Abstract
Candida infections continue to play a significant role not only in critically ill and immunocompromised patients but also in non-compromised patients. The incidence of systemic fungal infections in the United States has been on the rise for the past 30 years. Anidulafungin and all echinocandins inhibit glucan synthase thus inhibiting the formation of 1,3-β-D-glucan which is an essential component of the fungal cell wall. The decrease in 1,3-β-D-glucan results in the osmotic lysis of the cell, resulting in fungicidal activity against candida. Anidulafungin is active against most species of candida and resistance to it is very rare. Two potential mechanisms conferring reduced susceptibility to the echinocandins are efflux and target alteration. The efflux pump associated with fluconazole resistance in Candida albicans can confer higher minimum inhibitory concentrations to caspofungin. The second mechanism of resistance is via mutations in the genes which code for 1,3 β-D-glucan synthase, specifically FKS1. Because of its spectrum of activity, fungicidal nature, and tolerability it is an attractive first-line therapeutic choice for treating candidemia in both non-neutropenic and neutropenic patients. Because it is available only parenterally its role in treating mucocutaneous candidiasis is primarily in patients unable to take oral therapy.
Keywords:
Candida infections continue to play a significant role not only in critically ill and immunocompromised patients but also in non-compromised patients. The spectrum of disease caused by candida ranges from non-life-threatening mucocutaneous infections to life-threatening invasive candidiasis/candidemia. Candida remains the 4th and 5th leading cause of bloodstream infections in adult and pediatric patients, respectively.Citation1,Citation2 The incidence of systemic fungal infections in the United States has been on the rise the past 30 years. Between 1979 and 2000 the annual number of sepsis cases due to fungal organisms increased by 207%.Citation3 In addition the number of candida-related hospitalizations increased by 52% between 2000 and 2005.Citation4 Common risk factors for the development of systemic candidiasis or candidemia include: immunosuppression, use of broad-spectrum antibiotics, central venous catheters, TPN, disruption of mucosal membranes and extremes of age.Citation5
The echinocandin class of anti-fungal agents (anidulafungin, caspofungin, and micafungin) was introduced into the United States and European markets in 2001. They are the most recent addition joining the azoles, polyenes, allylamines, and anti-metabolites available for treating systemic fungal infections. The echinocandins are recommended as potential first-line therapy for candidemia in non-neutropenic and neutropenic patients and first- or second-line for several other candida infections.Citation6 This review will focus on anidulafungin and its role in candida infections.
Chemistry
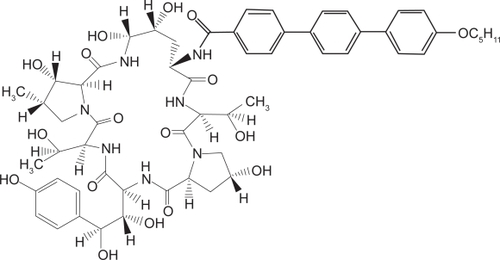
The echinocandins are synthetically modified lipopeptides which were identified from the fermentation broths of various fungi. Anidulafungin () is derived from Aspergillus nidulans. It is a 1-[(4R,5R)-4,5-Dihydroxy-N2-[[4″ (pentyloxy)[1,1′:4′,1″-terphenyl]-4-yl]carbonyl]-L-ornithine]echinocandin B. Its molecular formula is C58H73N7O17, and its molecular weight is 1140.3.Citation7 The echinocandins are only available parenterally and anidulafungin is available in both 50 and 100 mg vials.
Anidulafungin is initially reconstituted with a diluent containing 20% (w/w) dehydrated alcohol in water for injection and then diluted to its final concentration of either 0.36 or 0.43 mg/mL in either 5% dextrose or normal saline. Compatibility studies with other diluents or solutions have not been performed therefore they should not be used. The maximum rate of infusion for anidulafungin is 1.1 mg/min. Histamine-related adverse effects such as rash, urticaria, flushing, pruritus, dyspnea, and hypotension have been reported when the infusion rate exceeds 1.1 mg/min.Citation7
Mechanism of action, FDA-approved indications and dosing
Anidulafungin and all echinocandins inhibit glucan synthase thus inhibiting the formation of 1,3-β-D-glucan which is an essential component of the fungal cell wall. Glucan synthase is present in fungal cells but not mammalian cells. The decrease in 1,3-β-D-glucan results in the osmotic lysis of the cell, resulting in fungicidal activity against candida.Citation8 Anidulafungin is FDA approved for the treatment of esophageal candidiasis, candidemia, and invasive candidiasis (intra-abdominal abscess and peritonitis).Citation7
For the treatment of esophageal candidiasis, the recommended loading dose is 100 mg followed by the maintenance dose of 50 mg daily. The duration of treatment should be based on the patient’s clinical response with most patients being treated for ≥14 days, or for ≥7 days after the resolution of symptoms. For the treatment of candidemia, the recommended loading dose of 200 mg is followed by 100 mg daily for the duration of treatment of ≥14 days after the last positive blood culture results. No dosage adjustments are needed in patients with hepatic or renal impairment regardless of severity. Anidulafungin is not dialyzed during hemodialysis.Citation7
Spectrum of activity
The Clinical and Laboratory Standards Institute (CLSI) established susceptibility breakpoints for the echinocandins in 2007. The breakpoint for susceptible against Candida organisms is ≤2 μg/mL for all three echinocandins and given the extremely low number of isolates with minimum inhibitory concentrations (MICs) higher than 2 μg/mL no breakpoints for intermediate or resistant were established.Citation9 Organisms with MICs > 2 μg/mL are considered non-susceptible. The European Committee for Antimicrobial Susceptibility Testing (EUCAST) has not established breakpoints for the echinocandins.Citation10 The organisms to which the echinocandins are highly active include C. albicans, C. glabrata, C. tropicalis, C. dubliniensis, and C. krusei. In general, the MIC50 of anidulafungin against these pathogens is ≤0.03 μg/mL and the MIC90 is ≤0.13 μg/mL.Citation11,Citation12 In vitro anidulafungin is more active than caspofungin against these pathogens although this has not been clinically proven to be significant.Citation11–Citation14 The echinocandins are less active against C. parapsilosis, C. guilliermondii, and C. lusitaniae compared to the other Candida spp; the MIC50 and MIC90 of anidulafungin range from 0.06 to 2 and 0.25 to 2 μg/mL, respectively.Citation11–Citation13 Anidulafungin is highly active against azole-resistant candida with 99% of isolates inhibited at ≤1 μg/mL.Citation11 CLSI recommends further testing be performed on C. albicans, C. tropicalis, or C. glabrata isolates in which an echinocandin MIC of 1 or 2 μg/mL is obtained. None of the echinocandins are active against Cryptococcus neoformans (MICs 16 to 64 μg/mL) or other Cryptococcus spp.Citation15 The echinocandins are active against Aspergillus spp.Citation16–Citation20 The MIC90 for anidulfungin against A. fumigatus is ≤0.25 μg/mL.Citation16,Citation17 Activity against other species of Aspergillus is similar to that seen against fumigatus.
Resistance
Resistance to the echinocandins is rare amongst Candida spp. and identification of the mechanism(s) has resulted in contradictory information. The efflux pump associated with fluconazole resistance in C. albicans was suggested to confer higher MICs to caspofungin.Citation21 The increase in MICs to caspofungin was minor and the isolates were still considered susceptible by CLSI breakpoint. This cross-substrate of the echinocandins to the fluconazole efflux pump did not occur with all yeast isolates expressing or hyperexpressing the efflux pump.Citation22 The second mechanism of resistance to the echinocandins in C. albicans, C. parapsilosis and C. krusei is via mutations in the genes which code for 1,3 β-D-glucan synthase, specifically FKS1.Citation23–Citation25 Five candida isolates, which had MICs > 4 μg/mL to caspofungin were recovered from patients enrolled in a caspofungin clinical trial and all were found to have mutations in the FKS1 gene.Citation23 Within C. parapsilosis an intrinsic mutation in FKS1 appears to be responsible for the higher MICs for the echinocandins. The mutations in the conserved hot spot 1 region of fks1 appear to result in a glucan synthase which is less sensitive to the echinocandins and some isolates had MICs > 8 μg/mL.Citation26–Citation28 Another study failed to demonstrate mutations in the hot spot 1 of several isolates of C. parapsilosis which had higher MICs to caspofungin.Citation29 These isolates had caspofungin and micafungin MICs of ≥8 μg/mL and the anidulafungin MIC90 for anidulafungin was 2 μg/mL. Moudgal and colleagues also reported a C. parapsilosis isolate in which the caspofungin and micafungin MICs increased to >16 μg/mL while anidulafungin’s MIC was 2 mcg/mL.Citation30 The mechanism behind this disparity in MICs of anidulafungin compared to the other 2 agents is still unknown but mutations in FKS2 and/or FKS3 may play a role.
The incidence of developing resistance during therapy is still rare and a small number of cases regarding the development of higher MICs while receiving caspofungin have been reported. The first involved C. parapsilosis prosthetic valve endocarditis and the patient failed caspofungin therapy. The MICs during the first hospitalization were 2, 8 and 1 μg/mL for caspofungin, micafungin and anidulafungin, respectively which increased to >16 μg/mL for caspofungin and micafungin and 2 μg/mL for anidulafungin.Citation30 Reports of failed echinocandin therapy in HIV or AIDS patients with recurrent esophagitis caused by C. albicans have been published.Citation31–Citation33 Gene sequencing in two of the cases revealed C. albicans isolates with caspofungin MICs of ≥8 μg/mL with mutation of the FKS1 gene.Citation31,Citation32 One of the isolates was resistant to all 3 of the echinocandins.Citation32 Reports exist as well for other non-albicans candida (C. glabrata, C. krusei and C. tropicalis) developing resistance during caspofungin therapy.Citation34–Citation36 Increases in MICs to the three echinocandins are not necessarily uniform as demonstrated by these case reports.
Potential limitations or problems with the current CLSI breakpoint of ≤2 μg as susceptible and the methodologies recommended for susceptibility testing by both CLSI and EUCAST have been identified. Ardendrup and colleagues evaluated susceptibility methodologies on a C. albicans isolate from a patient who died from a fungal infection which had been treated with caspofungin.Citation37 In addition to the EUCASTCitation38,Citation39 and CLSICitation40 methods they evaluated Etest and agar dilution susceptibility methods. The EUCAST method resulted in a susceptible interpretation for both caspofungin and anidulafungin with MICs of ≤2 and ≤0.125 μg/mL, respectively. The CLSI method resulted in caspofungin and anidulafungin MICs of ≤2 and ≤0.5 μg/mL, respectively. Etest demonstrated MICs for both agents of >32 μg/mL and agar dilution showed growth at all dilutions including 2 μg/mL. Molecular characterization of the isolate revealed a mutation in the hot spot region of the FKS1 gene.Citation37 It has been demonstrated that in the presence of serum the MICs of caspofungin increase an average of 1- to 16-fold, micafungin 32- to 128-fold, and anidulafungin 8- to 256-fold compared to testing conditions without serum.Citation41,Citation42 Garcia-Effron and colleagues evaluated the susceptibility of the echinocandins in the absence and presence of serum against 14 isolates with FKS1 mutation.Citation43 CLSI and EUCAST methodologies don’t include serum in their methodologies and under these conditions 12/14 isolates were susceptible to anidulafungin, 10/14 to micafungin, and only 3/14 to caspofungin. In the presence of serum 2/14 were susceptible to anidulafungin, 1/14 to micafungin, and 0/14 to caspofungin.Citation43 Therefore, current susceptibility testing methods may not detect all echinocandin non-susceptible candida isolates with the FKS1 mutation. Further evaluation is needed to determine if changing the breakpoint for micafungin and anidulafungin is warranted to detect non-susceptible candida organisms. In addition there is a need to further evaluate the role of including serum in the methodologies for susceptibility testing as well as how to interpret the data. At present, Garcia-Effron and colleagues postulate that caspofungin can be used as a surrogate marker for predicting the susceptibility of all of the echinocandins based on the premise that the echinocandins share the same target, mechanism of resistance, spectrum of activity and in vitro potency.Citation43
Pharmacodynamics
Results from four phase 2 and 3 studies of anidulafungin in patients with esophageal or oropharyngeal candidiasis were examined to determine a pharmacokinetic-pharmacodynamic relationship. In this study, successful treatment was defined as either resolution of signs and symptoms or endoscopic response at the completion of therapy. Multiple pharmacokinetic parameters were associated with success and included the AUC at steady state (AUCss) greater than 35 mg*h/L, concentration at steady state (Css) greater than 1.5 μg/mL, and minimum concentration (Cmin) greater than 1 μg/mL. This study did not specify which of these pharmacokinetic parameters was most closely associated with success. Anidulafungin’s potent activity against Candida spp. and its favorable pharmacokinetics allow drug exposure to be in excess of these pharmacokinetic-pharmacodynamic targets with recommended doses. Anidulafungin at the approved maintenance dosage of 50 mg per day for esophageal candidiasis produces a Css of 2.2 μg/mL, an AUCss of 53 mg*h/L and a Cmin above 1 μg/mL throughout the dosing interval in a typical patient.Citation44
Similar findings were also reported in animal studies. When a pharmacokinetic-pharmacodynamic relationship was evaluated in persistently neutropenic rabbit infection model with disseminated candidiasis, 100% efficacy was achieved with a Cmax of approximately 2 μg/mL, an AUC0–24 of 8 μg*h/mL, and a time of 12 hours with plasma concentration above the minimum fungicidal concentration (MFC) for the test organism. Again, this model was not able to discern which parameter most closely associated with optimal anti-fungal activity.Citation45 Another study of pharmacodynamic characterization in a neutropenic murine model of disseminated candidiasis reported concentration-dependent efficacy against C. albicans and C. glabrata. In this study, the Cmax:MIC and the AUC0–24:MIC ratios were most strongly associated with antifungal activity.Citation46 A post anti-fungal effect (PAFE) exists for the echinocandins and candida. Against Candida spp., the PAFE is concentration-dependent with higher concentrations resulting in longer PAFEs.Citation47,Citation48 At concentrations equal to or greater than the MIC of the Candida organism the PAFE was greater than 12 hours for most isolates tested.Citation47,Citation48
An Eagle effect is an in vitro paradoxical effect, and above a particular concentration instead of a decrease in organism, an increase occurs. This effect has been observed with the echinocandins with both yeast and filamentous fungi.Citation49–Citation51 Stevens and colleagues postulated that the high concentrations derepressed resistance mechanisms.Citation52 The clinical significance of this phenomenon is unknown but appears to be negligible and further evaluation may be warranted.
Pharmacokinetics
Pharmacokinetic studies of anidulafungin have been conducted in healthy volunteers, patients with invasive fungal infection, renal or hepatically impaired patients, and in children. Results from these studies demonstrate that anidulafungin has poor and variable absorption after oral administration. However, when administered intravenously, absorption concentrations are predictable and exposure is increased linearly with dose.
Pharmacokinetics in healthy volunteers
The pharmacokinetics of [14C] anidulafungin at a mean dose 88.3 mg (range: 87.6 to 88.7 mg) and 95 μg Ci were evaluated in 9 healthy male volunteers. Following a single intravenous dose of anidulafungin, a mean Cmax of 3.63 μg/mL, mean AUC of 92.5 μg*h/mL, a large volume of distribution (Vd) of 32.6 L and a long-mean terminal elimination half-life (t1/2) of 27.7 hours were reported.Citation53
In addition to the aforementioned pharmacokinetic study in healthy volunteers, other experiments that included in vitro degradation, in vitro human cytochrome P450 inhibition, in vitro incubation with rat and human hepatocytes, and mass balance studies in rats were conducted to characterize anidulafungin clearance. The results revealed that anidulafungin undergoes slow chemical degradation to a primary inactive product, which is likely further degraded by plasma peptidases. The primary degradation product and subsequent ones produced by plasma peptidases are assumed to be void of antifungal activity. The products from degradation and less than 10% of the unchanged drug are eliminated into feces via biliary excretion. Although the intact drug has a t1/2 of approximately 1 day, the degradation products are thought to persist in the body for a longer period of time. Anidulafungin does not undergo hepatic metabolism nor interact with cytochrome P450 isoenzymes. Renal elimination of the drug is negligible.Citation53
Pharmacokinetics in patients with invasive fungal infections
Data from four different phase 2 and 3 clinical studies were combined to describe the pharmacokinetic characteristics of anidulafungin in patients with invasive fungal infections. A total of 225 patients received various anidulafungin regimens consisting of a loading dose of twice the daily maintenance dose (50, 75, 100 mg) as treatment for esophageal candidiasis (129 patients), invasive candidiasis (87 patients), invasive aspergillosis (7 patients) or azole-refractory mucosal candidiasis (2 patients). All doses were administered intravenously at a rate of 1 mg/min.Citation54
The results revealed that a two-compartment model with first-order elimination best described the disposition of anidulafungin. The estimated pharmacokinetic parameters were similar to those observed in healthy volunteers. The clearance was estimated to be 0.946 L/h, the Vd at steady state was 33.2 L, and the t1/2 was 25.9 hours. When demography (age, sex, weight, race), concomitant drugs, and study participation were taken into consideration, the central volume of distribution increased with increasing body weight. In addition, clearance was increased in male subjects, patients with increased body weight and patients who participated in the invasive candidiasis study. Patients in the invasive candidiasis study were hospitalized, older, had higher body weight, and were more acutely ill than those who participated in the esophageal candidiasis study which may have contributed to altered clearance of the drug. However, these predictors explained less than 20% of the difference in clearance rate and the differences were deemed to have little clinical significance.Citation54
Concomitant medications that were categorized as substrates, inducers, or inhibitors of cytochrome P450 isoenzymes, including rifampin were also evaluated in this study. None of these drugs had significant impact on anidulafungin population pharmacokinetic parameters, indicating lower potential for interactions with drugs that affect cytochrome P450 isoenzymes.Citation54
Renal and hepatic impairment
To evaluate anidulafungin pharmacokinetics in patients with hepatic insufficiency, a single intravenous dose of 50 mg was administered to 19 subjects (6 mild, 6 moderate, and 7 severe hepatic impairment patients). Pharmacokinetic parameters in patients with mild or moderate impairment were not significantly different from healthy controls. On the other hand, subjects with severe hepatic impairment showed statistically significant decreases in Cmax (36% decrease: mean ± SD 1.8 ± 0.8 vs 2.9 ± 0.7 μg/mL) and AUC (33% decrease 46.6 ± 14.1 vs 70.0 ±13.4 μg*g/mL) as well as, significant increases in clearance (57% increase: 1.16 ± 0.34 vs 0.74 ± 0.15 L/h) and volume of distribution at steady state (78% increase: 50.8 ± 17.0 vs 28.5 ± 6.5 L). However, the half-life was similar in both groups (severe hepatic impairment vs controls: 35.2 ± 7.1 vs 31.2 ± 1.5 hours). This decrease in exposure compared with control subjects were thought to be due to ascites and edema. Unfortunately, protein binding was not evaluated in this study. The reduced Cmax and AUC in these patients may be important factors to consider in the treatment of fungemia. However, anidulafungin 50 mg per day produces levels that exceed the MIC 90 of most Candida spp. throughout the dosing period. Consequently, no dosage adjustment of anidulafungin is currently recommended for any degree of hepatic impairment.Citation55
Anidulafungin’s pharmacokinetic profile was evaluated in 21 patients with varying degrees of renal function. Patients with mild (51 to 70 mL/min), moderate (31 to 50 mL/min), severe (≤30mL/min) renal impairment or patients with end-stage renal disease were given a single 50 mg dose of anidulafungin. In comparison to 8 healthy volunteers, pharmacokinetic profiles were similar among the groups. In addition, no measurable quantity of anidulafungin was present in dialysate. Therefore, due to minimal renal excretion and clearance by hemodialysis, no dosage adjustment of anidulafungin is needed in renal insufficiency.Citation55
Pediatric pharmacokinetics
The pharmacokinetic profile of anidulafungin was studied in immunocompromised, hospitalized children with neutropenia. Children aged 2 to 17 years were given either a loading dose of 1.5 mg/kg (maximum 100 mg) followed by 0.75 mg/kg per day (maximum 50 mg) or a loading dose of 3 mg/kg (maximum 200 mg) followed by 1.5 mg/kg per day (maximum 100 mg). The mean duration of therapy was 8.7 days (range of 1 to 23 days). As with adults, steady-sate concentration was achieved after the loading dose. Similar concentration profile is reported in pediatric patients receiving doses of 0.75 mg/kg per day and adults receiving 50 mg per day as well children receiving 1.5 mg/kg per day and adults 100 mg per day. The half-life was approximately 20 hours which was slightly less than those estimated in adults, but still supports once daily dosing. Body weight affected clearance and volume of distribution. Therefore, for children aged 2 years and older, anidulafungin should be dosed based on body weight and no dosage adjustment is recommended based on age.Citation56
Clinical trials
Esophagitis
One randomized, double-blind, non-inferiority trial comparing anidulafungin to fluconazole therapy was assessed for esophageal candidiasis.Citation57 Anidulafungin 100 mg loading dose followed by 50 mg once daily (n = 249 evaluable patients) and was compared to fluconazole 200 mg loading dose followed by 100 mg once daily (n = 255 evaluable patients). The endoscopic success rates at the end of therapy (EOT) for anidulafungin and fluconazole were 97.2% and 98.8%, respectively. The clinical success rates were 98.8% for anidulafungin 99.6% for fluconazole. The endoscopic exam at the 2-week follow-up of 462 patients revealed a success rate of 64.4% for anidulafungin compared to 89.5% for fluconazole which was statistically significant.Citation57
A phase 2 open-label trial of anidulafungin for the treatment of azole-refractory mucosal candidiasis was performed.Citation58 Nineteen patients were enrolled and received anidulafungin 100 mg loading dose followed by 50 mg once daily. Seventeen of 18 patients (94%) of patients with oropharyngeal candidiasis and 11/12 patients (92%) with esophageal candidiasis achieved clinical success at the end of therapy. The clinical success at the 10- to 14-day follow-up was 8/18 (44%) with oropharyngeal candidiasis and 6/12 patients (50%) with esophageal candidiasis.Citation58
Candidemia/invasive candidiasis
Two studies evaluating the efficacy of anidulafungin for candidemia or invasive candidiasis have been performed. The first was a randomized, dose ranging study in adult patients with doses of 50 mg, 75 mg, or 100 mg once daily of anidulafungin.Citation59 In the modified-intent-to-treat (MITT) analysis there were 37, 40, and 39 patients in the 50 mg, 75 mg, and 100 mg dosing groups, respectively. A loading dose of twice the maintenance dose was administered on day 1 in each dosage group. Candidemia was the most prevalent infection occurring in 94% of patients, 10% (12 patients) had positive tissue cultures, 4% (5 patients) had both positive tissue and blood cultures, and 1 patient had a prosthetic hip infection. C. albicans accounted for 53% of the infections followed by C. glabrata (31%), C. tropicalis (9%), C. parapsilosis (9%), C. krusei (4%), then others at 3%. Global response was defined as both clinical and microbiological success and was assessed at EOT and follow-up. At EOT the global response for the 83 evaluable patients was 84%, 90%, and 89% with the 50 mg, 75 mg, and 100 mg doses, respectively. At follow-up, the global response of the 68 evaluable patients decreased to 72%, 85%, and 83% with the 50 mg, 75 mg, and 100 mg doses, respectively.Citation59
The second study was a randomized, prospective, non-inferiority study comparing anidulafungin to fluconazole for candidemia or invasive candidiasis.Citation60 Patients aged 16 to 91 years received either 200 mg on day 1 followed by 100 mg once daily of anidulafungin or 800 mg on day 1 followed by 400 mg once daily of fluconazole for at least 14 days from improvement of symptoms and negative cultures. The primary outcome was a successful global response which was defined as both clinical success (resolution of signs and symptoms of invasive candidiasis and no need for additional systemic antifungal therapy) and microbiologic success (eradication of candida species present at baseline which was determined on follow-up culture or the presumed eradication if cultures were not available) at the end of intravenous therapy. Secondary outcomes were global response at the end of all therapy, 2 and 6 weeks follow-up. In the MITT analysis 127 patients received anidulafungin and 118 received fluconazole. Candidemia was the most prevalent infection occurring in 116/127 (91.3%) of patients receiving anidulafungin and 103/118 (87%) of patients receiving fluconazole. Seven (6%) and 11 patients (9%) had candida recovered from other sterile body fluids or sites in the anidulafungin and fluconazole groups, respectively. Three percent in both groups had candida in both the blood and a sterile site. C. albicans was the predominant pathogen in patients receiving either anidulafungin (64%) or fluconazole (59%). The other pathogens in the anidulafungin arm were C. glabrata (16%), C. tropicalis (12%), C. parapsilosis (10%). The pathogens in the fluconazole arm were slightly different in frequency with C. glabrata (25%), C. tropicalis (9%), C. parapsilosis (14%), however this was not statistically different. A successful global response at the end of intravenous therapy was 75.6% (96/127 patients) in the anidulafungin arm and 60.2% (71/118 patients) in the fluconazole arm which was statistically significant. Anidulafungin demonstrated higher successful global response than fluconazole at each of the secondary assessments: EOT (74.0% vs 56.8%), 2-week follow-up (64.6% vs 49.2%), and 6-week follow-up (55.9% vs 44.1%) although at 6 weeks the difference was not statistically significant. Mortality was higher in the patients receiving fluconazole (37/118, 31.4%) than anidulafungin (29/127, 22.8%) however this was not significant. In addition, more patients died in the first 10 days in the fluconazole arm (14) compared to 5 in the anidulafungin group. This study demonstrated anidulafungin was more efficacious than fluconazole at end of intravenous therapy for treating candidemia/invasive candidiasis. A curiosity of the study is the clinical response rates for C. albicans and C. glabrata. In the anidulafungin arm the success rates were 81.1% and 56.3% for albicans and glabrata, and in the fluconazole arm were 62.3% and 50%, respectively. It appears the primary difference in global response was due to the poor response in fluconazole treated patients infected with C. albicans. One study site enrolled 25 patients which accounted for 10% of the MITT population. Fifteen patients received anidulafungin and 14 had a successful global response and only 5 of the 10 patients who received fluconazole had a successful global response. Statistical analysis did not reveal a study site bias. However, if those 25 patients are removed from the analysis then there is no difference in global response between the two therapies. According to FDA guidelines, a second study demonstrating this exceptional outcome of anidulafungin over fluconazole would be required in order to prove superiority.
Invasive candidiasis/candidemia in neutropenic patients
In a neutropenic mouse model of invasive candidiasis, anidulafungin demonstrated good activity against 3 strains of C. glabrata; 1 was resistant to fluconazole and 1 was resistant to amphotericin B.Citation61 Clinical trials with anidulafungin enrolled so few patients with neutropenia that no assessment could be made therefore anidulafungin does not have a FDA indication for treating candidemia or invasive candidiasis in neutropenic patients. Despite this lack of indication the Infectious Diseases Society of America has recommended anidulafungin as a potential first-line therapy for the treatment of candidemia in neutropenic patients.Citation6 However, anidulafungin is not indicated in the guidelines for the empiric treatment of suspected invasive candidiasis in neutropenic patients, nor is it recommended for prophylaxis for solid-organ transplant recipients, patients hospitalized in intensive care units, neutropenic patients receiving chemotherapy, and stem cell transplant recipients at risk of candidiasis.Citation6
Drug interactions
Anidulafungin is neither a substrate nor an inhibitor of the cytochrome P450 enzyme system or of P-glycoprotein. Therefore, it is unlikely that anidulafungin will alter the pharmacokinetics of drugs that influence cytochrome P450 isoenzymes or be affected by them. Several studies evaluated the influence of anidulafungin on the metabolism of rifampin, cyclosporine, tacrolimus liposomal amphotericin B and voriconazole. These investigations do not report any significant alterations in the pharmacokinetics of either the tested agent or of anidulafungin.
Rifampin
In the aforementioned population pharmacokinetic study, concomitant medications taken by 225 patients were categorized as substrates, inducers, or inhibitors of cytochrome P450 and evaluated for their effect on clearance of anidulafungin. Rifampin is a potent inducer and therefore, was evaluated separately. A total of 27 patients (12%) were taking rifampin during the study. Anidulafungin clearance was not affected by concomitant treatment with substrates, inhibitors, or inducers of the cytochrome P450 isoenzymes, including rifampin.Citation54
Cyclosporine
The interaction of anidulafungin and cyclosporine was evaluated in 12 healthy volunteers. Subjects were given anidulafungin 200 mg on day 1 then 100 mg once daily intravenously on days 2 to 8 and cyclosporine 1.25 mg/kg orally twice daily on days 5 to 8. One subject was withdrawn from the study on day 6 due to slight increases in hepatic transaminase levels. After concomitant administration of cyclosporine, the mean AUC of anidulafungin was 22% higher, the mean Cmin was 43% higher, the clearance was 16% lower. These alterations in anidulafungin pharmacokinetics were not considered clinically significant and subsequently, no dosage adjustments were recommended. The effect of anidulafungin on cyclosporine pharmacokinetics was not evaluated in this study.Citation62
Tacrolimus
The potential interaction between anidulafungin and tacrolimus was evaluated in 36 healthy male volunteers. Subjects received tacrolimus 5 mg orally on days 1 and 13 and anidulafungin 200 mg on day 4 followed by 100 mg once daily intravenously on days 5 to 13. There were no significant differences in any of the pharmacokinetic parameters measured with or without co-administration of tacrolimus and anidulafungin. Therefore, no dosage adjustment is recommended.Citation63
Liposomal amphotericin B
The effect of co-administration of anidulafungin and liposomal amphotericin B was evaluated in 17 patients with invasive aspergillosis. Anidulafungin (100 mg once daily) and liposomal amphotericin B (5 mg/kg per day) were administered concurrently until resolution of signs or symptoms of aspergillosis or for a total of 90 days. Co-administration of these two antifungal agents was well tolerated by all subjects.Citation64
Voriconazole
A combination of anidulafungin and voriconazole was evaluated in 17 healthy male volunteers. In a blinded, randomized, crossover design, subjects received anidulafungin with placebo, voriconazole with placebo, and anidulafungin with voriconazole. Voriconazole was administered orally 400 mg every 12 hours on day 1 followed by 200 mg every 12 hours on days 2 to 4. Anidulafungin was given intravenously 200 mg on day 1 followed by 100 mg per day on days 2 to 4. There were no significant differences in pharmacokinetic parameters when subjects received anidulafungin alone or in combination with voriconazole or voriconazole alone or in combination with anidulafungin. Co-administration of anidulafungin and voriconazole was well tolerated.Citation65
Safety
Anidulafungin is well tolerated with few adverse effects. Abnormal liver function tests and hypokalemia are the most commonly reported adverse effects at 1.5% to 5% and 3% to 10%, respectively. Nausea, vomiting, and diarrhea have also been reported in 1% to 3% of patients.Citation57,Citation59,Citation60 Histamine-related adverse effects such as rash, urticaria, flushing, pruritus, dyspnea, and hypotension have been reported when the infusion rate exceeds 1.1 mg/min.Citation7
Summary
Anidulafungin is active against most species of candida and resistance to it is very rare. Because of its spectrum of activity, fungicidal nature, and tolerability it is an attractive first-line therapeutic choice for treating candidemia in both non-neutropenic and neutropenic patients. Because it is available only parenterally its role in treating mucocutaneous candidiasis is primarily in patients unable to take oral therapy. Further studies are needed to define the role of anidulafungin in the empiric treatment of suspected invasive candidiasis in neutropenic patients, or other immunocompromised patients, candida osteomyelitis, meningitis, and endocarditis.
Disclosures
The authors declare no conflicts of interest.
References
- WisplinghoffHBischoffTTallentSMSeifertHWenzelRPEdmondMBNosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance studyClin Infect Dis20043930931715306996
- SmithMJCatheter-related bloodstream infections in childrenAmer J Infect Contr200836S173.e171S173.e173
- MartinGSManninoDMEatonSMossMThe epidemiology of sepsis in the United States from 1979 through 2000N Engl J Med20033481546155412700374
- ZilberbergMDShorrAFKollefMHSecular trends in candidemia-related hospitalization in the United States, 2000–2005Infect Control Hosp Epidemiol20082997898018715153
- RichardsonMDChanging patterns and trends in systemic fungal infectionsJ Antimicrob Chemother200556i51116120635
- PappasPGKauffmanCAAndesDClinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of AmericaClin Infect Dis20094850319191635
- PfizerIEraxis (anidulafungin) package insertNew York, NY2007
- BartizalKGillCJAbruzzoGKIn vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872)Antimicrob Agents Chemother199741232623329371328
- Clinical and Laboratory Standards Institute (CLSI)Reference method for broth dilution antifungal susceptibility testing of yeasts: Approved standard3rd. edWayne, PA2008
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST)EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia–forming mouldsClin Microbiol Infect20081498298418828858
- PfallerMABoykenLHollisRJMesserSATendolkarSDiekemaDJIn vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazoleJ Clin Microbiol2005435425542716272464
- Ostrosky-ZeichnerLRexJHPappasPGAntifungal Susceptibility Survey of 2,000 Bloodstream Candida Isolates in the United StatesAntimicrob Agents Chemother2003473149315414506023
- PfallerMADiekemaDJMesserSAHollisRJJonesRNIn vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolatesAntimicrob Agents Chemother2003471068107112604543
- Cuenca-EstrellaMRodriguezDAlmiranteBIn vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002–2003J Antimicrob Chemother20055519419915618284
- MaligieMASelitrennikoffCPCryptococcus neoformans resistance to echinocandins: (1,3)beta-glucan synthase activity is sensitive to echinocandinsAntimicrob Agents Chemother2005492851285615980360
- PfallerMAMarcoFMesserSAJonesRNIn vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungiDiagn Microbiol Infect Dis1998302512559582584
- SerranoMdCValverde-CondeAChavezMMIn vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against aspergillus sppDiagn Microbiol Infect Dis20034513113512614985
- DiekemaDJMesserSAHollisRJJonesRNPfallerMAActivities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungiJ Clin Microbiol2003413623362612904365
- ArikanSYurdakulPHascelikGComparison of two methods and three end points in determination of in vitro activity of micafungin against Aspergillus sppAntimicrob Agents Chemother2003472640264312878531
- OdabasiZPaetznickVLRodriguezJRChenEOstrosky-ZeichnerLIn vitro activity of anidulafungin against selected clinically important mold isolatesAntimicrob Agents Chemother2004481912191515105159
- Schuetzer-MuehlbauerMWillingerBKrapfGEnzingerSPresterlEKuchlerKThe Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofunginMolecul Microbiol200348225235
- NiimiKMakiKIkedaFOverexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibilityAntimicrob Agents Chemother2006501148115516569823
- ParkSKellyRKahnJNSpecific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida spp. isolatesAntimicrob Agents Chemother2005493264327316048935
- DouglasCMD’IppolitoJASheiGJIdentification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitorsAntimicrob Agents Chemother199741247124799371352
- BalashovSVParkSPerlinDSAssessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1Antimicrob Agents Chemother2006502058206316723566
- Garcia-EffronGKatiyarSKParkSEdlindTDPerlinDSA Naturally Occurring Proline-to-Alanine Amino Acid Change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis Accounts for Reduced Echinocandin SusceptibilityAntimicrob Agents Chemother2008522305231218443110
- PerlinDSResistance to echinocandin-class antifungal drugsDrug Resistance Updates20071012117569573
- KahnJNGarcia-EffronGHsuMJParkSMarrKAPerlinDSAcquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthaseAntimicrob Agents Chemother200751187617325225
- GhannoumMAChenABuhariMDifferential in vitro activity of anidulafungin, caspofungin and micafungin against Candida parapsilosis isolates recovered from a burn unitClin Microbiol Iinfect200915274
- MoudgalVLittleTBoikovDVazquezJAMultiechinocandin- and Multiazole-Resistant Candida parapsilosis Isolates Serially Obtained during Therapy for Prosthetic Valve EndocarditisAntimicrob Agents Chemother20054976776915673762
- MillerCDLomaestroBWParkSPerlinDSProgressive esophagitis caused by Candida albicans with reduced susceptibility to caspofunginPharmacother200626877880
- LaverdiereMLalondeRGBarilJGSheppardDCParkSPerlinDSProgressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitisJ Antimicrob Chemother20065770570816464893
- HernandezSLopez-RibotJLNajvarLKMcCarthyDIBocanegraRGraybillJRCaspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitisAntimicrob Agents Chemother2004481382138315047549
- Krogh-MadsenMArendrupMCHesletLKnudsenJDAmphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patientClin Infect Dis20064293894416511756
- HakkiMStaabJFMarrKAEmergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapyAntimicrob Agents Chemother2006502522252416801435
- PasqualeTTomadaJRGhannounMDipersioJBonillaHEmergence of Candida tropicalis resistant to caspofunginJ Antimicrob Chemother20086121918024953
- ArendrupMCGarcia-EffronGBuzinaWBreakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testingAntimicrob Agents Chemother200953118519104024
- Cuenca-EstrellaMMooreCBBarchiesiFMulticenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST)Clin Microbiol Iinfect20039467
- Cuenca-EstrellaMArendrupMCChryssanthouEMulticentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST)Clin Microbiol Iinfect2007131018
- National Committee for Clinical Laboratory StandardsReference method for broth dilution antifungal susceptibility testing of yeasts: Approved standard M27-A22nd edNational Committee for Clinical Laboratory StandardsWayne, PA2002
- PaderuPGarcia-EffronGBalashovSDelmasGParkSPerlinDSSerum Differentially Alters the Antifungal Properties of Echinocandin DrugsAntimicrob Agents Chemother2007512253225617420211
- OdabasiZPaetznickVRexJHOstrosky-ZeichnerLEffects of serum on in vitro susceptibility testing of echinocandinsAntimicrob Agents Chemother2007514214421617785512
- Garcia-EffronGParkSPerlinDSCorrelating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpointsAntimicrob Agents Chemother20095311212218955538
- DowellJAStogniewMKrauseDSHenkelTAnidulafungin (ANID) pharmacokinetic (PK)/Pharmacodynamic (PD) correlation: Treatment of esophageal candidiasis (EC)43rd Interscience Conference on Antimicrob Agents ChemotherWashington, DCAmerican Society of Microbiology2003A1578
- GrollAHMickieneDPetraitieneRPharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma samplingAntimicrob Agents Chemother2001452845285511557479
- AndesDDiekemaDJPfallerMAIn vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis modelAntimicrob Agents Chemother20085253955018070979
- ErnstEJRolingEEPetzoldCRKeeleDJKlepserMEIn vitro activity of micafungin (FK-463) against Candida spp: microdilution, time-kill, and postantifungal-effect StudiesAntimicrob Agents Chemother2002463846385312435687
- ErnstEJKlepserMEPfallerMAPostantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformansAntimicrob Agents Chemother2000441108111110722525
- HallGSMylesCPrattKJWashingtonJACilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalisAntimicrob Agents Chemother198832133113353058017
- RamageGVandeWalleKBachmannSPWickesBLLopez-RibotJLIn vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studiesAntimicrob Agents Chemother2002463634363612384379
- WiederholdNPKontoyiannisDPChiJPrinceRATamVHLewisREPharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activityJ Infect Dis20041901464147115378439
- StevensDAEspirituMParmarRParadoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrationsAntimicrob Agents Chemother2004483407341115328104
- DamleBDDowellJAWalskyRLWeberGLStogniewMInskeepPBIn vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafunginAntimicrob Agents Chemother200953114919029327
- DowellJAKnebelWLuddenTStogniewMKrauseDHenkelTPopulation pharmacokinetic analysis of anidulafungin, an echinocandin antifungalJ Clin Pharmacol20044459059815145966
- DowellJAStogniewMKrauseDDamleBAnidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairmentJ Clin Pharmacol20074746147017389555
- BenjaminDKJrDriscollTSeibelNLSafety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infectionsAntimicrob Agents Chemother20065063263816436720
- KrauseDSSimjeeAEvan RensburgCA randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasisClin Infect Dis20043977015472806
- VazquezJASchranzJAClarkKGoldsteinBPReboliAFichtenbaumCA phase 2, open-label study of the safety and efficacy of intravenous anidulafungin as a treatment for azole-refractory mucosal candidiasisJ Acquir Immune Defic Syndr20084830430918545153
- KrauseDSReinhardtJVazquezJAPhase 2, randomized, dose-ranging study evaluating the safety and efficacy of anidulafungin in invasive candidiasis and candidemiaAntimicrob Agents Chemother2004482021202415155194
- ReboliACRotsteinCPappasPGAnidulafungin versus fluconazole for invasive candidiasisN Engl J Med20073562472248217568028
- GumboTDrusanoGLLiuWAnidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasisAntimicrob Agents Chemother200650369516954319
- DowellJAStogniewMKrauseDHenkelTWestonIEAssessment of the safety and pharmacokinetics of anidulafungin when administered with cyclosporineJ Clin Pharmacol20054522723315647416
- DowellJAStogniewMKrauseDHenkelTDamleBLack of pharmacokinetic interaction between anidulafungin and tacrolimusJ Clin Pharmacol20074730531417322142
- HerbrechtRGrahamDSchusterMSafety and tolerability of combination anidulafungin (ANID) and liposomal amphotericin B (LAmB) for the treatment of invasive aspergillosis (IA)Tandem Bone Marrow Transplantation MeetingsOrlando, FLAmerican Society for Blood and Marrow Transplantation200491
- DowellJASchranzJBaruchAFosterGSafety and pharmacokinetics of coadministered voriconazole and anidulafunginJ Clin Pharmacol2005451373138216291712