsClin Infect Dis200336213113912522744
- WeberJTCommunity-associated methicillin-resistant Staphylococcus aureusClin Infect Dis200541S269S27216032563
- StevensDLBisnoALChambersHFPractice guidelines for the diagnosis and management of skin and soft-tissue infectionsClin Infect Dis200541101373140616231249
- RuheJJSmithNBradsherRWCommunity onset of methicillin-resistant Staphylococcus aureus skin anad soft tissue infections: Impact of antimicrobial therapy outcomeClin Infect Dis20074477778417304447
- GorwitzRJJerniganDBPowersJHStrategies for clinical management of MRSA in the community: Summary of an experts’ meeting convened by the Centers for Disease Control and Prevention2006 Available at http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html. 2006
- Septra® [package insert]. Bristol, TN, USA: King Pharmaceuticals; 2007
- KakaASRuedaAMShelburneSAIIIBactericidal activity of orally available agents against methicillin-resistant Staphylococcus aureusJ Antimicrob Chemother20065868068316840428
- MarkowitzNQuinnELSaravolatzKDTrimethoprim-Sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infectionsAnn Int Med19921173903981503330
- SzumowskiJDChoenDEKanayaFTreatment and outcomes of infections by methicillin-resistant Staphylococcus aureus at an ambulatory clinicAntimicrob Agents Chemother20075142342817116664
- Cleocin® [package insert]. New York, NY, USA: Pfizer Inc; 2007
- DumitrescuOBoissetSBadrionCEffect of antibiotics on Staphylococcus aureus producing Panton-Valentine LeukocidinAntimicrob Agents Chemother2007511515151917242137
- SiberryGKTekleTCarollKFailure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitroClin Infect Dis2003371257126014557972
- PatelJBJevittLAHagemanJAn association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureusClin Infect Dis2006a421652165316652325
- Vibramycin® [package insert]New York, NY, USAPfizer Inc2001
- Minocin® [package insert]Philadelphia, PA, USAWyeth Pharmaceuticals2005
- RuheJJMonsonTBradsherRWUse of long-acting tetracyclines for methicillin-resistant Staphylococcus aureus infections: case series and review of the literatureClin Infect Dis200540101429143415844065
- Sumycin® [package insert]Spring Valley, NY, USAPar Pharmaceuticals2006
- TenoverFCMcDougalLKGoeringRVCharacterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United StatesJ Clin Microbiol200644110811816390957
- Zyvox® [package insert]New York, NY, USAPfizer Inc2008
- WeigeltJItaniKStevensDLinezolid versus vancomycin in treatment of complicated skin and soft tissue infectionsAntimicrob Agents Chemother2005171388416
- TsiodrasSGoldHSSakoulasGLinezolid resistance in a clinical isolate of Staphylococcus aureusLancet200135820720811476839
- StevensDLMaYSalmiDBMcIndooEWallaceRJBryantAEImpact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureusJID200719520221117191165
- StrausbaughLJJacobsonCSewellDLAntimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affair nursing home care unitInfect Control Hosp Epidemiol19921331511591564313
- Rifadin® [package insert]Bridgewater, NJ, USASanofi-aventis US LCC2007
- StryjewskiMEChambersHFSkin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureusClin Infect Dis200846S368S37718462092
- HooperDCMechanisms of quinolone resistanceDrug Resistance Updates19992385511504468
- TenoverFCMoelleringRCThe rationale for revising the clinical and laboratory standards institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureusClin Infect Dis2007441208121517407040
- Synercid® [package insert]Bristol, TN, USAMonarch Pharmaceuticals2003
- SaravolatzLDPawlakJJohnsonLBComparative activity of telavancin against isolates of community-associated methicillin-resistant Staphylococcus aureusJ Antimicrob Chemother200760240640917586562
- MendesRESaderHSDeshpandeLAntimicrobial activity of tigecycline against community-acquired methicillin-resistant Staphylocoous aureus isolates recovered from North AmericaDiagn Microbiol Infect Dis200860443343618068326
- NicholsRLGrahamDRBarriereSLTreatment of hospitalized patients with complicated gram-positive skin and skin structure infections: two randomized, multicentre studies of quinupristin/dalfopristin versus cefazolin, oxacillin or vancomycin. Synercid Skin and Skin Structure Infection GroupJ Antimicrob Chemother199944226327310473234
- MoelleringRCLindenPKReinhardtJThe efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin-resistant Enterococcus faecium. Synercid Emergency-Use Study GroupJ Antimicrob Chemother199944225126110473233
- Cubicin® [package insert]Lexington, MA, USACubist Pharmaceuticals2007
- JohnsonLBSaeedSPawlakJClinical and laboratory features of community-associated methicillin-resistant Staphylococcus aureus: is it really new?Infect Control Hosp Epidemiol200627213313816465629
- CuiLTominagaENeohHMCorrelation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureusAntimicrob Agents Chemother2006501079108216495273
- PatelMWaitesKBMoserSAPrevalence of inducible clindamycin resistance among community and hospital-associated Staphylococcus aureus isolatesJ Clin Microbiol2006b442481248216825368
- SakoulasGAlderJThauvin-EliopoulosCInduction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycinAntimicrob Agents Chemother2006501581158516569891
- PillaiSKGoldHSSakoulasGDaptomycin nonsusceptability in Staphylococcus aureus with reduced Vancomycin susceptibility is independent of alterations in MprFAntimicrob Agents Chermother20075122232226
- ArbeitRDMakiDTallyFPDaptomycin 98–01 and 99–01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infectionsClin Infect Dis200438121673168115227611
- FowlerVGJrBoucherHWCoreyGRDaptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureusN Engl J Med2006355765366516914701
- KatzDEMartoneWJCommunity-phenotype-methicillin-resistant Staphylococcus aureus infections: a retrospective chart review of outcomes after treatment with daptomycinClin Ther200729112440244718158084
- Tygacil® [package insert]Philadelphia, PA, USAWyeth Pharmaceuticals2007
- BreedtJTerasJGardovskisJTigecycline 305 c Study Group. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonamAntimicrob Agents Chemother200549114658466616251309
- SacchidanandSPennRLEmbilJMEfficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trialInt J Infect Dis20059525126116099700
- McAleeseFMurphyEBabinchakTUse of ribotyping to retrospectively identify methicillin-resistant Staphylococcus aureus isolates from phase 3 clinical trials for tigecycline that are genotypically related to community-associated isolatesAntimicrob Agents Chemother200549114521452916251291
- BaileyJSummersKMDalbavancin: a new lipoglycopeptide antibioticAm J Health Syst Pharm200865759961018359966
- SeltzerEDorrMBGoldsteinBPDalbavancin Skin and Soft-Tissue Infection Study Group. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infectionsClin Infect Dis200337101298130314583862
- JaureguiLEBabazadehSSeltzerERandomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infectionsClin Infect Dis200541101407141516231250
- RaadIDarouicheRVazquezJEfficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogensClin Infect Dis200540337438015668859
- GoldsteinEJCitronDMWarrenYAIn vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infectionsAntimicrob Agents Chemother20065082875287916870792
- AttwoodRJLaPlanteKLTelavancin: a novel lipoglycopeptide antimicrobial agentAm J Health Syst Pharm20076422233517989443
- StryjewskiMEGrahamDRWilsonSEAssessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organismsClin Infect Dis200846111683169318444791
- FowlerVGJrRudeTHNelsonCLActivity of telavancin against Staphylococcus aureus isolates carrying the Panton-Valentine leukocidin gene in the ATLAS studies [abstract 847]Program and abstracts of the 17th European Congress of Clinical Microbiology and Infectious Diseases (Munich)2007
- PoulakouGGiamarellouHOritavancin: a new promising agent in the treatment of infections due to Gram-positive pathogensExpert Opin Investig Drugs2008172225243
- GiamarellouHO’RiordanWHarrisHPhase 3 trial comparing 3–7 days of oritavancin vs 10–14 days of vancomycin/cephalexin in the treatment of patients with complicated skin and skin structure infections (c) [abstract]Program and abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago)Washington, DCAmerican Society for Microbiology2003
- WasilewskiMDishDMcGillJEquivalence of shorter course of therapy with oritavancin vs vancomycin/cephalexin in complicated skin and skin structure infections (c) [abstract UL-18]Program and abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago)Washington, DCAmerican Society for Microbiology2001
- FluitACSchmitzFJVerhoefJSCCmec activity of AZD2563, a novel oxazolidinone, against European Gram-positive cocciJ Antimicrob Chemother20025027127612161411
- McKeeEEFergusonMBentleyATInhibition of mammalian mitochondrial protein synthesis by oxazolidinonesAntimicrob Agent and Chemo200650620422049
- ChungMAntignacAKimCComparative study of the susceptibilities of major epidemic clones of methicillin-resistant Staphylococcus aureus to oxacillin and to the new broad-sepctrum cephalosporin ceftobiproleAntimicrob Agents Chemother20085282709271718505853
- FritscheTRSaderHSJonesRNAntimicrobial activity of ceftobiprole, a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, tested against contemporary pathogens: results from the SENTRY antimicrobial surveillance program (2005–2006)Diag Microb Infect Dis2008618695
- SaderHSFritscheTRJonesRNAntimicrobial activities of Ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureusAntimicrob Agents Chemother20085231153115518180353
- LoTSWelchJMAlontoAMA review of the carbapenems in clinical use and clinical trialsRecent Patents Anti-Infect Drug Disc200832123131
- BradyALoughlinRGilpinDIn vitro activity of tea-tree oil against clinical skin isolates of methicillin-resistant and sensitive Staphylococcus aureus and coagulase-negative staphylococci growing planktonically and as biofilmsJ Medical Microbiol20065513751380
- DrydenMSDaillySCrouchMA randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonizationJ Hosp Infect20045628328615066738
- McMahonMABlairIMooreJHabituation to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia) is associated with reduced susceptibility to antibiotics in human pathogensJ Antimicrob Chemother20075912512717071952
- HanLMcDougalLGorwitzRHigh frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health centerJ Clin Microbiol2007451350135217287335
- DiepBAChambersHFGrabersCJEmergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with menAnn Intern Med2008148424925718283202
- MoranGJKrishnadasanAGorwitzRJMethicillin-resistant Staphylococcus aureus infections among patients in the emergency departmentN Engl J Med200635566667416914702
- StevensDLBisnoALChambersHFPractice guidelines for the diagnosis and management of skin and soft-tissue infectionsClin Infect Dis200541101373140616231249
- Abbott. Sterile vancomycin hydrochloride, USP ADD-Vantage® vials prescribing informationNorth Chicago, IL1998
- CrumNFLeeRUThorntonSAFifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureusAm J Med20061191194395117071162
- FridkinSKHagemanJCMorrisonMActive Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communitiesN Engl J Med2005352141436144415814879
- HuangHFlynnNMKingJHComparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, CaliforniaJ Clin Microbiol20064472423242716825359
- KingMDHumphreyBJWangYFEmergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infectionsAnn Intern Med2006144530931716520471
- NaimiTSLeDellKHBoxrudDJEpidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996–1998Clin Infect Dis200133799099611528570
- TsujiBTRybakMJCheungCMCommunity- and health care-associated methicillin-resistant Staphylococcus aureus: a comparison of molecular epidemiology and antimicrobial activities of various agentsDiagn Microbiol Infect Dis2007581414717300912